Abstract
Background
DNA methylation regulates gene expression, through the inhibition/activation of gene transcription of methylated/unmethylated genes. Hence, DNA methylation profiling can capture pivotal features of gene expression in cancer tissues from patients at the time of diagnosis. In this work, we analyzed a breast cancer case series, to identify DNA methylation determinants of metastatic versus non-metastatic tumors.
Methods
CpG-island methylation was evaluated on a 56-gene cancer-specific biomarker microarray in metastatic versus non-metastatic breast cancers in a multi-institutional case series of 123 breast cancer patients. Global statistical modeling and unsupervised hierarchical clustering were applied to identify a multi-gene binary classifier with high sensitivity and specificity. Network analysis was utilized to quantify the connectivity of the identified genes.
Results
Seven genes (BRCA1, DAPK1, MSH2, CDKN2A, PGR, PRKCDBP, RANKL) were found informative for prognosis of metastatic diffusion and were used to calculate classifier accuracy versus the entire data-set. Individual-gene performances showed sensitivities of 63–79 %, 53–84 % specificities, positive predictive values of 59–83 % and negative predictive values of 63–80 %. When modelled together, these seven genes reached a sensitivity of 93 %, 100 % specificity, a positive predictive value of 100 % and a negative predictive value of 93 %, with high statistical power. Unsupervised hierarchical clustering independently confirmed these findings, in close agreement with the accuracy measurements. Network analyses indicated tight interrelationship between the identified genes, suggesting this to be a functionally-coordinated module, linked to breast cancer progression.
Conclusions
Our findings identify CpG-island methylation profiles with deep impact on clinical outcome, paving the way for use as novel prognostic assays in clinical settings.
Similar content being viewed by others
Background
Breast cancer predictive and prognostic procedures have a significant impact on current medical care. However, traditional prognostic parameters (lymph node diffusion, tumor size, grading, estrogen receptor expression) cannot adequately predict tumor relapse. As an example, 10–20 % of the patients with the best prognosis, i.e. with small size tumors, expressing estrogen receptors and without lymph node invasion, still experience relapse within 5 years [1, 2]. At the time of diagnosis, progressing cases cannot be distinguished from those that do not relapse by any conventional prognostic parameter. Therefore, effective markers, with better performance than traditional prognostic indicators, are urgently needed.
By merging biological insight and cluster analysis for experimental immunoistochemistry (IHC) parameters, we have previously succeeded in subgrouping breast cancers with distinct outcomes [3-5], and response to therapy [4, 6], indicating the clinical usefulness of such procedures. DNA methylation regulates gene expression, through the inhibition/activation of gene transcription of methylated/unmethylated genes, respectively [7, 8]. This largely occurs through methylation of CpG islands, most frequently in the promoter region of the genes [7, 9-11]. Broad hypomethylation with focal hypermethylation are frequently found in cancer [8, 12], thus affecting the expression of tumor suppressor genes, e.g. TP53, DCC, SOCS2, DLEU7 [13-16], and favoring the mutation of oncogenes [17]. In turn, tumor suppressors have been shown to modulate DNA methylation levels, genome stability and DNA methylation-dependent gene amplification [18, 19], suggesting key interplays between alterations of DNA methylation and tumor progression. Indeed, DNA methylation-mediated loss of expression has been shown to cause functional ablation of hemizygous alleles at loss of heterozygosity (LOH) loci, encoding transcription factors (TF), e.g. MOS, TTF-1 [20, 21], or proteins associated with DNA repair [22, 23], proteolytic processing [24], morphogenesis [25], control of cell cycle, signal transduction or apoptosis [26]. In breast cancer, CpG-island methylation was shown to inhibit PTCH1 [27], EFEMP1 [28] and ESR1 [29] expression.
DNA methylation patterns can be assessed in formalin-fixed paraffin-embedded tissues (FFPE) tumor samples [30], allowing to profile gene expression regulatory mechanisms in tumors at the time of surgery, through methylation-sensitive restriction enzyme-analysis over a 56-gene cancer-specific biomarker microarray (MethDet-56) [31]. Long-term follow-up then permits to dissect correlations between DNA methylation profiles and biological outcome [32-35]. In this work we identified CpG-island methylation profiles of cancer biomarker regulatory regions, with a deep impact on prognostic determination in breast cancer, and the ability to distinguish cases with limited or nil risk for progression from those at high risk.
Methods
Breast cancer case series
A multi-institutional case series of breast cancer patients was collected from the University of Udine, the Venice and Rovigo hospitals, and Rush University (Additional file 1: Table S1). 123 breast cancer patients were analyzed; 19 cases showed metastases or metastatic relapse within 5 years from surgery (15.4 %) (Additional file 1: Table S1). Metastatic/relapsing cases were compared with patients that did not progress. Clinical and pathological data were obtained [36, 37] (Additional file 1: Table S1). Carcinoma grading was performed as described [38]. This project was approved by the Italian Ministry of Health (RicOncol RF-EMR-2006–361866), and by the Institutional Review Board of Rush University Medical Center. No written consent was needed for this study.
DNA isolation
Different procedures were compared for efficiency of DNA isolation from FFPE breast cancer samples from mastectomy or excision biopsy [31, 39, 40]. Samples were then processed for DNA extraction as described [31, 40]. Briefly, xylene deparaffination was followed by deproteinization with proteinase K in SDS-containing buffer at 56 °C. DNA was purified using DNAeasy Tissue kits (Qiagen). DNA was quantified using Hoechst 33258 or ethidium bromide fluorescence [41]. Agarose gel electrophoresis profiled DNA size distributions for sample quality assessment (Fig. 1).
Breast cancer genomic DNA from the breast cancer case series. DNA was extracted from FFPE tissue samples as described and assessed by agarose gel/ethidium bromide electrophoresis. Numbers above each lane indicate individual patient’ cancer samples. mw: molecular weight markers in kilobases (left side of the panels)
Microarray-mediated methylation assay
Bisulfite-based modification permits analysis of all cytosines in a sample; however, it leads to excessive fragmentation of DNA [42]. Affinity-based techniques require a substantial amount of starting sample, and their efficiency depends on the density of methylation marks within each specific fragment [43]. Restriction enzyme-dependent methods are more flexible for analysis of small samples, and are focused on assessing methylation of selected restriction sites [8, 31]. As DNA from human cancer samples is a limiting factor, we utilized previously developed procedures of methylation-sensitive restriction enzyme-cleavage [31].
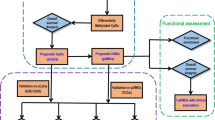
For each patient, DNA methylation was tested over a 56-gene cancer-specific biomarker microarray as previously described [44] (a flowchart is provided in Fig. 2a). Briefly, each DNA sample was split into two aliquots; one of these was digested with Hin6I, while the second one was mock-digested. Both samples were amplified by nested PCR, and 5-aminoallyl dUTP (Biotium Inc.) was added to the second amplification run. Products of the Hin6I-digested DNA were then labeled with Cy3, while products of the mock-digested DNA were labeled with Cy5. Labeled DNAs were mixed and competitively hybridized to DNA microarrays. Slides were scanned using a GenePix 4000B Microarray Scanner (Molecular Devices). Intensity of fluorescence was determined using the GenePix Pro 6.1.0.2 software. Raw GenePix data were imported as the ratio of signal intensity of Control hybridization and Test hybridization for each spot, and processed using Agilent Genespring 12.5, with lowess normalization and base 2 log transformation. Microarray data are available in the ArrayExpress database [45] under accession number E-MTAB-3153.
Data processing
Each microarray contained three identical subarrays of 64 (8 × 8) spots [31] (Fig. 2b). Gene CpG-island probes corresponded to 56 spots; three spots contained positive control DNAs and 2 spots contained hybridization control DNAs, to quantify specific versus nonspecific binding; three empty areas were used to quantify background intensity. A multi-step filtering was applied as follows: spots were removed from analysis if signals were <2 times the average of the control spots, as calculated for each slide over the three subarrays. Next, data that had less than two informative spots across the three subarrays were removed. Genes with missing data in more than 25 % of the samples were removed from the analysis. For continuous-variable approaches, the mean of the ratios was calculated for each gene of every sample. The methylation ratio (Cy5/Cy3) was then calculated, and the methylation status of each CpG island was categorized as either methylated or unmethylated.
Statistical analysis
The initial lowess-normalized, log-transformed ratio data were grouped according to breast cancer progression status. Log-transformed data distributions were shown to follow a normal distribution. Hence, the means of values from the tumors that progressed were compared to the corresponding measurement from non-progressing tumors by independent sample student’s t-test of fold changes (FC). Cutoffs of absolute FC ≥2, p < 0.05 were used to filter-out genes that were not highly differentially methylated. The remaining genes were submitted to feature selection models utilizing JMP Genomics algorithms, with 5-fold, ten-runs cross-validation. The models utilized were discriminant analysis, general linear model selection, k-nearest neighbors, logistic regression, partial least squares (PLS) and partition trees. Based on the highest average area (AUC) under the receiver-operating-characteristic curve (ROC) value across ten runs, a model was chosen as the optimal binary classifier. That model was then used to select the genes with greatest effect on the classifier results. To confirm these results, individual-gene PLS analysis was performed using R’s pls package, and statistical power computations, as previously described [46, 47]. Hierarchical clustering using euclidean distance and centroid linkage were performed with Genespring. Statistical power was computed for each individual gene and for the averaged 7-gene panel using PASS 12.0 with a t-test model and no assumption of equal variance [46, 47]. A significance level (alpha) of ≥0.05 and a threshold of ≥80 % statistical power were adopted as analytical thresholds.
Network analysis
Signaling hubs and connectivity networks were obtained using Ingenuity Pathway analysis [48] and STRING 9.1 [49] software. To increase specificity, the IPA analysis was confined to molecules and/or relationships observed in breast tissues and cell lines. STRING parameters included: Active Prediction Methods: Experiments ~ Databases ~ Textmining; required high-confidence (0.700); no more than ten interactors.
Results
CpG-island methylation analysis
DNA extraction was performed on FFPE breast cancer samples from 123 patients from a multi-institutional case serie with a minimum follow-up of 5 years. (Additional file 1: Table S1). Ethidium bromide gel electrophoresis (Fig. 1) and amplification of RAS and TP53 exons (manuscript in preparation) benchmarked DNAs as viable for additional DNA methylation analysis. Relapsing cases were extracted from the registry and matched with non relapsing patients on the basis of clinico-pathological data (tumor diameter, pathological stage, tumor histotype, age, hormone receptors and grading). DNA methylation of transcription-regulatory regions in the selected case-control group was analyzed through methylation-sensitive restriction enzyme-cleavage, followed by PCR amplification and competitive hybridization of fluorescence-labelled PCR products on custom DNA microarrays containing CpG-island gene-probes for 56 cancer specific biomarkers, as described [31]. Samples were analyzed through triplicate spot arrays (eight by eight), each one containing three positive hybridization controls, two negative controls (A. thaliana and HLTF) and thre empty spots, to measure background fluorescence (Fig. 2). Hybridization raw data are available as indicated in Methods [45]. Microarray fluorescence measurements were acquired as test (Hin6I-digested DNA labeled with Cy3) versus control hybridization intensity (mock-digested DNA labeled with Cy5). Background fluorescence was subtracted, and Cy3/Cy5 intensity ratios were obtained for each spot. Intensity ratios were lowess normalized against global signal intensity of the array, and transformed as base 2 logs. For continuous-variable approaches, means of ratios were obtained for each gene analyzed. Spot signals were filtered according to absolute signal intensity (threshold of ≥2 times versus control spots), viable spots numbers (≥2) and missing data (spot series with missing data in ≥25 % of the samples were discarded).
Gene CpG-island methylation ranking
Filtered methylation ratios (Cy5/Cy3) were utilized to define the methylation status of each CpG island. These were categorized as either methylated or unmethylated, using cutoffs of absolute FC ≥2, p <0.05. Normalized fluorescence ratios/differential methylation categorization were then assigned to the relapsing or non-relapsing patients groups. A first comparison between the means of the values from each group extracted 21 genes, that met the cutoff for significant differential methylation (Table 1). PLS K nearest-neighbors with a radial basis machine was applied to obtain a first ranking of significantly differentially-methylated genes (Additional file 2: Table S2). This identified DAPK1, MDGI, BRCA1, P15, PGK1, PGR, SYK, THBS1, 14-3-3σ, APAF1, CALCA and CCND2 as the highest differentially methylated genes between progressing and non-progressing breast cancers. This group of genes contained controllers of cell proliferation (P15, CCND2, PGR) and apoptosis (APAF1, DAPK1), p53 interactors (THBS1, DAPK1), signaling kinases (SYK, PGK1, DAPK1), drivers of tumor development (BRCA1, 14-3-3 [50]), suggesting direct relevance of differentially-methylated/regulated genes for breast cancer development or progression.
Marker-gene profiling
These findings led us to further refine our breast cancer prognostic model, through procedures of best-model fitting of differentially methylated gene profiles. The 21 genes that had been previously filtered (Table 1) were thus modelled using discriminant analysis, general linear model selection, k-nearest neighbors, logistic regression, PLS and partition trees. Model’ performances were evaluated on the basis of AUC values across five-fold ten-runs cross-validation. The PLS model generated the highest AUC (mean AUC ≈ 0.8) and was chosen as the best binary classifier of progressing versus non-progressing breast cancers. Genes selected by the PLS model were BRCA1, DAPK1, MSH2, CDKN2A/P16, PGR, PRKCDBP/SRBC, RANKL/TNFSF11/TRANCE (Fig. 3). These seven genes were shown to provide the maximal overall contribution to AUC measurements (Fig. 4a, Table 1), i.e. the greatest impact on the classifier, across all ten comparison runs (Fig. 4a, Table 1).
The 7-gene classifier. (a) ROC curve for all the PLS-selected genes. Highest AUC value across ten runs, for optimal binary classifier model. (b) Hierarchical clustering with euclidean distance. Sample colors represent deviation from the median (black is missing datapoint). Green bars indicate cancer-progressing samples, brown bars correspond to non-progressing samples. Notably, tight clustering of 13/19 relapsing cases was observed on one branch (left side), while 18/19 non-relapsing cases were clustered on the second branch (right side)
Measuring the global accuracy of prognostic gene markers
We then went on to utilize model-fitting marker genes to generate accuracy measurements for binary classification of breast cancer relapse risk. The seven genes selected by PLS were subjected to whole-model fit using the entire data-set. Individual-gene data were then analyzed using R statistics. Individual-gene analyses highlighted sensitivities of 63–79 %, with 53–84 % specificity, positive predictive value of 59–83 % and a negative predictive value of 63–80 %. Modeling all seven genes together then allowed to reach remarkable sensitivity (93 %) and specificity (100 %), a positive predictive value of 100 % and a negative predictive value of 93 %. These findings indicated that while individual genes had effectiveness at classification, global analyses were much more proficient (Fig. 4a, Table 2). Notably, the ROC curve for all PLS-selected genes together had an AUC = 0.9643 (Fig. 4a).
Statistical power of the CpG-island methylation analysis
Statistical power analysis indicated that the methylation patterns of five of the seven best predictors, i.e. BRCA1, DAPK1, MSH2, PGR, PRKCDBP, possessed a high discriminating power of relapsing cancer cases versus non-relapsing controls (Additional file 2: Table S3). CDKN2A and RANKL showed trends toward the high threshold and contributed non-redundantly to prediction when combined in the 7-gene set. Remarkably, the power of the 7-gene set was 1 (Table 3), strongly supporting the combined use of the seven gene markers.
Hierarchical clustering
Unsupervised hierarchical clustering of the tumor samples, based on the 7-gene classifier, was performed to independently assess the prognostic-association performance above. Hierarchical clustering demonstrated close agreement with the accuracy measurements. Of interest, tight clustering of 13 relapsing cases was oberved. At variance, 6 other progressing cases appeared broadly distributed among tumors with benign outcome (Fig. 4b), consistent with a distinct heterogeneity in biological trajectories to metastatization (manuscript in preparation).
Gene pathways associated to prognostic determinants
Pathway analysis was performed on differentially-methylated genes (Additional file 2: Table S4; Additional file 3, 4: Figures. S1, S2). Most genes/gene relationships were found to map over key cancer and cell-signaling biological pathways (Additional files 3, 4: Figures S1B, S2). Ingenuity pathway analysis (IPA) highlighted a statistically significant network of interactions (score = 28, Fisher’s exact test p = 1x10−28) (Additional file 3: Figure S1A). 14 of the differentially methylated genes appeared closely connected, suggesting a functionally-relevant signaling module. This module converged on three major hubs: ERBB2, PRG and BRCA1 (Additional file 3: Figure S1A). This analysis revealed the p53 signaling network as the most relevant one (p = 0.0000091); this encompassed the CDKN2A, CCND2, THBS1 and BRCA1 genes (Additional file 2: Table S4). These findings were independently validated by STRING network analysis (Additional file 4: Figure S2A) (p53 signaling network, KEGG entry: map04115, p-value = 6.17 E-22). Molecular Mechanisms of Cancer (IPA p = 0.000977) (Additional file 2: Table S4; Additional file 3: Figure S1B) and Pathways in cancer (KEGG entry: map05200 STRING p = 8.72 E-13) (Additional file 4: Figure S2B) appeared as additional relevant canonical network. Of interest, gene networks appeared related to broadly different cancer histotypes (Additional file 2: Table S4), suggesting a wide significance of this cancer-associated module, and potential relevance also in histotypes other than breast cancer.
Discussion
In this work, we have identified a gene methylation panel for binary classification of breast cancer progression. Utilizing IHC parameters we had previously succeeded in subgrouping breast cancers [3-5] for prognostic and therapeutic use [6]. Profiles of DNA methylation/regulation of expression of pivotal cancer drivers [51] were expected to provide additional valuable information, and to critically complement current prognostic procedures. Hence, we went on to identify gene methylation profiles that could bear significant value for prognostic determination [31].
We first identified gene markers whose methylation patterns differed between progressing and non-progressing breast tumors. PLS k-nearest neigbors radial basis machine identified DAPK1, MDGI, BRCA1, P15, PGK1, PGR, SYK, THBS1, 14-3-3σ, APAF1, CALCA and CCND2, as differentially methylated genes between progressing and non-progressing cancers. These genes included cell cycle regulators, signaling kinases, cytoplasmic scaffold/regulatory molecules and p53 interactors, suggesting direct relevance for breast tumor progression.
Hence, we went on to further refine our breast cancer prognostic model, through procedures of best-model fitting of differentially methylated gene profiles. PLS models were shown to provide the highest AUC and were chosen as the best binary classifiers of progressing versus non-progressing breast cancers. The genes that contributed most to a PLS binary classification model were BRCA1, DAPK1, MSH2, CDKN2A, PGR, PRKCDBP, RANKL. Individual-genes assessments showed 63–79 % sensitivity, 53–84 % specificity, positive predictive values of 59–83 % and negative predictive values of 63–80 %. A 5-fold cross-validation in selected models and PLS analysis through distinct procedures (JMP Genomics, Genespring and R) were used to assess gene clusters versus individual genes. Remarkably, when modelled together, the seven genes reached a sensitivity of 93 %, with 100 % specificity, a positive predictive value of 100 % and a negative predictive value of 93 %. The 97 % estimates for the AUC of the 7-gene panel model supported it as a reliable predictor of breast cancer progression, as did the normality of the log-transformed data distributions. Statistical power analysis supported the strength of our analytical strategies. The majority of the predictors (i.e. BRCA1, DAPK1, MSH2, PGR, PRKCDBP) demonstrated high statistical power, with a threshold of ≥80 % and an alpha significance level of 0.05. CDKN2A and RANKL were close to high statistical power thresholds and were shown to provide non-redundant information to prognosis when combined with BRCA1, DAPK1, MSH2, PGR and PRKCDBP. To assess the overall statistical power of the 7-gene set, an average for the seven prognostic genes was computed for each individual sample. Then, the power calculation was performed, as based on the distance between the mean of relapsing cancer samples versus that on non-relapsing cases. A remarkable power of 1 was obtained, strongly supporting the efficiency of 7-genes panel. Unsupervised hierarchical clustering of the tumor samples, demonstrated close agreement with the accuracy measurements. Of note, tight clustering of 13 relapsing cases was oberved, whereas 6 additional cases distributed among tumors with favourable outcome. These findings suggested heterogeneity in the biological paths that are followed to reach pro-metastatic states, in spite of the sharing of candidate causal genes.
Our model predicted that promoter DNA methylation, with subsequent transcriptional inactivation of the 7-gene set would be detrimental and associated with tumor progression. Individual genes findings fully supported this model.
BRCA1 is a tumor suppressor gene [52, 53] involved in DNA repair, cell cycle checkpoint control, and maintenance of genomic stability [54]. Germline mutations in BRCA1 predispose women to breast and ovarian cancers [55], with a 50–85 % lifetime risk of developing breast cancer [56]. Promoter hypermethylation was shown to cause loss of BRCA1 expression both in sporadic ovarian cancer [57] and in hereditary ovarian carcinomas [58]. Promoter methylation was detected in 31 % of carcinomas but in none of the benign or borderline tumors [59]. Levels of methylation in ovarian tumors quantitatively correlated with decreased BRCA1 expression [60, 61]. Hypermethylation of BRCA1 was detected at a significantly higher frequency in serous carcinomas than in tumors of the other histological types [62], with earlier onset of high-grade serous ovarian cancer. BRCA1 promoter methylation was frequently found in triple negative breast cancers and identified a significant fraction of patients with poor outcomes [63]. Notably, promoter methylation of BRCA1 was also found in 46 % of pancreatic neoplasms [64] suggesting a broader impact of this alteration, beyond ovarian and breast cancers.
Death-associated protein kinase (DAPK) is a pro-apoptotic determinant which is dysregulated in a wide variety of cancers [65]. Hypermethylation of DAPK1 is the most frequent molecular alteration identified in immunodeficiency-related lymphomas [66], and was detected in almost all cases of chronic lymphocytic leukemia [67]. Hypermethylation patterns of DAPK were found in head and neck cancers [68], bladder tumors [69], and brain metastases of solid tumors [70], and were associated with poor outcome. The DNA methyltransferase inhibitor 5-Azacytidine (5-Aza) was shown to induce promoter demethylation and to restore mRNA expressions of DAPK in osteosarcoma cells [71], confirming DNA methylation as a determinant of transcriptional inactivation of this gene.
PGR (progesterone receptor) is a member of the steroid receptor family and mediates the gene transcription regulatory effects of progesterone. The PGR status yields prognostic information in patients with node-negative breast cancer [72]. Lack of expression of PGR, together with loss of estrogen receptors and of Her-2/neu, identifies ‘triple negative’ breast cancers, which are an aggressive, poor-outcome breast cancer subgroup [4]. PGR was inactivated by promoter methylation in tamoxifen-resistant breast cancer cells. Following promoter demethylation with 5-Aza, the co-addition of oestradiol (E2) restored gene expression, and inhibited cell proliferation [73]. PGRß was found hypermethylated in 56 % of melanoma cell lines [74], and in acute myeloid leukemias [75].
Protein kinase C δ-binding protein is encoded by the PRKCDBP (SRBC) gene. Frequent epigenetic or mutational inactivation of PRKCDBP was observed in sporadic breast, lung, ovarian, and other types of adult cancers as well as childhood tumors [76]. The expression of the PRKCDBP protein was down-regulated in about 70 % of breast, lung, and ovarian cancer cell lines, whereas a strong expression of the protein is detected in normal mammary and lung epithelial cells [76]. PRKCDBP is frequently shut-down in glioblastoma multiforme [77] and in colorectal cancer [78] by promoter hypermethylation [79]. PRKCDBP methylation in neuroblastoma was associated with unfavourable outcome [80]. PRKCDBP is a proapoptotic tumor suppressor which is activated by NF-κB in response to TNFα, suggesting that PRKCDBP inactivation may contribute to tumor progression by reducing cellular sensitivity to TNFα. Loss of expression of the PRKCDBP protein was associated to hypermethylation in non-small-cell lung cancers and breast cancer cells; re-expression was observed after treatment with 5-Aza [76, 81].
p16 is a cyclin-dependent kinase inhibitor and a tumor suppressor protein. Loss of the corresponding locus (CDKN2A) is among the most frequent cytogenetic alteration events in human cancer [82]. The frequency of inactivation of p16 by DNA methylation is even higher than that by genetic changes in many cancers, e.g. in gastric carcinomas (32–42 % of cases), where this is an early event and is associated with poor clinical outcome [83]. Correspondingly, p16 methylation is detected in precancerous and inflammatory lesions of colon, lung, liver, oral cavity [84], and is associated with malignant progression [85, 86]. p16 methylation is associated with lower overall survival and disease-free survival in non-small cell lung cancer patients [87], melanomas [88] and paragangliomas [85]. p16 is frequently methylated/inactivated in haematopoietic malignancies, such as acute lymphoid leukaemia (ALL), lymphomas and multiple myeloma [89]. 5-Aza was shown to restore gene transcription of hypermethylated CDKN2A genes [90]. Taken together, these findings have led to the FDA approval of 5-Aza for treatment of patients with myelodysplastic syndromes [89].
MSH2 is a tumor suppressor protein involved in DNA repair, e.g. base excision, and transcription-coupled homologous recombination [91-93]. Heterozygous LOH germline mutations of MSH2 are causal factors of the Lynch syndrome (hereditary non-polyposis colorectal cancer, HNPCC) [94]. Heritable transmission of propensity to MSH2 methylation in a family with HNPCC has been reported [95]. Aberrant DNA methylation and epigenetic inactivation of MSH2 play a role in the development of ALL, through induction of cell growth and survival [96]. CpG island methylation in MSH2 associates with carcinogenesis in colorectal carcinomas presenting with a conventional adenoma-carcinoma sequence. Therefore, the detection of MSH2 methylation may have clinical significance in the evaluation of colon cancer patients and in a precision-medicine management of the disease [97].
RANKL (TNFSF11, TRANCE) is a TNF family member, and, together with its receptor RANK, is a key regulator of cell survival. The RANKL/RANK system is modulated by osteoprotegerin (OPG) which binds to RANKL and prevents its interaction with RANK. RANKL activates Akt1 through a signaling complex involving Src and TRAF6 [98]. RANK is found expressed on cancer cell lines and breast cancer cells in patients [99]. The RANK/RANKL signaling plays an essential role in progestin-induced breast cancer development [100] and stimulates breast cancer metastasis [101]. Corresponding, RANKL triggers the migration of cancer and melanoma cells that express the RANK receptor [99]. The methylation status of both RANKL and OPG quantitatively controls their levels of expression [102]. Consistent, RANKL expression in myeloma cells was shown to be driven by TNFα-induced gene demethylation [103]. Thus, RANKL and OPG act as a cancer/metastasis control module, whose balance is determined by epigenetic regulation.
Network analysis was used to identify functional interrelationship across the tumor progression predictors identified. Notably, most of these prognostic genes and their direct interactors in the network were found to map over key cancer cell-signaling pathways. These close relationships suggested the existence of a functional signaling module, which converged on the ERBB2, PRG and BRCA1 hubs. Remarkably, this network module appeared most related to the p53 signaling pathway (p-value = 0.0000091). TP53 is the most frequently mutated gene in cancer [104-106] and TP53 mutations are specifically associated to tumor subgroups with distinct biological features, particularly in breast cancer [4, 5, 33, 107]. Notably, though, the 7-gene-driven network was shown to be active also in cancer histotypes other than breast (non-small cell lung cancer, bladder, ovary), suggesting an even broader relevance for tumor progression.
Conclusions
Our findings identify CpG-island methylation profiles of seven genes, i.e. BRCA1, DAPK1, MSH2, CDKN2A, PGR, PRKCDBP, RANKL, as having a deep impact on clinical outcome. Our findings candidate the 7-gene methylation profile as a tool for quantifying the risk of relapse of breast cancers, paving the way for use as a novel prognostic assay in clinical settings.
Abbreviations
- ALL:
-
Acute lymphoid leukaemia
- AUC:
-
Area under the curve
- FFPE:
-
Formalin-fixed paraffin-embedded
- IHC:
-
Immunohistochemistry
- IPA:
-
Ingenuity pathway analysis
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LOH:
-
Loss of heterozygosity
- PCR:
-
Polymerase chain reaction
- PLS:
-
Partial least squares
- ROC:
-
Receiver operating characteristic
- STRING:
-
Search Tool for the Retrieval of Interacting Genes/Proteins
References
Oncofem P. Carcinoma della mammella in Italia. Federazione Medica. 1992;9:11–6.
Group EBCTC. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–42.
Ambrogi F, Fornili M, Boracchi P, Trerotola M, Relli V, Simeone P, et al. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9:e96993.
Biganzoli E, Coradini D, Ambrogi F, Garibaldi JM, Lisboa P, Soria D, et al. p53 status identifies two subgroups of triple-negative breast cancers with distinct biological features. Jpn J Clin Oncol. 2011;41:172–9.
Ambrogi F, Fornili M, Alberti S, Querzoli P, Piantelli M, Pedriali M, et al. EpCAM expression is an indicator of increased incidence of relapse in p53-positive breast cancer. Cancer Clin Oncol. 2013;2:41–50.
Cimoli G, Malacarne D, Ponassi R, Valenti M, Alberti S, Parodi S. Meta-analysis of the role of p53 status in isogenic systems tested for sensitivity to cytotoxic antineoplastic drugs. Biochim Biophys Acta. 2004;1705:103–20.
Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–59.
Alberti S, Herzenberg LA. DNA methylation prevents transfection of genes for specific surface antigens. Proc Natl Acad Sci U S A. 1988;85:8391–4.
Simeone P, Alberti S. Epigenetic heredity of human height. Physiol Rep. 2014;2:e12047.
Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921.
Illingworth RS, Bird AP. CpG islands - ‘A rough guide’, FEBS Lett. 2009.
He X, Chang S, Zhang J, Zhao Q, Xiang H, Kusonmano K, et al. MethyCancer: the database of human DNA methylation and cancer. Nucleic Acids Res. 2008;36:D836–41.
Momparler RL, Bovenzi V. DNA methylation and cancer. J Cell Physiol. 2000;183:145–54.
Park HL, Kim MS, Yamashita K, Westra W, Carvalho AL, Lee J, et al. DCC promoter hypermethylation in esophageal squamous cell carcinoma. Int J Cancer. 2008;122:2498–502.
Martinez-Chantar ML, Vazquez-Chantada M, Ariz U, Martinez N, Varela M, Luka Z, et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–9.
Hammarsund M, Corcoran MM, Wilson W, Zhu C, Einhorn S, Sangfelt O, et al. Characterization of a novel B-CLL candidate gene–DLEU7–located in the 13q14 tumor suppressor locus. FEBS Lett. 2004;556:75–80.
Denissenko MF, Chen JX, Tang MS, Pfeifer GP. Cytosine methylation determines hot spots of DNA damage in the human P53 gene. Proc Natl Acad Sci U S A. 1997;94:3893–8.
Alberti S, Nutini M, Herzenberg LA. DNA methylation prevents the amplification of TROP1, a tumor associated cell surface antigen gene. Proc Natl Acad Sci U S A. 1994;91:5833–7.
Nasr AF, Nutini M, Palombo B, Guerra E, Alberti S. Mutations ofTP53 induce loss of DNA methylation and amplification of the TROP1 gene. Oncogene. 2003;22:1668–77.
Scholz C, Nimmrich I, Burger M, Becker E, Dorken B, Ludwig WD, et al. Distinction of acute lymphoblastic leukemia from acute myeloid leukemia through microarray-based DNA methylation analysis. Ann Hematol. 2005;84:236–44.
Kondo T, Nakazawa T, Ma D, Niu D, Mochizuki K, Kawasaki T, et al. Epigenetic silencing of TTF-1/NKX2-1 through DNA hypermethylation and histone H3 modulation in thyroid carcinomas. Lab Invest. 2009;89(7):791–9.
Suter CM, Martin DI, Ward RL. Germline epimutation of MLH1 in individuals with multiple cancers. Nat Genet. 2004;36:497–501.
Agrelo R, Cheng WH, Setien F, Ropero S, Espada J, Fraga MF, et al. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proc Natl Acad Sci U S A. 2006;103:8822–7.
Dunn JR, Panutsopulos D, Shaw MW, Heighway J, Dormer R, Salmo EN, et al. METH-2 silencing and promoter hypermethylation in NSCLC. Br J Cancer. 2004;91:1149–54.
Wen XZ, Akiyama Y, Baylin SB, Yuasa Y. Frequent epigenetic silencing of the bone morphogenetic protein 2 gene through methylation in gastric carcinomas. Oncogene. 2006;25:2666–73.
Baylin SB, Esteller M, Rountree MR, Bachman KE, Schuebel K, Herman JG. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet. 2001;10:687–92.
Wolf I, Bose S, Desmond JC, Lin BT, Williamson EA, Karlan BY, et al. Unmasking of epigenetically silenced genes reveals DNA promoter methylation and reduced expression of PTCH in breast cancer. Breast Cancer Res Treat. 2007;105:139–55.
Sadr-Nabavi A, Ramser J, Volkmann J, Naehrig J, Wiesmann F, Betz B, et al. Decreased expression of angiogenesis antagonist EFEMP1 in sporadic breast cancer is caused by aberrant promoter methylation and points to an impact of EFEMP1 as molecular biomarker. Int J Cancer. 2009;124:1727–35.
Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–5.
Levenson VV, Melnikov AA. The MethDet: a technology for biomarker development. Expert Rev Mol Diagn. 2011;11:807–12.
Melnikov AA, Scholtens DM, Wiley EL, Khan SA, Levenson VV. Array-based multiplex analysis of DNA methylation in breast cancer tissues. J Mol Diagn. 2008;10:93–101.
The Cancer Genome Atlas (TGCA), NCI/NHGRI [http://cancergenome.nih.gov/].
Ambrogi F, Biganzoli E, Querzoli P, Ferretti S, Boracchi P, Alberti S, et al. Molecular subtyping of breast cancer from traditional tumor marker profiles using parallel clustering methods. Clin Cancer Res. 2006;12:781–90.
Querzoli P, Pedriali M, Rinaldi R, Lombardi AR, Biganzoli E, Boracchi P, et al. Axillary lymph node nanometastases are prognostic factors for disease-free survival and metastatic relapse in breast cancer patients. Clin Cancer Res. 2006;12:6696–701.
Biganzoli E, Pedriali M, Querzoli P, Nenci I, Iacobelli S, Piantelli M, et al. Sentinel node and bone marrow micrometastases and nanometastases. Curr Breast Cancer Rep. 2010;2:96–106.
Wold LE, Ingle JN, Pisansky TM, Johnson RE, Donohue JH. Prognostic factors for patients with carcinoma of the breast. Mayo Clin Proceedings. 1995;70:678–9.
Silverstein MJ, Lagios MD, Groshen S, Waisman JR, Lewinsky BS, Martino S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999;340:1455–61.
Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham prognostic index in primary breast cancer. Breast Cancer Res Treat. 1992;22:207–19.
Alberti S, Fornaro M. Higher transfection efficency of genomic DNA purified with a guanidinium-thiocyanate-based procedure. Nucleic Acids Res. 1990;18:351–3.
Castrilli G, Piantelli M, Artese L, Perfetti G, Rubini C, Fioroni M, et al. Expression of hMSH2 and hMLH1 proteins of the human DNA mismatch repair system in ameloblastoma. J Oral Pathol Med. 2001;30:305–8.
Bonasera V, Alberti S, A.c S. Protocol for high-sensitivity/long linear-range spectrofluorimetric DNA quantification using ethidium bromide. BioTechniques. 2007;43:173–6.
Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, et al. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008;454(7205):766–70.
Bock C, Tomazou EM, Brinkman AB, Muller F, Simmer F, Gu H, et al. Quantitative comparison of genome-wide DNA methylation mapping technologies. Nat Biotechnol. 2010;28:1106–14.
Liggett T, Melnikov A, Yi QL, Replogle C, Brand R, Kaul K, et al. Differential methylation of cell-free circulating DNA among patients with pancreatic cancer versus chronic pancreatitis. Cancer. 2010;116:1674–80.
ArrayExpress Archive of Functional Genomics Data, EMBL-EBI [http://www.ebi.ac.uk/arrayexpress].
Wang C, Gong B, Bushel PR, Thierry-Mieg J, Thierry-Mieg D, Xu J, et al. The concordance between RNA-seq and microarray data depends on chemical treatment and transcript abundance. Nat Biotechnol. 2014;32:926–32.
Melson J, Li Y, Cassinotti E, Melnikov A, Boni L, Ai J, et al. Commonality and differences of methylation signatures in the plasma of patients with pancreatic cancer and colorectal cancer. Int J Cancer. 2014;134:2656–62.
Ingenuity Pathway Analysis [http://www.ingenuity.com/].
STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) [http://string-db.org/].
Simeone P, Trerotola M, Urbanella A, Lattanzio R, Ciavardelli D, Di Giuseppe F, et al. A unique four-Hub protein cluster associates to glioblastoma progression. PLoS One. 2014;9:e103030.
Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486:400–4.
Duncan JA, Reeves JR, Cooke TG. BRCA1 and BRCA2 proteins: roles in health and disease. Mol Pathol. 1998;51:237–47.
Yoshida K, Miki Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004;95:866–71.
Wang B, Hurov K, Hofmann K, Elledge SJ. NBA1, a new player in the Brca1 A complex, is required for DNA damage resistance and checkpoint control. Genes Dev. 2009;23:729–39.
Bochar DA, Wang L, Beniya H, Kinev A, Xue Y, Lane WS, et al. BRCA1 is associated with a human SWI/SNF-related complex: linking chromatin remodeling to breast cancer. Cell. 2000;102:257–65.
Hartman AR, Ford JM. BRCA1 induces DNA damage recognition factors and enhances nucleotide excision repair. Nat Genet. 2002;32:180–4.
McCoy ML, Mueller CR, Roskelley CD. The role of the breast cancer susceptibility gene 1 (BRCA1) in sporadic epithelial ovarian cancer. Reprod Biol Endocrinol. 2003;1:72.
Rzepecka IK, Szafron L, Stys A, Bujko M, Plisiecka-Halasa J, Madry R, et al. High frequency of allelic loss at the BRCA1 locus in ovarian cancers: clinicopathologic and molecular associations. Cancer Genet. 2012;205:94–100.
Wang C, Horiuchi A, Imai T, Ohira S, Itoh K, Nikaido T, et al. Expression of BRCA1 protein in benign, borderline, and malignant epithelial ovarian neoplasms and its relationship to methylation and allelic loss of the BRCA1 gene. J Pathol. 2004;202:215–23.
Chan KY, Ozcelik H, Cheung AN, Ngan HY, Khoo US. Epigenetic factors controlling the BRCA1 and BRCA2 genes in sporadic ovarian cancer. Cancer Res. 2002;62:4151–6.
Wang YQ, Yan Q, Zhang JR, Li SD, Yang YX, Wan XP. Epigenetic inactivation of BRCA1 through promoter hypermethylation in ovarian cancer progression. J Obstet Gynaecol Res. 2013;39:549–54.
Yang HJ, Liu VW, Wang Y, Tsang PC, Ngan HY. Differential DNA methylation profiles in gynecological cancers and correlation with clinico-pathological data. BMC Cancer. 2006;6:212.
Sharma P, Stecklein SR, Kimler BF, Sethi G, Petroff BK, Phillips TA, et al. The prognostic value of promoter methylation in early stage triple negative breast cancer. J Cancer Ther Res. 2014;3:1–11.
Guo M, Jia Y, Yu Z, House MG, Esteller M, Brock MV, et al. Epigenetic changes associated with neoplasms of the exocrine and endocrine pancreas. Discov Med. 2014;17:67–73.
Michie AM, McCaig AM, Nakagawa R, Vukovic M. Death-associated protein kinase (DAPK) and signal transduction: regulation in cancer. FEBS J. 2010;277:74–80.
Rossi D, Gaidano G, Gloghini A, Deambrogi C, Franceschetti S, Berra E, et al. Frequent aberrant promoter hypermethylation of O6-methylguanine-DNA methyltransferase and death-associated protein kinase genes in immunodeficiency-related lymphomas. Br J Haematol. 2003;123:475–8.
Raval A, Tanner SM, Byrd JC, Angerman EB, Perko JD, Chen SS, et al. Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia. Cell. 2007;129:879–90.
Rosas SL, Koch W, da Costa Carvalho MG, Wu L, Califano J, Westra W, et al. Promoter hypermethylation patterns of p16, O6-methylguanine-DNA-methyltransferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res. 2001;61:939–42.
Tada Y, Wada M, Taguchi K, Mochida Y, Kinugawa N, Tsuneyoshi M, et al. The association of death-associated protein kinase hypermethylation with early recurrence in superficial bladder cancers. Cancer Res. 2002;62:4048–53.
Gonzalez-Gomez P, Bello MJ, Alonso ME, Lomas J, Arjona D, Aminoso C, et al. Frequent death-associated protein-kinase promoter hypermethylation in brain metastases of solid tumors. Oncol Rep. 2003;10:1031–3.
Li Y, Geng P, Jiang W, Wang Y, Yao J, Lin X, et al. Enhancement of radiosensitivity by 5-Aza-CdR through activation of G2/M checkpoint response and apoptosis in osteosarcoma cells. Tumour Biol. 2014;35:4831–9.
Sigurdsson H, Baldetorp B, Borg A, Dalberg M, Ferno M, Killander D, et al. Indicators of prognosis in node-negative breast cancer. N Engl J Med. 1990;322:1054–3.
Stone A, Valdes-Mora F, Gee JM, Farrow L, McClelland RA, Fiegl H, et al. Tamoxifen-induced epigenetic silencing of oestrogen-regulated genes in anti-hormone resistant breast cancer. PLoS One. 2012;7:e40466.
Tellez CS, Shen L, Estecio MR, Jelinek J, Gershenwald JE, Issa JP. CpG island methylation profiling in human melanoma cell lines. Melanoma Res. 2009;19:146–55.
Kroeger H, Jelinek J, Estecio MR, He R, Kondo K, Chung W, et al. Aberrant CpG island methylation in acute myeloid leukemia is accentuated at relapse. Blood. 2008;112:1366–73.
Xu XL, Wu LC, Du F, Davis A, Peyton M, Tomizawa Y, et al. Inactivation of human SRBC, located within the 11p15.5-p15.4 tumor suppressor region, in breast and lung cancers. Cancer Res. 2001;61:7943–9.
Martinez R, Martin-Subero JI, Rohde V, Kirsch M, Alaminos M, Fernandez AF, et al. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics. 2009;4:255–64.
Lee JH, Kang MJ, Han HY, Lee MG, Jeong SI, Ryu BK, et al. Epigenetic alteration of PRKCDBP in colorectal cancers and its implication in tumor cell resistance to TNFalpha-induced apoptosis. Clin Cancer Res. 2011;17:7551–62.
Moutinho C, Martinez-Cardus A, Santos C, Navarro-Perez V, Martinez-Balibrea E, Musulen E, et al. Epigenetic inactivation of the BRCA1 interactor SRBC and resistance to oxaliplatin in colorectal cancer. J Natl Cancer Inst. 2014;106:djt322.
Caren H, Djos A, Nethander M, Sjoberg RM, Kogner P, Enstrom C, et al. Identification of epigenetically regulated genes that predict patient outcome in neuroblastoma. BMC Cancer. 2011;11:66.
Zochbauer-Muller S, Fong KM, Geradts J, Xu X, Seidl S, End-Pfutzenreuter A, et al. Expression of the candidate tumor suppressor gene hSRBC is frequently lost in primary lung cancers with and without DNA methylation. Oncogene. 2005;24:6249–55.
Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–75.
Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clin Chim Acta. 2013;424:53–65.
Deng D, Liu Z, Du Y. Epigenetic alterations as cancer diagnostic, prognostic, and predictive biomarkers. Adv Genet. 2010;71:125–76.
Kiss NB, Geli J, Lundberg F, Avci C, Velazquez-Fernandez D, Hashemi J, et al. Methylation of the p16INK4A promoter is associated with malignant behavior in abdominal extra-adrenal paragangliomas but not pheochromocytomas. Endocr Relat Cancer. 2008;15:609–21.
Matsuda Y, Ichida T. p16 and p27 are functionally correlated during the progress of hepatocarcinogenesis. Med Mol Morphol. 2006;39:169–75.
Lou-Qian Z, Rong Y, Ming L, Xin Y, Feng J, Lin X. The prognostic value of epigenetic silencing of p16 gene in NSCLC patients: a systematic review and meta-analysis. PLoS One. 2013;8:e54970.
Kazianis S, Coletta LD, Morizot DC, Johnston DA, Osterndorff EA, Nairn RS. Overexpression of a fish CDKN2 gene in a hereditary melanoma model. Carcinogenesis. 2000;21:599–605.
Boultwood J, Wainscoat JS. Gene silencing by DNA methylation in haematological malignancies. Br J Haematol. 2007;138:3–11.
Chim CS, Liang R, Kwong YL. Hypermethylation of gene promoters in hematological neoplasia. Hematol Oncol. 2002;20:167–76.
Pitsikas P, Lee D, Rainbow AJ. Reduced host cell reactivation of oxidative DNA damage in human cells deficient in the mismatch repair gene hMSH2. Mutagenesis. 2007;22:235–43.
de Wind N, Dekker M, Berns A, Radman M, te Riele H. Inactivation of the mouse Msh2 gene results in mismatch repair deficiency, methylation tolerance, hyperrecombination, and predisposition to cancer. Cell. 1995;82:321–30.
Mellon I, Rajpal DK, Koi M, Boland CR, Champe GN. Transcription-coupled repair deficiency and mutations in human mismatch repair genes. Science. 1996;272:557–60.
Hitchins MP. The role of epigenetics in Lynch syndrome. Fam Cancer. 2013;12:189–205.
Chan TL, Yuen ST, Kong CK, Chan YW, Chan AS, Ng WF, et al. Heritable germline epimutation of MSH2 in a family with hereditary nonpolyposis colorectal cancer. Nat Genet. 2006;38:1178–83.
Wang CX, Wang X, Liu HB, Zhou ZH. Aberrant DNA methylation and epigenetic inactivation of hMSH2 decrease overall survival of acute lymphoblastic leukemia patients via modulating cell cycle and apoptosis. Asian Pac J Cancer Prev. 2014;15:355–62.
Lee KH, Lee JS, Nam JH, Choi C, Lee MC, Park CS, et al. Promoter methylation status of hMLH1, hMSH2, and MGMT genes in colorectal cancer associated with adenoma-carcinoma sequence. Langenbecks Arch Surg. 2011;396:1017–26.
Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–9.
Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–6.
Schramek D, Leibbrandt A, Sigl V, Kenner L, Pospisilik JA, Lee HJ, et al. Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature. 2010;468:98–102.
Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–53.
Delgado-Calle J, Sanudo C, Fernandez AF, Garcia-Renedo R, Fraga MF, Riancho JA. Role of DNA methylation in the regulation of the RANKL-OPG system in human bone. Epigenetics. 2012;7:83–91.
Yuan L, Chan GC, Fung KL, Chim CS. RANKL expression in myeloma cells is regulated by a network involving RANKL promoter methylation, DNMT1, microRNA and TNFalpha in the microenvironment. Biochim Biophys Acta. 1843;2014:1834–8.
Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30:413–21.
Hainaut P, Hernandez T, Robinson A, Rodriguez-Tome P, Flores T, Hollstein M, et al. IARC Database of p53 gene mutations in human tumors and cell lines: updated compilation, revised formats and new visualisation tools. Nucleic Acids Res. 1998;26:205–13.
Sidransky D, Tokino T, Helzlsouer K, Zehnbauer B, Rausch G, Shelton B, et al. Inherited p53 gene mutations in breast cancer. Cancer Res. 1992;52:2984–6.
Network. CGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70.
Acknowledgments
We thank C.A. Beltrami, C. Di Loreto, E. Bianchini and B. Murer for help with the cancer pathology analyses. We gratefully acknowledge the support of Oncoxx Biotech, Compagnia di San Paolo, Fondazione CARIChieti, and the Italian Ministries for the University and Research (RBNE0157EH, SCN_00558), Development (MI01_00424) and Health (RicOncol RF-EMR-2006–361866).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
VL is co-Founder and Chief Scientific Officer of US Biomarkers Inc.; AAM is co-Founder, Vice-President of Research and Development and Chief Technology Officer of US Biomarkers Inc.; US Biomarkers Inc. conducts business related to DNA methylation analysis. All other authors declare that they have no financial conflict of interest regarding the contents of this manuscript. The sponsors had no role in the design and conduct of this study, nor in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript.
Authors’ contributions
SA and VL conceived the project; EG performed the DNA extraction and viability analyses; VL and AAM carried out the methylation assays; YD and YL performed the data processing and statistical analyses; PS performed the network analyses. All authors drafted the manuscript and approved the final version.
Additional files
Additional file 1: Table S1.
Pathological and clinical parameters of the breast cancer case series.
Additional file 2: Table S2.
PLS K nearest-neigbors, radial basis machine ranked genes. Table S3: Statistical power analysis of single-gene DNA-methylation patterns. Table S4: Pathway analyses.
Additional file 3: Figure S1.
Gene-protein interaction network - IPA analysis. (A) Network display. Red nodes are directly linked to input genes (Tables 1, 2); white nodes indicate higher iteration/depth. Edges are predicted functional links, and are indicated by arrows. Continuous lines: direct interactions; dashed lines: indirect interactions. (B) Overlay on the Canonical cancer signaling Pathway. Proteins directly linked to input genes (Tables 1, 2) are highlighted in red. Nuclear or cytoplasmic localization is indicated. Relationship with apoptotic pathways is detailed.
Additional file 4: Figure S2.
Gene-protein interaction network - STRING analysis. Networks including input proteins and protein bridges. (A) p53 signaling pathway (KEGG entry: map04115 p = 6.17 E−22) proteins are highlighted in blue. (B) Pathways in cancer (KEGG entry: map05200 p = 8.72 E−13) proteins are highlighted in red; additional cancer-relevant proteins are highlighted in pink.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Li, Y., Melnikov, A.A., Levenson, V. et al. A seven-gene CpG-island methylation panel predicts breast cancer progression. BMC Cancer 15, 417 (2015). https://doi.org/10.1186/s12885-015-1412-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-015-1412-9