Abstract
Background
Maternal obesity is associated with adverse outcome for pregnancy and childbirths. While bariatric surgery may improve fertility and reduce the risk of certain pregnancy-related complications such as hypertension and gestational diabetes mellitus, there is a lack of evidence on the optimal nutritional monitoring and supplementation strategies in pregnancy following bariatric surgery. We aimed to assess the impact of bariatric surgery on micronutrients in post-bariatric pregnancy and possible differences between gastric bypass surgery and sleeve gastrectomy.
Methods
In this prospective case control study, we recruited 204 pregnant women (bariatric surgery n = 59 [gastric bypass surgery n = 26, sleeve gastrectomy n = 31, missing n = 2] and controls n = 145) from Akershus university hospital in Norway. Women with previous bariatric surgery were consecutively invited to study participation at referral to the clinic for morbid obesity and the controls were recruited from the routine ultrasound screening in gestational week 17–20. A clinical questionnaire was completed and blood samples were drawn at mean gestational week 20.4 (SD 4.5).
Results
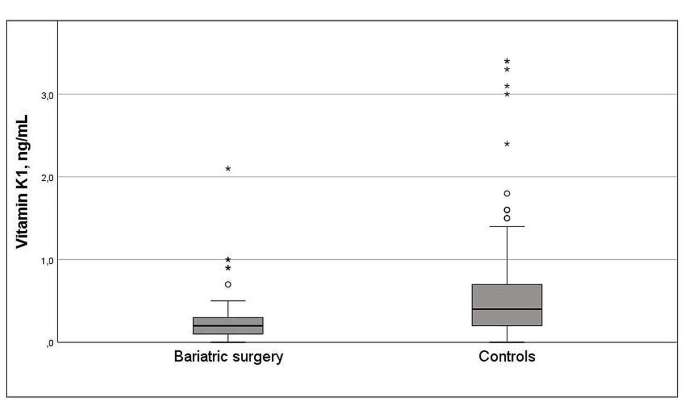
The women with bariatric surgery had a higher pre-pregnant BMI than controls (30.8 [SD 6.0] vs. 25.2 [5.4] kg/m2, p < 0.001). There were no differences between groups regarding maternal weight gain (bariatric surgery 13.3 kg (9.6) vs. control 14.8 kg (6.5), p = 0.228) or development of gestational diabetes (n = 3 [5%] vs. n = 7 [5%], p = 1.000). Mean levels of vitamin K1 was lower after bariatric surgery compared with controls (0.29 [0.35] vs. 0.61 [0.65] ng/mL, p < 0.001). Multiadjusted regression analyses revealed an inverse relationship between bariatric surgery and vitamin K1 (B -0.26 ng/mL [95% CI -0.51, -0.04], p = 0.047) with a fivefold increased risk of vitamin K1 deficiency in post-bariatric pregnancies compared with controls (OR 5.69 [1.05, 30.77] p = 0.044). Compared with sleeve gastrectomy, having a previous gastric bypass surgery was associated with higher risk of vitamin K1 deficiency (OR 17.1 [1.31, 223.3], p = 0.030).
Conclusion
Post-bariatric pregnancy is negatively associated with vitamin K1 with a higher risk of vitamin K1 deficiency in pregnancies after gastric bypass surgery compared with after sleeve gastrectomy. Vitamin K1 deficiency in post-bariatric pregnancy have potential risk of hypocoaguble state in mother and child and should be explored in future studies.
Similar content being viewed by others
Background
Obesity is common in women of reproductive age, increasing the risk of several complications for mother and child [1, 2]. Maternal metabolism in obesity may reduce the likelihood of successful pregnancy [3]. Moreover, given that weight loss before pregnancy mitigates the adverse outcomes of pregnancy related outcomes from obesity, bariatric surgery in women of reproductive age in increasing [4, 5]. However, although bariatric surgery may reduce the risks of certain obesity related complications in pregnancy, pregnancy after bariatric surgery may carry adverse events such as malnutrition, vitamin deficiencies and inadequate weight gain as well as changes in endocrine and metabolic homeostasis [6,7,8,9,10]. Pregnancy following bariatric surgery has been associated with increased risk of preterm birth, nutritional deficiency and small for gestational age [7, 8, 11,12,13,14]. The causality of these effects are not known, but personalized nutritional counseling during post-bariatric pregnancy has been shown to improve nutrient intake of mothers and may contribute to higher weight of offspring [15].
There is a growing body of evidence suggesting that maternal nutrition and lifestyle affect fetal growth and development [16, 17]. Micronutrients are vitamins and minerals that enable the body to produce enzymes, hormones and other substances essential for normal growth and development [18]. Micronutrient deficiencies contribute to poor growth, intellectual impairments and increased risk of morbidity and mortality [19]. Widespread global micronutrient deficiencies exist, with pregnant women and young children at highest risk [19]. Micronutrient interventions such as supplementation of folate to prevent neural tube defects zinc to reduce risk of preterm birth, and iron to reduce the risk of low birthweight are established [20,21,22]. The micronutritional deficiencies seen after bariatric surgery might be explained by poor dietary pattern in combination with gastrointestinal modification and reduced intestinal transit time [23,24,25,26,27]. Deficiencies of fatty soluble vitamins seem to be particularly prevalent in post-bariatric pregnancies, with potential risks of impaired vision, neuronal disorders, impairment of the immune system and hypocoagulability for mother and child [24, 28,29,30].
While sleeve gastrectomy is the most common surgical procedure for the treatment of obesity worldwide, there is conflicting evidence on the optimal surgical procedure before subsequent pregnancy [10, 31]. A large registry study showed no difference between gastric bypass and sleeve gastrectomy for preterm birth or small for gestational age [12]. Studies indicates increased risk of prematurity in pregnancy occurring less than 2 years after bariatric surgery [12, 32]. However, other studies have not confirmed increased risks in pregnancies related to time-interval between bariatric surgery and conception [13, 33]. As such, there is an evident knowledge gap on the impact of bariatric surgery on micronutrient status in pregnancies as well as outcomes for mother and child in order to provide optimal obstetric care in this group.
The aim of this study was to assess the impact of bariatric surgery on concentrations of micronutrients in post-bariatric pregnancies compared with non-surgical controls. Specifically, we hypothesized that fatty soluble micronutrients, including vitamin K1, was impaired after bariatric surgery. We also wanted to assess differences in maternal micronutrients concentrations following sleeve gastrectomy versus gastric bariatric surgery.
Materials and methods
Design and study population
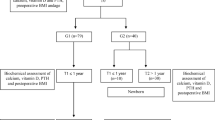
This observational case control study compared micronutritional status in pregnancy after bariatric surgery with non-surgical controls. Study participants were recruited from Akershus university hospital, between October 18th 2018 and December 9th 2022. Pregnant women with previous bariatric surgery were consecutively invited to study participation at referral to the clinic for morbid obesity and the controls were recruited from the routine ultrasound screening in gestational week 17–20. A total of 59 women with a previous bariatric surgery was included in the study and information on surgical procedure was available for 57 women (gastric bypass surgery n = 26 and sleeve gastrectomy n = 31). All women with post-bariatric pregnancies were closely monitored individually by a clinical doctor and a registered clinical dietitian focusing on micronutrient status and gestational weight gain. The controls received standard hospital care and dietary advice with additional advice if the blood samples revealed deficiencies.
A total of 204 women were included in this study with 92% of Caucasian ethnicity (n = 185). We compared micronutrient status in pregnancy in women with previous bariatric surgery (n = 59) to controls (n = 145). Women with known intestinal conditions (i.e. known inflammatory bowel disease, uncontrolled coeliac disease) were not included in the study. The study was approved by the Regional Committee for Medical and Health Research Ethics (reference 25829). All study participants provided written informed consent before study commencement, and the study was performed in accordance with the Declaration of Helsinki [34].
Definitions
The reference intervals for micronutrients in non-pregnant women and the chosen cut-offs defining micronutrient deficiencies in pregnancy are presented in Table 1. We defined micronutrient deficiency according to known physiological changes in blood during pregnancy combined with established reference intervals in a non-pregnant population [30, 35,36,37]. Time interval between bariatric surgery and conception was categorized into < 18 and ≥ 18 months.
Data collection
Clinical and laboratory data were retrieved at mean gestational week 20.4 (SD 4.5) (Bariatric surgery 23.9 [6.5] vs. controls 19.0 [2.0] weeks, p < 0.001). Follow-up blood sample was available in a subgroup of 32 women with post-bariatric pregnancies at mean 30.4 (SD 5.6) gestational week. All patients completed a questionnaire on comorbidities, medications and dietary supplements. Additional information including maximum weight, time of bariatric surgery, type of bariatric surgery was retrieved during the first visit.
Blood samples and analysis
The blood samples were obtained by venipuncture and collected in Vacuette® tubes. EDTA tubes were used for analysis of hemoglobin, hemoglobin A1c and thiamine (vitamin B1). Lithium heparin gel tubes were used for analysis of zinc and selenium, and serum gel tubes for the remaining analyses. All the blood samples were non-fasting. After blood collection, all tubes were handled according to established procedures. The standard clinical chemistry parameters were analysed at the laboratory at Akershus University Hospital. Hemoglobin was analysed on Sysmex instruments (Sysmex Corporation, Kobe, Japan) and hemoglobin A1c on Tosoh instruments (Tosoh Corporation, Tokyo, Japan). Magnesium and homocysteine were analysed on Vitros 5.1 FS (Ortho Clinical Diagnostics, Raritan, NJ) until May 2021, thereafter on cobas c503 (Roche Diagnostics, Mannheim, Germany). Folate, cobalamin, ferritin and vitamin D were analysed on cobas e801 (Roche Diagnostics). Zinc and selenium were analysed using inductive coupled plasma – mass spectrometry (ICP-MS) and methylmalonic acid (MMA) with a liquid chromatography – mass spectrometry method (LC-MS/MS). Thiamine, pyridoxal 5-phosphate (vitamin B6), vitamin A and vitamin E were analysed at Oslo University Hospital, Aker and vitamin K1 was analysed at Fürst Medical Laboratorium, Oslo, all with chromatographic methods.
Statistical analysis
We estimated that the prevalence of micronutrient deficiency would be 30% in post-bariatric pregnancies and 5% in controls. To confirm a similar difference with a statistical power of more than 80% and a significance level (α) of 0.05, a total of 200 patients had to be included in the study with a 4:1 ratio of cases vs. controls (40 post-bariatric pregnancies and 160 controls). Proportions are reported as numbers with percent, continuous variables as mean ± standard deviation (SD) as appropriate. Differences between treatment groups were analysed using Pearson’s chi-square test or Fishers exact test for categorical data and Student’s t-test for continuous data. Paired sample t-test was used to assess paired observations of micronutrients in baseline and follow-up blood samples. Skewed distributed data were log-transformed to achieve normal distribution. Correlations between possible confounders and vitamin K1 variables were assessed by Spearman’s correlation (rho). Two-sided P values < 0.05 were considered statistically significant. The Bonferroni Holm correction was applied to mitigate the risk of type 1 statistical error. We used linear regression analyses to explore possible associations between bariatric surgery and vitamin K1 and logistic regression analyses to explore possible associations between bariatric surgery and vitamin K1 deficiency. Possible confounders were identified using a stepwise selection approach in which variables with p-values below 0.10 were included along with clinically significant confounders. Coefficients and odds ratio (OR) from regression analysis are presented with 95% confidence interval (CI). The analyses were performed using IBM SPSS Statistics (version 729.0.0).
Results
We included 204 women in the study (bariatric surgery n = 59 and controls n = 145). Data on the specific type of surgical procedure were available for 57 women who had undergone bariatric surgery prior to conception (gastric bypass surgery n = 26 and sleeve gastrectomy = 31). The women in the surgical group lost on average 39.0 (16.9) kg from the time of surgery to the start of pregnancy and the time interval from bariatric surgery to pregnancy was mean 63.7 (39.2) months. Patients’ characteristics by surgical status are presented in Table 2.
The women with bariatric surgery had a higher pre-pregnant body mass index (BMI) compared with controls (30.8 [SD 6.0] vs. 25.2 [5.4] kg/m2, p < 0.001). There was no difference between groups regarding age (32.1 [5.7] vs. 31.2 [4.2] years, p = 0.215), maternal weight gain (13.3 [9.6] vs. 14.8 [6.5] kg, p = 0.228), HbA1c (30.2 [7.1] vs. 31.1[3.6] mmol/mol, p = 0.234) or development of gestational diabetes (5% vs. 5%, p = 1.000). Fewer women with bariatric surgery had completed higher education and more women with bariatric surgery currently smoked compared with controls (24 [43%] vs. 103 [72%], p < 0.001 and 5 [9%] vs. 0, p = 0.001, respectively. Children of post-bariatric pregnancies had lower gestational age and lower birthweight, however neither reached statistical significance (38.5[3.1] vs. 39.3[2.1] weeks, p = 0.054 and 3363 [624] vs. 3520 [521] g, p = 0.081, respectively).
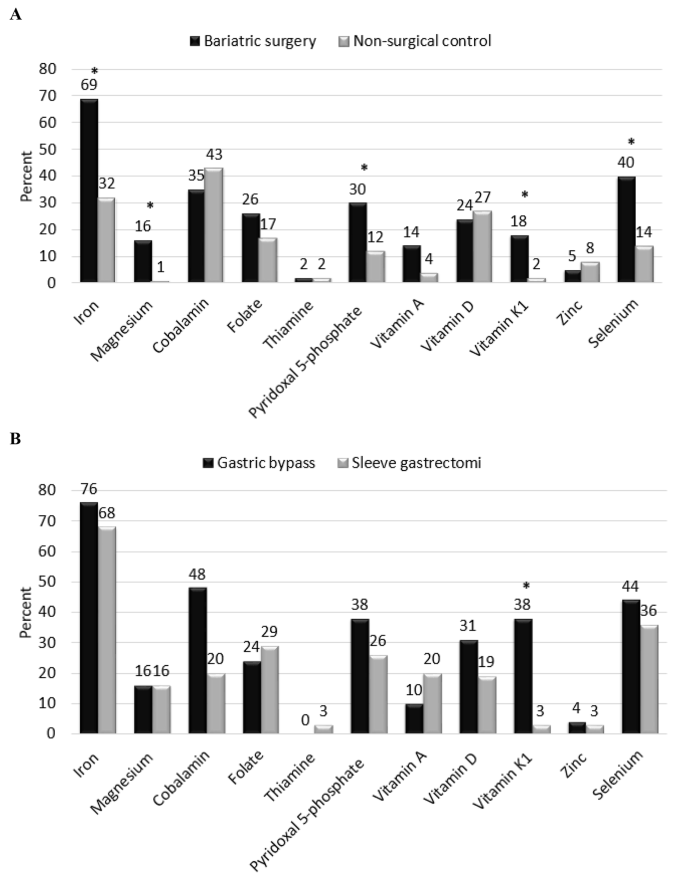
Dietary supplements and micronutrient status by surgical status are presented in Table 3. Concentrations of ferritin, magnesium, pyridoxal 5-phosphate, vitamin A, E and K1 and selenium were significantly lower post-bariatric pregnancies compared with controls. Using micronutrients as categorical variables (deficiency yes/no) conferred a higher prevalence of micronutrient deficiencies such as iron, magnesium, pyridoxal 5-phosphate, vitamin K1 and selenium in pregnancies after bariatric surgery compared with controls and a higher prevalence of vitamin K1 deficiency after gastric bariatric surgery vs. sleeve gastrectomy (Fig. 1). The distribution of vitamin K1 concentrations in women with post-bariatric pregnancies and controls is presented in Fig. 2. Paired sample t-test showed increased concentrations vitamin K1 in a subgroup of women with post-bariatric pregnancies (0.29 [0.29] ng/mL to 0.64 [0.92] ng/mL, p = 0.070).
The women with gastric bariatric surgery underwent surgery at a younger age and with a longer time-interval between surgery and conception compared with the women with sleeve gastrectomy (23.5 vs. 27.5 years, p = 0.002 and 85 [40] vs. 45 [28] months, p = < 0.001, respectively). One woman (4%) after gastric bariatric surgery and five women (16%) after sleeve gastrectomy, p = 0.205 became pregnant < 18 months after surgery. Both surgical groups had lost comparable weight since surgery (gastric bypass surgery 41.4 [17.1] vs. sleeve gastrectomy 37.0 [16.8] kg, p = 0.342) and they had comparable pre-pregnant BMI (gastric bypass surgery 31.9 [5.5] vs. sleeve gastrectomy 29.9 [6.4] kg/m2, p = 0.222). The proportion of women with vitamin K1 deficiency was higher after gastric bariatric surgery compared with sleeve gastrectomy (gastric bypass surgery 9 [38%] vs. 1 [3%], p = 0.003 and Fig. 1).
Univariate linear regression analysis showed that bariatric surgery was inversely associated with vitamin K1 levels (B -0.33 [95% CI -0.51, -0.15, p < 0.001]. The result remained statistically significant after multivariable adjustments (-0.26 ng/mL [-0.51, -0.04], p = 0.047) (Table 4A). In addition, compared with sleeve gastrectomy, gastric bariatric surgery was inversely associated with vitamin K1 in univariate linear regression analysis (0.20 [0.019, 0.387], p = 0.031), but not after multivariate adjustment (Table 4B). Using vitamin K1 as a categorical variable (deficiency yes/no), bariatric surgery was associated with a fivefold increased risk of vitamin K1 deficiency compared with controls and that gastric bariatric surgery was associated with higher adjusted risk of vitamin K1 deficiency compared with sleeve gastrectomy (Table 5).
Discussion
In this study, we compare micronutrient concentrations in post-bariatric pregnancy with matched non-surgical controls. The study shows that the concentrations of vitamin K1, magnesium, and selenium were significantly impaired in post-bariatric pregnancies vs. controls. Moreover, our results show that bariatric surgery was consistently associated with vitamin K1 levels, both as a continuous outcome variable and as a categorical variable (vitamin K1 deficiency) in post-bariatric pregnancy compared with controls. Moreover, the associations might be driven by gastric bariatric surgery rather than sleeve gastrectomy. However, the number of pregnant women with vitamin K1 concentration below the lower reference limit was overall small and the confidence intervals were large. Thus, these results should be interpreted with caution.
Maternal nutrition and micronutrients in pregnancy after bariatric surgery
In pregnancy, there is an increased need for nutrients to support fetal and placental growth and development [20]. A detailed dietary information was not available in this study and we cannot exclude that the women with bariatric surgery had a different nutritional composition compared with controls. In a subgroup of women with post-bariatric pregnancies, an increment in vitamin K was seen. However, the changes did not reach statistical significance. Follow-up blood samples for the controls were not available. A healthy diet after bariatric surgery may differ from the general population in the composition of lean protein, fruits and vegetables and starchy carbohydrates. Nonetheless, the combination of diet, intestinal modifications and increased metabolism in pregnancy might explain the deficiencies in fatty soluble vitamins seen in this study [23,24,25,26,27]. Improved nutrient intake of mothers was seen after personalized nutritional counseling during post-bariatric pregnancy and might contribute to higher birth weight of offspring [15]. Given the complexity and heterogeneity of nutritional status in post-bariatric pregnancies, focusing on sub-groups including pre-gestational nutritional deficiencies, and type of surgery performed is of vital importance. A recent consensus report recommended specialized care in pregnancies after bariatric surgery [38]. There is however a paucity of data to support clinical practice [38, 39]. As such, there is an imperative need to identify pregnancy and trimester specific reference intervals and clinical decision limits in order to help clinical advice on dietary supplement.
Lifelong dietary supplement is recommended after bariatric surgery, however adherence to adequate dietary supplements seems to decrease over time [26, 40, 41]. Our study also confers inadequate use of dietary supplements in pregnancy after bariatric surgery with 30–70% of the women not taking recommended post-bariatric surgery dietary supplements (Table 3). Thus, a need for increased awareness to ensure adequate microntutrional care before, during and after pregnancy is imperative.
The role of vitamin K1 in pregnancy after bariatric surgery
In line with our results, a systematic review on vitamin K1 concentrations in patients with a history of bariatric surgery reported high risk of vitamin K1 deficiency after bariatric surgery and opted for better monitoring [23]. Our results also cohere with another study of 49 pregnant women with previous bariatric surgery, showing that vitamin K1 concentrations were lower in women with a history of bariatric surgery compared with 27 controls [30]. The increased fat storage in pregnancy may lead to less bioavailability for activation of fatty soluble vitamins [42]. Furthermore, the highly fat-soluble vitamin K1 depend upon conjugated bile salts for adequate absorption. Consequently, reduced stomach acid production, reduced absorption surface and shorter interaction time between conjugated bile salts and vitamin K1 might explain the lower serum concentrations of vitamin K1 after bariatric surgery [43]. Screening for vitamin K1 deficiency is usually recommended after malabsorptive surgical procedures including biliopancreatic diversion with or without duodenal switch [43]. However, restrictive procedures may also cause vitamin deficiencies due to digestive symptoms such as vomiting and food intolerance. Interestingly, lower levels of vitamin K1 were found in the first trimester compared to a control group of women without bariatric surgery [30]. Vomiting and food intolerance may also be the main symptoms of hyperemesis gravidarum, which calls for increased vigilance of vitamin K1 insufficiency in post-bariatric pregnancies in women with symptoms of hyperemesis in pregnancy.
The impact of vitamin K1 deficiency in post-bariatric pregnancies is not clear. Low circulating levels of vitamin K1 might lead to a hypocoaguble state in mother and child [30]. Some cases of neonatal intracranial bleeding have been reported, possible due to vitamin K1 deficiency [44]. Another study reported that obesity had stronger impact on hypercoagulability than pregnancy itself [45]. Nonetheless, insufficient data exist in order to recommend interventions of vitamin K1 deficiency in post-bariatric pregnancy [38]. While optimal monitoring of vitamin K1 during pregnancy following bariatric surgery remains unclear, a major concern is raised about the consistent finding of vitamin K1 deficiency in post-bariatric pregnancy.
Bariatric surgery before pregnancy: timing and selection of procedure – dose it matter?
Few studies have assessed the impact of different surgical procedures before pregnancy. One study of 119 pregnant women found no effect of maternal weight gain on maternal and perinatal outcome after sleeve gastrectomy [46]. However, the study did not include pregnancies after gastric bariatric surgery for comparison. Another retrospective observational study showed no differences between gastric bariatric surgery and sleeve gastrectomy regarding re-interventions or obstetric outcomes [4]. Conflicting evidence exists on the possible adverse effects of sleeve gastrectomy such as dyspepsia and weight regain as compared with gastric bariatric surgery [47,48,49]. Our study adds important knowledge about the different surgical procedures, suggesting that gastric bariatric surgery holds greater risk of vitamin K1 deficiency compared with sleeve gastrectomy. The optimal surgical procedure for obesity treatment in women of reproductive age is however not clear and a person-centered approach should be advocated in future guidelines.
The timing of pregnancy after bariatric surgery is moreover under debate. Current recommendations suggest waiting at least 12 months after bariatric surgery before planning a pregnancy [12, 38, 50]. In our study, women with previous sleeve gastrectomy had a shorter time interval between surgery and conception than the women with gastric bariatric surgery. This might reflect that the women who underwent gastric bariatric surgery underwent surgery in an era where gastric bariatric surgery was the most common surgical procedure for weight loss [31]. Interestingly, after adjustments for the time interval since bariatric surgery, gastric bariatric surgery was not associated with vitamin K1 in the linear regression model (Table 4B). Thus, as adherence to dietary supplements is reduced with time after bariatric surgery, we cannot rule out that patient’ adherence to dietary supplement might have influenced the differences between surgical procedure seen in the present study [26, 40, 41]. On the other hand, the time interval between sleeve gastrectomy and conception did not impact maternal and neonatal outcomes in a study of 15 women conceived > 18 months after surgery. The authors concluded that pregnancy after sleeve gastrectomy was overall safe and well-tolerated [33]. Furthermore, a study of 30 women who became pregnant within a mean time of 17 months after gastric bariatric surgery did not appear to confer any serious risks in pregnancy with 90% of the children were born at term with normal birthweight [13]. In our study, only six patients (11%) became pregnant earlier than 18 months after surgery and the study was not designed to assess pregnancy or birth related complications.
Future implications?
The results of this study underscore the need for increased awareness of nutritional and microntutrional status to ensure adequate obstetric care both before and during post-bariatric pregnancies. Also, this study present important information on adherence to dietary supplement that should be considered in the planning of post-bariatric pregnancies. Moreover, the results of our study rises important questions on the impact of micronutrients deficiencies on future child development.
Strengths and limitations
The strengths of this study include the prospective design with matched controls. Moreover, definitions for the chosen cut-offs for micronutrient deficiency were chosen according to pregnancy specific reference intervals if established. However, we cannot rule out that the concentrations of the micronutrients change in pregnancy. Thus, the validity of the chosen cut-offs for defining micronutrient deficiency should be assessed in future studies. This study was a small single center study and did not have the statistical power to assess pregnancy related or birth related complications. The majority of the women in this study was Caucasian and the results may not be valid in populations of other ethnicities. The observational design does not provide any causality between variables. Also, we cannot rule out if the difference in gestational week for blood sampling or non-fasting blood samples might have influenced the micronutrient analyses. Finally, use of dietary supplements was self-reported and we cannot be sure that all the study participants adhered with the recommendation.
Conclusion
This study shows that concentrations of the micronutrients vitamin K1, magnesium, and selenium were significantly impaired in post-bariatric pregnancies compared with controls. We found a negative association between bariatric surgery and vitamin K1 and a higher risk of vitamin K1 deficiency after gastric bariatric surgery compared with sleeve gastrectomy. Vitamin K1 deficiency in post-bariatric pregnancy have potential risk of hypocoaguble state in mother and child and should be assessed in future studies.
Data availability
The data used in the present study is not open access or publicly available. The datasets are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SD:
-
Standard deviation
References
Obesity in Pregnancy. ACOG Practice Bulletin, Number 230. Obstet Gynecol. 2021;137(6):e128–44.
Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16(8):621–38.
Bou Nemer L, Shi H, Carr BR, Word RA, Bukulmez O. Effect of Body Weight on metabolic hormones and fatty acid metabolism in follicular fluid of women undergoing in Vitro fertilization: a pilot study. Reprod Sci. 2019;26(3):404–11.
Chao GF, Yang J, Peahl AF, Thumma JR, Dimick JB, Arterburn DE, Telem DA. Comparative effectiveness of sleeve gastrectomy vs Roux-en-Y gastric bypass in patients giving birth after bariatric surgery: reinterventions and obstetric outcomes. Surg Endosc. 2022;36(9):6954–68.
Fisher SA, Stetson BT, Kominiarek MA. Pregnancy after bariatric surgery. JAMA. 2023;329(9):758–9.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, et al. Bariatric surgery versus Intensive Medical Therapy for diabetes – 5-Year outcomes. N Engl J Med. 2017;376(7):641–51.
Johansson K, Cnattingius S, Näslund I, Roos N, Trolle Lagerros Y, Granath F, Stephansson O, Neovius M. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372(9):814–24.
Bloomberg RD, Fleishman A, Nalle JE, Herron DM, Kini S. Nutritional deficiencies following bariatric surgery: what have we learned? Obes Surg. 2005;15(2):145–54.
Yi XY, Li QF, Zhang J, Wang ZH. A meta-analysis of maternal and fetal outcomes of pregnancy after bariatric surgery. Int J Gynaecol Obstet. 2015;130(1):3–9.
Akhter Z, Rankin J, Ceulemans D, Ngongalah L, Ackroyd R, Devlieger R, Vieira R, Heslehurst N. Pregnancy after bariatric surgery and adverse perinatal outcomes: a systematic review and meta-analysis. PLoS Med. 2019;16(8):e1002866.
Stephansson O, Johansson K, Näslund I, Neovius M. Bariatric surgery and Preterm Birth. N Engl J Med. 2016;375(8):805–6.
Roos N, Neovius M, Cnattingius S, Trolle Lagerros Y, Sääf M, Granath F, Stephansson O. Perinatal outcomes after bariatric surgery: nationwide population based matched cohort study. BMJ. 2013;347:f6460.
Chagas C, Saunders C, Pereira S, Silva J, Saboya C, Ramalho A. Perinatal outcomes and the influence of maternal characteristics after Roux-en-Y gastric bypass surgery. J Womens Health (Larchmt). 2017;26(1):71–5.
Hammeken LH, Betsagoo R, Jensen AN, Sørensen AN, Overgaard C. Nutrient deficiency and obstetrical outcomes in pregnant women following Roux-en-Y gastric bypass: a retrospective Danish cohort study with a matched comparison group. Eur J Obstet Gynecol Reprod Biol. 2017;216:56–60.
Araki S, Shani Levi C, Abutbul Vered S, Solt I, Rozen GS. Pregnancy after bariatric surgery: effects of personalized nutrition counseling on pregnancy outcomes. Clin Nutr. 2022;41(2):288–97.
Barker M, Dombrowski SU, Colbourn T, Fall CHD, Kriznik NM, Lawrence WT, Norris SA, Ngaiza G, Patel D, Skordis-Worrall J, et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet. 2018;391(10132):1853–64.
Jain S, Maheshwari A, Jain SK. Maternal Nutrition and Fetal/Infant development. Clin Perinatol. 2022;49(2):313–30.
Tam E, Keats EC, Rind F, Das JK, Bhutta AZA. Micronutrient Supplementation and Fortification interventions on Health and Development outcomes among children under-five in low- and Middle-Income countries: a systematic review and Meta-analysis. Nutrients 2020, 12(2).
Bailey RL, West KP Jr., Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(Suppl 2):22–33.
Gernand AD, Schulze KJ, Stewart CP, West KP Jr., Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. 2016;12(5):274–89.
Carducci B, Keats EC, Bhutta ZA. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2021;3(3):Cd000230.
Kaska L, Kobiela J, Abacjew-Chmylko A, Chmylko L, Wojanowska-Pindel M, Kobiela P, Walerzak A, Makarewicz W, Proczko-Markuszewska M, Stefaniak T. Nutrition and pregnancy after bariatric surgery. ISRN Obes. 2013;2013:492060.
Sherf-Dagan S, Goldenshluger A, Azran C, Sakran N, Sinai T, Ben-Porat T. Vitamin K-what is known regarding bariatric surgery patients: a systematic review. Surg Obes Relat Dis. 2019;15(8):1402–13.
Sherf-Dagan S, Buch A, Ben-Porat T, Sakran N, Sinai T. Vitamin E status among bariatric surgery patients: a systematic review. Surg Obes Relat Dis. 2021;17(4):816–30.
Lewis CA, de Jersey S, Hopkins G, Hickman I, Osland E. Does bariatric surgery cause vitamin A, B1, C or E Deficiency? A systematic review. Obes Surg. 2018;28(11):3640–57.
Krzizek EC, Brix JM, Stöckl A, Parzer V, Ludvik B. Prevalence of Micronutrient Deficiency after bariatric surgery. Obes Facts. 2021;14(2):197–204.
Guelinckx I, Devlieger R, Donceel P, Bel S, Pauwels S, Bogaerts A, Thijs I, Schurmans K, Deschilder P, Vansant G. Lifestyle after bariatric surgery: a multicenter, prospective cohort study in pregnant women. Obes Surg. 2012;22(9):1456–64.
Falcone V, Stopp T, Feichtinger M, Kiss H, Eppel W, Husslein PW, Prager G, Göbl CS. Pregnancy after bariatric surgery: a narrative literature review and discussion of impact on pregnancy management and outcome. BMC Pregnancy Childbirth. 2018;18(1):507.
Devlieger R, Guelinckx I, Jans G, Voets W, Vanholsbeke C, Vansant G. Micronutrient levels and supplement intake in pregnancy after bariatric surgery: a prospective cohort study. PLoS ONE. 2014;9(12):e114192.
Jans G, Guelinckx I, Voets W, Galjaard S, Van Haard PM, Vansant GM, Devlieger R. Vitamin K1 monitoring in pregnancies after bariatric surgery: a prospective cohort study. Surg Obes Relat Dis. 2014;10(5):885–90.
English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and bariatric surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surg Obes Relat Dis. 2018;14(3):259–63.
Parent B, Martopullo I, Weiss NS, Khandelwal S, Fay EE, Rowhani-Rahbar A. Bariatric surgery in women of Childbearing Age, timing between an operation and birth, and Associated Perinatal complications. JAMA Surg. 2017;152(2):128–35.
Sancak S, Çeler Ö, Çırak E, Karip AB, Tumiçin Aydın M, Esen Bulut N, Mahir Fersahoğlu M, Altun H, Memişoğlu K. Timing of Gestation after laparoscopic sleeve gastrectomy (LSG): does it influence obstetrical and neonatal outcomes of pregnancies? Obes Surg. 2019;29(5):1498–505.
Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000;284(23):3043–5.
Means RT. Iron Deficiency and Iron Deficiency Anemia: implications and impact in pregnancy, Fetal Development, and early childhood parameters. Nutrients 2020, 12(2).
Berger MM, Shenkin A, Schweinlin A, Amrein K, Augsburger M, Biesalski HK, Bischoff SC, Casaer MP, Gundogan K, Lepp HL, et al. ESPEN micronutrient guideline. Clin Nutr. 2022;41(6):1357–424.
Nasjonal brukerhåndbok i medisinsk biokjemi. Available from https://metodebok.no/index.php?action=book&book=biokjemi. Version 2.4. Last updated 23.06.2022. Accessed 20.06.2023.
Shawe J, Ceulemans D, Akhter Z, Neff K, Hart K, Heslehurst N, Stotl I, Agrawal S, Steegers-Theunissen R, Taheri S, et al. Pregnancy after bariatric surgery: Consensus recommendations for periconception, antenatal and postnatal care. Obes Rev. 2019;20(11):1507–22.
Makrides M, Crosby DD, Bain E, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database Syst Rev. 2014;2014(4):Cd000937.
Smelt HJM, Pouwels S, Smulders JF, Hazebroek EJ. Patient adherence to multivitamin supplementation after bariatric surgery: a narrative review. J Nutr Sci. 2020;9:e46.
Ben-Porat T, Elazary R, Goldenshluger A, Sherf Dagan S, Mintz Y, Weiss R. Nutritional deficiencies four years after laparoscopic sleeve gastrectomy-are supplements required for a lifetime? Surg Obes Relat Dis. 2017;13(7):1138–44.
Weissgerber TL, Wolfe LA. Physiological adaptation in early human pregnancy: adaptation to balance maternal-fetal demands. Appl Physiol Nutr Metab. 2006;31(1):1–11.
Pournaras DJ, le Roux CW. After bariatric surgery, what vitamins should be measured and what supplements should be given? Clin Endocrinol (Oxf). 2009;71(3):322–5.
Eerdekens A, Debeer A, Van Hoey G, De Borger C, Sachar V, Guelinckx I, Devlieger R, Hanssens M, Vanhole C. Maternal bariatric surgery: adverse outcomes in neonates. Eur J Pediatr. 2010;169(2):191–6.
Sharma S, Uprichard J, Moretti A, Boyce H, Szydlo R, Stocks G. Use of thromboelastography to assess the combined role of pregnancy and obesity on coagulation: a prospective study. Int J Obstet Anesth. 2013;22(2):113–8.
Sancak S, Altun H, Çeler Ö, Çırak E, Er C, Karip AB, Okuroğlu N, Bulut NE, Fersahoğlu MM, Sertbaş Y, et al. Impact of Gestational Weight Gain on maternal and perinatal outcomes after laparoscopic sleeve Gastrectomy. Obes Surg. 2022;32(12):4007–14.
Carabotti M, Silecchia G, Greco F, Leonetti F, Piretta L, Rengo M, Rizzello M, Osborn J, Corazziari E, Severi C. Impact of laparoscopic sleeve gastrectomy on upper gastrointestinal symptoms. Obes Surg. 2013;23(10):1551–7.
Mandeville Y, Van Looveren R, Vancoillie PJ, Verbeke X, Vandendriessche K, Vuylsteke P, Pattyn P, Smet B. Moderating the enthusiasm of Sleeve Gastrectomy: up to 50% of reflux symptoms after ten years in a Consecutive Series of one hundred laparoscopic sleeve gastrectomies. Obes Surg. 2017;27(7):1797–803.
Silveira FC, Poa-Li C, Pergamo M, Gujral A, Kolli S, Fielding GA, Ren-Fielding CJ, Schwack BF. The effect of laparoscopic sleeve gastrectomy on gastroesophageal reflux disease. Obes Surg. 2021;31(3):1139–46.
Ciangura C, Coupaye M, Deruelle P, Gascoin G, Calabrese D, Cosson E, Ducarme G, Gaborit B, Lelièvre B, Mandelbrot L, et al. Clinical practice guidelines for childbearing female candidates for bariatric surgery, pregnancy, and Post-partum Management after bariatric surgery. Obes Surg. 2019;29(11):3722–34.
Acknowledgements
We acknowledge the work of the staff at the Section for Morbid Obesity at Akershus University Hospital HF for the persistent effort of data collection.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
TGV and EMRS designed the study. BTB, EMRS and TGV collected the data for the study. TGV analysed the data. BTB and TGV drafted the manuscript. CS ad GK were responsible for the laboratory analyses. TGV and JAW were responsible for the statistical analyses. All authors contributed to the interpretation of data, reviewed and edited the manuscript and gave their final approval of the final version to be published.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by the Regional Committee for Medical and Health Research Ethics (reference 25829). The study was performed in accordance with the Declaration of Helsinki. All study participants provided written informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bechensteen, B.T., Sithiravel, C., Strøm-Roum, E.M. et al. Post-bariatric pregnancy is associated with vitamin K1 deficiency, a case control study. BMC Pregnancy Childbirth 24, 229 (2024). https://doi.org/10.1186/s12884-024-06407-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06407-0