Abstract
Background
Circulating cell-free DNA (cfDNA) is emerging as a surrogate sample type for mutation analyses. To improve the clinical utility of cfDNA, we developed a sensitive peptide nucleic acid (PNA)-based method for analyzing EGFR and KRAS mutations in the plasma cfDNA of patients with advanced non-small cell lung cancer (NSCLC).
Methods
Baseline tissue and plasma samples were collected from treatment-naïve advanced NSCLC patients participated in a randomized phase II study, which was registered with ClinicalTrials.gov at Feb. 2009 (NCT01003964). EGFR and KRAS mutations in the plasma cfDNA were analyzed retrospectively using a PNA clamping-assisted fluorescence melting curve analysis. The results were compared with those obtained from tissue analysis performed using the direct sequencing. Exploratory analyses were performed to determine survival predicted by the plasma and tissue mutation status.
Results
Mutation analyses in matched tissue and plasma samples were available for 194 patients for EGFR and 135 patients for KRAS. The mutation concordance rates were 82.0 % (95 % confidence interval [CI], 76.5–87.4) for EGFR and 85.9 % (95 % CI, 80.1–91.8) for KRAS. The plasma EGFR mutation test sensitivity and specificity were 66.7 % (95 % CI, 60.0–73.3) and 87.4 % (95 % CI, 82.7–92.1), respectively, and the plasma KRAS mutation test sensitivity and specificity were 50.0 % (95 % CI, 41.6–58.4) and 89.4 % (95 % CI, 84.2–94.6), respectively. The predictive value of the plasma EGFR and KRAS mutation status with respect to survival was comparable with that of the tissue mutation status.
Conclusions
These data suggest that plasma EGFR and KRAS mutations can be analyzed using PNA-based real-time PCR methods and used as an alternative to tumor genotyping for NSCLC patients when tumor tissue is not available.
Similar content being viewed by others
Background
The paradigm of diagnosis and treatment for advanced non-small cell lung cancer (NSCLC) has changed since epidermal growth factor receptor (EGFR) mutations were identified as the best predictive biomarkers for EGFR-tyrosine kinase inhibitor (TKI) efficacy [1, 2]. Decisions on first-line treatments are based on the target oncogenes identified in tumor tissues; thus, the tumors of patients with NSCLC should be tested for EGFR mutations to determine whether an EGFR-TKI is the appropriate first-line therapy [3]. However, obtaining adequate tissue samples for molecular testing can be difficult. Consequently, efforts have been made to evaluate surrogate sample types for molecular testing [4].
Circulation cell-free (cf) DNA in the plasma of cancer patients offers an easily obtainable and repeatedly available source of biological material for mutation analyses [5, 6]. Several methods have been reported for the detection of EGFR mutations in cfDNA isolated from plasma, including high performance liquid chromatography, allele-specific polymerase chain reaction (PCR) with Scorpion amplification, peptide nucleic acid (PNA)-mediated PCR clamping, BEAMing, droplet digital PCR (ddPCR), and next-generation sequencing (NGS) [7–9]. The pooled sensitivity and specificity of EGFR mutations in cfDNA has been reported at 67.4 and 93.5 %, respectively [10]. Compared with EGFR mutations, KRAS mutations are predictive for a lack of benefit from EGFR-TKI therapy. Although erlotinib has been approved for second or third–line therapy for advanced NSCLC irrespective of EGFR mutation status, many studies have demonstrated that patients with KRAS mutations show inferior outcomes compared with those with wild-type KRAS [11–13]. Thus, KRAS genotyping can be considered for patients scheduled to receive EGFR-TKI therapy. Recent data indicate that the cfDNA in plasma could represent a new sample type for the analysis of KRAS mutations in tumors and act as a potential biomarker for anti-EGFR therapy efficacy in colorectal cancer [14–17].
PANAMutyper™ R EGFR and KRAS kits are newly developed sensitive kits that apply a PNA clamping-assisted fluorescence melting curve analysis to perform mutation detection and genotyping. The PNA clamp-assisted melting curve method can discriminate mutant from wild-type alleles through a relatively large melting temperature difference and has a sensitivity of 0.1–0.01 % [18, 19]. Additionally, this method can easily detect the presence of a mutant sequence without an additional data analysis process. The objective of this study was to compare the plasma analysis performed using PANAMutyper™ R EGFR and KRAS kits with tumor tissue analysis performed using routine EGFR and KRAS mutation tests. The aim of this analysis was to validate the use of cfDNA as a surrogate sample type for the detection of EGFR and KRAS mutations in advanced NSCLC.
Methods
Patients
Patients with advanced NSCLC who participated in a randomized phase II study that compared gemcitabine and cisplatin (GP) with irinotecan and cisplatin (IP) as first-line therapies were tested in this study. The trial is registered with ClinicalTrials.gov (NCT01003964). The main eligibility criteria included histologic confirmation of advanced NSCLC, no prior chemotherapy, age ≥ 18 years, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) less than 2, and measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST). Adequate organ function was required. All of the patients who received at least one cycle of chemotherapy were considered assessable for the progression-free survival (PFS), overall survival (OS), and safety. Archival plasma and tissue samples obtained prior to treatment were used for the EGFR and KRAS mutation tests. All patients provided written informed consent for the provision of tumor and plasma samples for the biomarker analysis. The protocol was approved by the National Cancer Center Institutional Review Board (study ID number: NCCCTS08371) and conducted in accordance with the Declaration of Helsinki, Good Clinical Practice.
Clinical assessment
The tumors were assessed by computed tomography of the targeted lesions every two cycles of therapy, every 6 weeks during chemotherapy and every 8 weeks during EGFR-TKI therapy. The objective tumor response was determined using RECIST software, version 1.0 [20]. The PFS was calculated from the start date of each therapy to the date of tumor progression or death. The OS was calculated from the start date of first-line therapy to the date of death or last follow-up.
EGFR and KRAS mutation analysis using tumor tissues
Genomic DNA was extracted from 10 % neutral buffered formalin-fixed, paraffin-embedded (FFPE) tumor tissue blocks using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany). We analyzed EGFR and KRAS mutations using the polymerase chain reaction (PCR)-based direct DNA sequencing method [21].
Plasma DNA extraction and mutation analysis
Circulating cell-free DNA (cfDNA) was extracted from the plasma using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany). The assays used to detect 47 different EGFR variants and 29 KRAS variants were obtained using the PANAMutyper™ R EGFR and KRAS kit (Panagene, Daejeon, Korea).
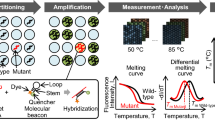
The PNA clamp-assisted melting curve method, the real-time genotyping of somatic mutations, can detect multiple mutations and analyze both curves in a single PCR experiment by using a PNA clamp and PNA detection probe [18, 19, 22]. A detection probe was designed for competitive hybridization with clamping PNA on the same strand (either sense or antisense DNA strand). In our system, two complementary PNA oligomers were designed to hybridize with either a sense or antisense strand (Fig. 1a, b). The PNA detection probes produce an amplification curve and are used to analyze the melting curve. When we take a look closely, all reactions were performed in total volumes of 25 μ l containing 10–25 ng template DNA, primers, PNA probe set, and PCR master mix. The PCR assay was performed under the following conditions: two holding periods of 50 °C for 2 min and 95 °C for 15 min; (i) 15 cycles of 95 °C for 30 s, 70 °C for 20 s, and 63 °C for 60 s; and (ii) 35 cycles of 95 °C for 10 s, 53 °C for 20 s, and 73 °C for 20 s. A melting curve step was performed (from 35 °C to 75 °C with temperature increments of 0.5 °C for 3 s) to acquire fluorescence values on all four channels (FAM, ROX, CY5 and HEX). The melting peaks were derived from the melting curve data. In summary, two specific designed PNA oligomers were used in qPCR: one was a clamping PNA, which suppresses the amplification of an undesired or wild-type allele, and the other is a PNA detection probe, which has a fluorophore and a quencher group at each terminus of the Probe. The mutations detected by the melting temperature of each tube of fluorescent dye are summarized in Additional file 1: Table S1. The plasma test was performed by Choi J-J, who was blind to the results obtained from the tissue samples.
Schematic representation of EGFR and KRAS mutation detection using PANAMutyperTM: EGFR (a) and KRAS (b). Sensitivity of the EGFR L858R and E19del (c) and KRAS G12V and G12R (d) mutants according to their cellularity by diluting to 100, 10, 1, 0.1, 0.01, 0 % with respect to the wild cell line DNA and mutant cell line DNA. The data presented here are representative obtained from sensitivity test conducted more than 50 times. MT, mutant type
Statistical analysis
The PFS and OS were evaluated using the Kaplan-Meier method. The log-rank test was used to compare the PFS with the OS according to the EGFR or KRAS mutation status. The 95 % confidence intervals for concordance, sensitivity and specificity were calculated by the Wilson Score method. All tests were two-sided, and P-values less than 0.05 were considered statistically significant. All statistical analyses were conducted using SPSS 21 software (IBM SPSS Chicago, IL, USA).
Results
Determination of mutation status in the plasma samples
The mutation status was determined with PCR clamping, which can specifically block the chain elongation step on a perfectly matched template (wild type [WT]) without interfering with the templates containing mismatched bases (mutant EGFR or KRAS). PNA detection probes with fluorescent dyes and quencher labels were also used in this technique. This hybridization probe system consists of a pair of probes (Fig. 1a, b). To determine the PANAMutyper™ R EGFR and KRAS sensitivity for each tested mutation, genomic DNA (gDNA) from the mutant cell line containing the tested mutation was serially diluted 5 times into highly concentrated WT gDNA from the human lung cancer cell line A549. The 0.005 and 0.001 % sensitivity values of EGFR and KRAS, respectively, corresponded to a specific detection of 2 and 1 mutated copies in 40,000 copies of WT DNA, respectively (Fig. 1c, d). All experimental points were obtained in triplicate, and all plasma samples that met the inclusion criteria were analyzed correctly (success rate of 100 %).
Patient characteristics
Of the 289 patients who participated in the randomized phase II study, 208 cases with a known EGFR or KRAS mutation status in their tumor tissues were examined. The median age was 58 years (range: 29 to 82). Most of the patients were male (65.4 %) and had a history of smoking (63 %) and presented an adenocarcinoma histology (78.8 %), stage IV disease (72.6 %), and a good performance status (PS) of 0 or 1 (72.6 %). All patients received GP or IP as the first-line therapy. Among the 208 patients, 37 had EGFR exon 19 deletions (19DEL) or L858R mutations and 12 patients had KRAS codon 12 mutations. A total of 98 patients received EGFR-TKI as a second- or third-line therapy upon disease progression. The characteristics of the study population are shown in Table 1.
Tumor tissue and plasma EGFR and KRAS mutation analyses
Matched tumor tissue and plasma mutation statuses were available for 194 patients for EGFR and 135 patients for KRAS. A comparison of the EGFR and KRAS mutation status between the tumor biopsies and plasma samples is summarized in Table 2. The concordance of EGFR mutation status between the tumor tissue and plasma was 82 % (95 % confidence interval [CI], 76.5–87.4), and it presented a sensitivity of 66.7 % (95 % CI, 60.0–73.3) and a specificity of 87.4 % (95 % CI, 82.7–92.1). The positive and negative predictive values were 65.4 % (95 % CI, 58.7–72.1) and 88.0 % (83.5–92.6), respectively.
The concordance of KRAS mutation status between the tumor tissue and plasma was 85.9 % (95 % CI, 80.1–91.8), and it presented a sensitivity of 50.0 % (95 % CI, 41.6–58.4) and a specificity of 89.4 % (95 % CI, 84.2–94.6). The positive and negative predictive values were 31.6 % (95 % CI, 58.7–72.1) and 94.8 % (83.5–92.6), respectively.
To validate the additional mutations that were identified in only the plasma samples, we tested the plasma samples using a digital PCR assay. Six of 7 T790M and 2 of 3 L858R EGFR mutations that were detected in only the plasma samples were confirmed by the digital PCR assay. We were unable to confirm the results in the remaining two samples because the total droplet number in the digital PCR was too low.
EGFR and KRAS mutation status and clinical outcome
The cut-off for the OS update was June 29, 2015, and the median duration of follow-up investigations was 16.7 months (range: 0.5 to 70.9 months). The median OS was 24.6 months (95 % CI, 20.7–28.5) in patients with plasma EGFR 19DEL or L859R mutations and 27.0 months (95 % CI, 25.0–29.0)) in patients with these mutations in their tumor tissue. The median OS was 10.2 months (95 % CI, 4.9–15.5) in patients with plasma KRAS mutations and 8.4 months (95 % CI, 0.0–22.5) in patients with these mutations in their tumor tissue. The Kaplan-Meier curves for the OS according to the tissue and plasma mutation status are shown in Fig. 2.
Because EGFR and KRAS mutations are predictive for the efficacy of EGFR-TKI therapy, we analyzed the association between the mutation status and the therapeutic benefits of this treatment. Of the 208 patients enrolled in this study, 98 received EGFR-TKI therapy as a second- or third-line therapy. We compared the efficacy of EGFR-TKI therapy in the patients carrying common sensitive EGFR mutations, including 19DEL and L858R with the patients carrying wild type EGFR. The median PFS for EGFR-TKI therapy was 8.4 (95 % CI, 5.8–11.0) months for the patients with plasma EGFR 19DEL or L858R mutations and 9.2 (95 % CI, 6.3–12.1) months for the patients with tissue EGFR 19DEL or L858R mutations. The median PFS of the patients with plasma KRAS mutations was 1.6 months (95 % CI, 0.1–3.1), a result that was comparable with the PFS of patients with tumor-tissue KRAS mutations (median PFS, 1.8 months [95 % CI, 0.0–3.6]). The Kaplan-Meier curves for the PFS according to the tissue and plasma mutation status are shown in Fig. 3. However, the correlation of mutations with outcome is of limited interest due to the concordance between mutational status in tissue and plasma.
Patients with additional EGFR mutations found in plasma, such as exon20ins and S768I, and multiple mutations (19DEL, L858R, and exon 20ins) showed primary resistance to EGFR-TKI therapy and presented a median PFS of 1.7 months (95 % CI, 0.6–2.8). We also found 8 de novo T790M mutations in plasma samples and three received EGFR-TKI therapy. One patient showed primary resistance and two showed partial responses with PFS values of 9.8 and 15 months.
Discussion
This report describes the first demonstration of a PNA clamping-assisted fluorescence melting curve analysis for the detection of EGFR and KRAS mutations in the plasma cfDNA of NSCLC patients. Our data revealed a relatively high concordance (82.0 % for EGFR and 85.9 % for KRAS) and specificity (87.4 % for EGFR and 89.4 % for KRAS) compared with other reported techniques such as the amplification refractory mutation systems, denaturing high-performance liquid chromatography, and PNA-PCR [23]. The EGFR mutation status has clinical significance as a biomarker and can be used to determine the best treatment for advanced NSCLC; thus, most studies that have evaluated novel analysis techniques have focused on detecting EGFR mutations in blood samples from NSCLC patients. The best reported data for patients with NSCLC were obtained with an EGFR mutation test that uses digital PCR, which resulted in a 92 % sensitivity and 100 % specificity [10]; however, the authors used only 35 samples from NSCLC patients to evaluate the accuracy of the technique. Recently, Douillard et al. reported a high global concordance (94.3 %), specificity (99.8 %) and sensitivity (67.5 %) of the Scorpion ARMS-based EGFR detection kit using 652 samples from patients with advanced NSCLC [24], and the sensitivity was similar to our results (66.7 %). Because of its low sensitivity, the plasma EGFR mutation status appeared to be less predictive for EGFR-TKI therapy benefits than the tissue EGFR mutation status (Fig. 3a, b). The sensitivity of test can be dependent on the condition of samples. The low sensitivity in our study may be attributed from long period of sample storage and multiple freeze and thaw cycles. The lower concordance observed in our study resulted from the additional detection of rare EGFR mutations in the plasma samples that were not detected using routine methods. We were able to validate 8 of 10 additional EGFR mutations using the digital PCR assay. Two mutations identified in the plasma samples could not be validated because the sample was insufficient (total droplet number was too low for the digital PCR). Unfortunately, additional matched tissue samples were unavailable for testing. However, patients with additional EGFR mutations, such as exon20ins and S768I, and multiple mutations (19DEL, L858R, and exon 20ins) showed primary resistance to EGFR-TKI therapy with a median PFS of 1.7 months (95 % CI, 0.6–2.8). These findings indicate that the additional rare EGFR mutations found in only the plasma samples may be true positive results. In our study, 8 de novo T790M mutations were found in the plasma samples only. However, the clinical impact of the de novo T790M mutation on EGFR-TKI therapy is complicated. Previously, we analyzed 124 EGFR-mutant NSCLC cases using mass spectrometry and identified 31 (25 %) patients with de novo T790M mutations. Although the patients with the de novo T790M mutations showed a shorter time to progression (TTP) following EGFR-TKI therapy than those without this mutation, significant differences were not observed in the response rate (RR). The median TTP and RR of the patients with the de novo T790M mutations were 6.3 months and 72 %, respectively. Furthermore, the de novo T790M mutations showed a dose-dependent effect on the efficacy of EGFR-TKI therapy [25]. Thus, it is difficult to conclude whether the additional T790M mutations found in only the plasma are true positives. Considering the clinical relevance of T790M mutations with respect to disease progression after EGFR-TKI therapy, further confirmation of the accuracy of T790M mutation detection using our method is required.
To date, most of the blood samples used in KRAS mutation tests have been obtained from colorectal cancer (CRC) patients [14–17]. Thierry et al. reported that a quantitative PCR-based method exhibited 92 % sensitivity and 98 % specificity for CRC [26]. Very recently, Sacher et al. reported that plasma ddPCR exhibited high specificity of 100 % (62 of 62) but modest specificity of 64 % (16 of 25) for the detection of KRAS G12X in lung cancer patients [7]. In our study, the concordance, specificity and sensitivity of the KRAS mutation test were 85.9, 89.4, and 50.0 %.
Mutation analyses of circulating tumor DNA (ctDNA) require highly sensitive techniques because of the small fraction of tumor-specific DNA relative to background levels of normal cfDNA. The sensitivity of conventional analytical methods, such as Sanger sequencing, is not sufficient to detect low-frequency variants. Recent advances in genomics technologies have provided new opportunities for analyzing ctDNA. Therefore, advanced technologies, such as PANAMutyper, BEAMing, castPCR, NGS and digital PCR, can be of clinical utility because they can identify multiple mutations with high sensitivity. These advanced technologies are extremely sensitive (0.01–5 % limit of detection) and suitable for analyzing circulating ctDNA in cancer patients; however, each technology has advantages and disadvantages. The advanced technologies that are currently deployed for analyzing circulating ctDNA in cancer patients are summarized in Table 3. The technologies using digital PCR, such as droplet-based systems and Beads, Emulsions, Amplification and Magnetics (BEAMing), provide quantitative analyses and single-molecule amplification. However, these methods are expensive, have longer assay times and can detect only a limited set of mutations [6, 17, 26–29]. Next-generation sequencing (NGS) technologies have the potential to provide cost-effective alternatives for high-throughput analyses of multiple mutations over wider genomic regions. However, NGS is less sensitive and more complex than other technologies and requires an expensive system and longer assay times [30]. PANAMutyper™ and castPCR are capable of effectively removing background wild-type DNA [18, 22, 26]; therefore, these techniques can detect a single copy of mutant DNA. Moreover, these approaches can be completed within 3 h, and the analysis can be performed with only a real-time PCR instrument; thus, additional specialized or expensive equipment is not required. Although the castPCR system cannot simultaneously genotype multiple mutations [22, 26], the PANAMutyper™ can simultaneously perform multiple mutation detections and genotype determinations, and these outstanding abilities are realized through the use of a PNA clamping-assisted fluorescence melting curve analysis [18].
Conclusions
We demonstrated that plasma EGFR and KRAS mutation testing using PNA-based real-time PCR methods is feasible and can be applied as an alternative to tumor genotyping when deciding upon the optimal treatment of NSCLC patients. Further studies are required to increase the testing accuracy and determine the clinical applications of this technique.
Abbreviations
- BEAMing:
-
Beads, Emulsions, Amplification and Magnetics
- cfDNA:
-
Circulating cell-free DNA
- ctDNA:
-
Circulating Tumor DNA
- EGFR:
-
Epidermal Growth Factor Receptor
- FFPE:
-
Formalin-fixed, Paraffin-embedded
- GP:
-
Gemcitabine and Cisplatin
- IP:
-
Irinotecan and Cisplatin
- NSCLC:
-
Non-small Cell Lung Cancer
- OS:
-
Overall Survival
- PCR:
-
Polymerase Chain Reaction
- PFS:
-
Progression-free Survival
- PNA:
-
Peptide Nucleic Acid
- TKI:
-
Tyrosine Kinase Inhibitor
References
Zhou C, Wu Y-L, Chen G, Feng J, Liu X-Q, Wang C, Zhang S, Wang J, Zhou S, Ren S, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–42.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8.
Masters GA, Temin S, Azzoli CG, Giaccone G, Baker Jr S, Brahmer JR, Ellis PM, Gajra A, Rackear N, Schiller JH, et al. Systemic therapy for stage IV non-small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33(30):3488–515.
Mao C, Yuan JQ, Yang ZY, Fu XH, Wu XY, Tang JL. Blood as a substitute for tumor tissue in detecting EGFR mutations for guiding EGFR TKIs treatment of nonsmall cell lung cancer: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(21), e775.
Hampton T. Methods to detect circulating tumor DNA may help early diagnosis of cancer. JAMA. 2007;298(17):1993–4.
Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–90.
Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O'Connell A, Feeney N, Mach SL, Janne PA, Oxnard GR. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2016. doi:10.1001/jamaoncol.2016.0173.
Karachaliou N, Mayo-de las Casas C, Queralt C, de Aguirre I, Melloni B, Cardenal F, Garcia-Gomez R, Massuti B, Sanchez JM, Porta R, et al. Association of EGFR L858R mutation in circulating free DNA with survival in the EURTAC trial. JAMA Oncol. 2015;1(2):149–57.
Marchetti A, Palma JF, Felicioni L, De Pas TM, Chiari R, Del Grammastro M, Filice G, Ludovini V, Brandes AA, Chella A, et al. Early prediction of response to tyrosine kinase inhibitors by quantification of EGFR mutations in plasma of NSCLC patients. J Thorac Oncol. 2015;10(10):1437–43.
Luo J, Shen L, Zheng D. Diagnostic value of circulating free DNA for the detection of EGFR mutation status in NSCLC: a systematic review and meta-analysis. Sci Rep. 2014;4:6269.
Garassino MC, Martelli O, Broggini M, Farina G, Veronese S, Rulli E, Bianchi F, Bettini A, Longo F, Moscetti L, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14(10):981–8.
Mao C, Qiu LX, Liao RY, Du FB, Ding H, Yang WC, Li J, Chen Q. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung Cancer. 2010;69(3):272–8.
Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J Clin Oncol. 2010;28(31):4769–77.
Kuo YB, Chen JS, Fan CW, Li YS, Chan EC. Comparison of KRAS mutation analysis of primary tumors and matched circulating cell-free DNA in plasmas of patients with colorectal cancer. Clin Chim Acta. 2014;433:284–9.
Tabernero J, Lenz HJ, Siena S, Sobrero A, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: a retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015;16(8):937–48.
Miyano S, Hanazawa K, Kitabatake T, Fujisawa M, Kojima K. Detecting KRAS mutations in peripheral blood of colorectal cancer patients by peptide nucleic acid clamp PCR. Exp Ther Med. 2012;4(5):790–4.
Diaz Jr LA, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486(7404):537–40.
Kim YT, Kim JW, Kim SK, Joe GH, Hong IS. Simultaneous genotyping of multiple somatic mutations by using a clamping PNA and PNA detection probes. Chembiochem. 2015;16(2):209–13.
Guha M, Castellanos-Rizaldos E, Makrigiorgos GM. DISSECT method using PNA-LNA clamp improves detection of EGFR T790m mutation. PLoS One. 2013;8(6):e67782.
Sohaib SA, Turner B, Hanson JA, Farquharson M, Oliver RT, Reznek RH. CT assessment of tumour response to treatment: comparison of linear, cross-sectional and volumetric measures of tumour size. Br J Radiol. 2000;73(875):1178–84.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500.
Kamat AA, Baldwin M, Urbauer D, Dang D, Han LY, Godwin A, Karlan BY, Simpson JL, Gershenson DM, Coleman RL, et al. Plasma cell-free DNA in ovarian cancer: an independent prognostic biomarker. Cancer. 2010;116(8):1918–25.
Sun W, Yuan X, Tian Y, Wu H, Xu H, Hu G, Wu K. Non-invasive approaches to monitor EGFR-TKI treatment in non-small-cell lung cancer. J Hematol Oncol. 2015;8:95.
Douillard JY, Ostoros G, Cobo M, Ciuleanu T, Cole R, McWalter G, Walker J, Dearden S, Webster A, Milenkova T, et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: circulating-free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol. 2014;9(9):1345–53.
Lee Y, Lee GK, Hwang JA, Yun T, Kim HT, Lee JS. Clinical likelihood of sporadic primary EGFR T790M mutation in EGFR-mutant lung cancer. Clin Lung Cancer. 2015;16(1):46–50.
Thierry AR, Mouliere F, El Messaoudi S, Mollevi C, Lopez-Crapez E, Rolet F, Gillet B, Gongora C, Dechelotte P, Robert B, et al. Clinical validation of the detection of KRAS and BRAF mutations from circulating tumor DNA. Nat Med. 2014;20(4):430–5.
Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D. BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions. Nat Methods. 2006;3(7):551–9.
Alix-Panabieres C, Schwarzenbach H, Pantel K. Circulating tumor cells and circulating tumor DNA. Annu Rev Med. 2012;63:199–215.
Taly V, Pekin D, Benhaim L, Kotsopoulos SK, Le Corre D, Li X, Atochin I, Link DR, Griffiths AD, Pallier K, et al. Multiplex picodroplet digital PCR to detect KRAS mutations in circulating DNA from the plasma of colorectal cancer patients. Clin Chem. 2013;59(12):1722–31.
Gullapalli RR, Desai KV, Santana-Santos L, Kant JA, Becich MJ. Next generation sequencing in clinical medicine: challenges and lessons for pathology and biomedical informatics. J Pathol Inform. 2012;3:40.
Acknowledgements
We thank for all patients and their families who consented to participate in this study.
Funding
This study was supported by grants 1410660 and 1410682 from the National Cancer Center.
Availability of data and materials
Detailed data of the patients will not be shared at the moment since these data are used for further publications.
Authors’ contributions
JJC participated in the development of the methodology and performed the statistical analysis. JYK, YLH, and GKL provided technical support about immunohistochemistry and participated in the data discussion, JYH was involved in the study design and data discussion, helped to draft the manuscript and gave a final approval of the version to be published. All authors read and approved the final manuscript.
Competing interests
Jae-Jin Choi is a full-time employee of PANAGENE Inc. Daejeon, Korea. Other authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The protocol was approved by the National Cancer Center Institutional Review Board (study ID number: NCCCTS08371) and conducted in accordance with the Declaration of Helsinki, Good Clinical Practice. All patients provided written informed consent for the provision of tumor and plasma samples for the biomarker analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1186/s12885-016-2760-9.
Additional file
Additional file 1: Table S1.
The criteria of the mutation detection according to the fluorescent dye and melting temperature. (DOCX 25 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Han, JY., Choi, JJ., Kim, J.Y. et al. PNA clamping-assisted fluorescence melting curve analysis for detecting EGFR and KRAS mutations in the circulating tumor DNA of patients with advanced non-small cell lung cancer. BMC Cancer 16, 627 (2016). https://doi.org/10.1186/s12885-016-2678-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-016-2678-2