Abstract
Background
Weight management has been an important component of the service in obstetric care offered to pregnant women. Current gestational weight gain recommendations were primarily for the general obstetric population, raising concern about the applicability to women with gestational diabetes mellitus (GDM). We aimed to assess the difference in weight progression and adherence to the recommended gestational weight gain targets between women with gestational diabetes mellitus (GDM) and women with normal glucose tolerance (NGT).
Methods
This was a hospital-based retrospective study of 56,616 pregnant women (9,430 GDM women and 47,186 NGT women) from Guangzhou between 2017 and 2021. The average change in weight progression was estimated based on serial weight measurements throughout pregnancy, using a mixed effects model with a random intercept to account for repeated measures of the same individual.
Results
Women with GDM gained less weight (12.07 [SD 5.20] kg) than women with NGT (14.04 [SD 5.04] kg) throughout pregnancy. Before OGTT, a small difference was observed in the average change in weight progression between the two groups (GDM, 0.44 kg/week vs. NGT, 0.45 kg/week, p < 0.001), however, this gap widened significantly after the test (0.34 vs. 0.50 kg/week, p < 0.001). GDM individuals were identified with an approximately 4-fold increased proportion of insufficient weight gain (41.1% vs. 10.4%) and a 2-fold decreased proportion of excessive weight gain (22.6% vs. 54.2%) compared to NGT individuals. These results were consistently observed across different BMI categories, including underweight (insufficient: 52.7% vs. 19.9%; excessive: 15.6% vs. 35.3%), normal weight (insufficient 38.2% vs. 7.4%; excessive: 22.2% vs. 57.3%), and overweight/obese (insufficient: 43.1% vs. 9.8%; excessive: 30.1% vs. 68.8%).
Conclusion
Weight progression varied significantly between GDM and NGT individuals, resulting in a substantial difference in identifying insufficient and excessive weight gain between the two groups under current gestational weight gain guidelines.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Gestational weight gain has been recognized as a crucial modifier factor for maternal and fetal outcomes [1,2,3,4]. Inappropriate gestational weight gain (GWG), especially excessive GWG, has been linked to a variety of short- and long-term adverse health consequences for mothers and their offspring, including the increased risks of gestational diabetes mellitus (GDM) [5, 6], fetal macrosomia [7, 8], obesity, and adverse cardiometabolic profiles in the offspring [9,10,11,12].
In 2009, the Institute of Medicine (IOM) published national guidelines for GWG [13]. These guidelines established specific recommendations stratified by pre-pregnancy BMI class and were quickly adopted widely in many countries [13]. In China, weight management has been an important component of the service in obstetric care offered to pregnant women. Recently, given the ethnic variation in body mass and stature, the Chinese nutrition society released lower GWG and GWR targets for the Asian population in October 2021 [14]. However, both guidelines were primarily for the general obstetric population and do not differentiate between women with normal glucose tolerance (NGT) and those with gestational diabetes mellitus (GDM). GDM is an increasingly common chronic disease in pregnancy that affects about 15% of pregnant women [15, 16]. Women affected by GDM usually receive more intensive and comprehensive health interventions than those with NGT as a part of the glycemic control program [17,18,19]. As a result of these interventions, it can be hypothesized that gestational weight may progress in distinctly different ways between women with GDM and those with NGT. In previous studies, it was noted that women with GDM gained fewer amounts of weight [20, 21] than the general obstetric population through pregnancy [22,23,24]. However, it is unknown how gestational weight changes during pregnancy and to what extent the weight progression differs between the two groups.
The objective of this study was to assess the difference in weight progression between women with GDM and NGT in the total cohort and across different BMI categories. A further objective was to evaluate adherence to the current gestational weight guidelines for these two groups.
Materials and methods
Study design and population
This hospital-based retrospective cohort study was conducted at the Guangzhou Women and Children’s Medical Center (GWCMC), Guangzhou, south China [25], which serves in general about 100,000 pregnant women every year with comprehensive obstetrical care. Adult women (≥ 18 years) were considered eligible for inclusion if they had completed a singleton pregnancy between 1 and 2017 and 1 January 2021 and undergone a 2-h 75-g oral glucose tolerance test (OGTT) during pregnancy. GDM was diagnosed based on the International Association of Diabetes and Pregnancy Study Groups criteria [26]. Women with incomplete information on childbirth, such as gestational age and birth weight, and those with an OGTT performed < 20 weeks or > 29 weeks of gestation were excluded from the study. Also excluded were women with pregestational diabetes or overt diabetes, since their earlier intensive interventions would have a significant impact on weight progression. We further excluded women with fewer than two weight measurements both in the second trimester and third trimester to ensure the accuracy of weight gain rate (WGR) calculations. According to the Chinese pre-pregnancy and pregnancy care guidelines, it is recommended that pregnant women attend 7–11 routine antenatal care visits throughout their pregnancy to assess potential risks regarding both the mother and the developing fetus. These visits are typically scheduled before the 13th week for the first time, and then at regular intervals of 14–19, 20–24, 25–28, 29–32, 33–36, and 37–41 weeks of gestational age [27]. Information on demographic characteristics, pre-existing health conditions, previous reproductive history, measured blood pressure, and self-reported pre-pregnancy weight and height were collected at the initial visit. During the following antenatal visits, maternal weight in lightweight clothing was routinely monitored, along with blood pressure, using the same set of calibrated electronic scales. Maternal baseline health records and longitudinal data including blood pressure, weight measurements, maternal complications, hospitalization before delivery, and childbirth were obtained from electronic medical records via a unique membership identifier. Ethical permission for this study was provided by the institutional review boards at Guangzhou Women and Children’s Medical Center. Given all maternal and neonatal data were extracted from the hospital EMR system by a unique identifier with no participant involved in the design, the written informed consent was waived.
Outcome measures
Outcomes of interest were the average change in weight progression and adherence to gestation weight gain guidelines, which was assessed by total GWG and WGR in the second and third trimesters. To calculate GWG, we subtracted pre-pregnancy weight from weight at the end of pregnancy, independent of gestational age. We also calculated WGR before and after the OGTT accounting for changes in gestational age, based on serial weight measurements taken from the 13th week to the test, and from the test to delivery, respectively. To ensure the accuracy of WGR calculation, we took the initial weight measurement in the specified time window as the baseline weight for each individual, subtracted it from each subsequent weight, divided the results by the respective interval weeks as individual WGR, and averaged the WGRs to determine the final WGR in the predefined time window. The GWG and WGR were categorized into insufficient, adequate, and excessive according to Chinese weight guidelines [14].
Other variables
The covariates included age at delivery, education, pre-pregnancy BMI, chronic hypertension, hospitalization before delivery, number of prenatal visits, gestational weeks for OGTT, gestational age, parity, and fetal sex. Chronic hypertension, defined as hypertension diagnosed or present before pregnancy or before 20 weeks of gestation, was identified based on systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg, or both [28]. Pre-pregnancy BMI was calculated as weight in kilograms (kg) divided by height in meters squared (m2) and categorized into underweight (BMI < 18.5 kg/ m2), normal weight (BMI 18.5–23.9 kg/m2), overweight (BMI 24.0-27.9 kg/m2), and obese (BMI ≥ 28 kg /m2) based on Chinese BMI criteria [29]. In the stratified analysis by BMI, we included overweight and obese into one category as there were limited obese cases.
Statistical analyses
We summarized the demographic and clinical variables for the whole cohort and compared subgroups with GDM and NGT using the student-t test for continuous variables or the chi-square test for categorical variables. We depicted weight progression throughout pregnancy and the corresponding density distribution of measured gestational age for both non-GDM and GDM populations. Furthermore, we ran a mixed effects model to assess the difference in weight progression between the two groups using a random intercept to account for repeated measures of the same individual. The fixed effects in the model included the presence of GDM, gestational weeks for weight measurements, and a multiplicative interaction term between the two variables. Covariates with a statistical difference between the groups were to be adjusted in the model, including maternal age, parity, pre-pregnancy BMI, chronic hypertension, and hospitalization before delivery. In stratification analyses by pre-pregnancy BMI, adjustments were further made for pre-pregnancy weight. Additionally, we compared the adherence to current weight recommendations between the two groups regarding total GWG, and WGR before and after the OGTT.
There were missing values for several variables including sex (29 [0.5%]), parity (29 [0.5%]), education (5368 [9.5%]), pre-pregnancy weight (4788 [8.5%]), and height (1620 [2.9%]), which resulted in a missing proportion of 10.1% (n = 5693) for pre-pregnant BMI. We used random imputation to address missing values for variables with low missing proportions, such as sex (male and female) and parity (primiparous and multiparous), based on their actual prevalence within the total cohort, which ensured that the imputed values were representative of the overall characteristics of the cohort. Missing values for education were considered a new category. For pre-pregnant weight and height, missing values were handled by multivariate imputation using chained equations (MICE) [30] based on the presence of GDM, and WGR before and after the OGTT. To test the robustness of our study, we conducted a sensitivity analysis by excluding those individuals with incomplete data on pre-pregnancy BMI, those with chronic hypertension, and those who were hospitalized before delivery. We further repeated the comparison for compliance with the IOM recommendations between GDM and NGT individuals. P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using the statistical software program R, version 4.0.2.
Results
A total of 56,616 pregnancies (mean maternal age 30.9 [SD 4.3] years of age) were included in the main analysis, out of which 9,430 (16.7%) individuals were affected by GDM (Fig. 1). The characteristics of individuals with GDM and NGT are shown in Table 1. Individuals with GDM were older than NGT (32.5 [SD 4.4] years vs. 30. 6 [SD 4.2] years), more likely to be overweight/obese, and multiparous. Absolute weight change during gestation was 12.1 (SD 5.2) kg and 14.0 (SD 5.0) kg in individuals with GDM and NGT, respectively. However, individuals with GDM had a shorter gestation length than those with NGT (Table 1).
Figure 2 presents a visualization of weight progression throughout pregnancy and the corresponding density distribution of measured gestational age. The distribution of measured gestational age was similar between GDM and NGT groups, and weight progression seemed aligned before the OGTT, however, individuals with GDM exhibited a lower rate of weight gain thereafter. Table 2 provides estimates of average change in weight progression among GDM and NGT individuals during pregnancy, before and after the OGTT, respectively. Throughout gestation, GDM individuals gained weight at a slower rate of 0.39 kg per week than NGT individuals (0.51 kg/week) (Table 2). Before the OGTT, WGRs were barely different between GDM (0.44 kg/week) and NGT individuals (0.45 kg/week). However, individuals experienced a substantial decrease in the rate of weight gain following the diagnosis of GDM compared with NGT individuals (0.34 vs. 0.50 kg/week), which was consistently shown across different BMI categories, including underweight class (0.39 vs. 0.51 kg/week), normal weight class (0.35 vs. 0.50 kg/week) and overweight/obese class (0.29 vs. 0.45 kg/week). These results were also observed in the sensitivity analysis (Table S1).
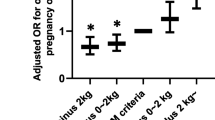
By absolute weight change, the percentages of insufficient GWG were 19.6% and 10.7% between GDM and NGT individuals, while those of excessive GWG were 32.3% and 47.0%, respectively (Fig. 3). This was more evident after the OGTT when using WGR as an indicator, with a four-fold increased percentage of insufficient weight gain and a two-fold decreased percentage of excessive weight gain in GDM individuals as compared to NGT individuals (insufficient, 41.1% vs. 10.4%; excessive, 22.6% vs. 54.2%), including in normal weight class (insufficient, 38.2% vs. 7.4%; excessive, 22.2% vs. 57.3%) and overweight/obese class (insufficient, 43.1% vs. 9.8%; excessive, 30.1% vs. 68.8%) (Fig. 3). In the second and third trimesters before OGTT, however, there was no difference in the distribution of insufficient and excessive weight gain between GDM and NGT individuals. Per IOM recommendations, while similar results were observed, the proportion of insufficient GWG was remarkably higher both in GDM and NGT groups (Figure S1).
Discussion
In this longitudinal hospital-based study of pregnant women, GDM individuals experienced different weight progression patterns from NGT individuals, manifesting as gaining weight at a similar rate before the OGTT but at a significantly slower rate after the test. Consequently, per current gestational weight gain recommendations, GDM individuals presented a considerably higher incidence of insufficient weight gain and a reduced proportion of excessive weight gain as compared to NGT individuals. These findings indicate the need for distinct weight gain recommendations for GDM and NGT populations that take into account their differences in weight progression patterns.
Several previous studies have assessed the GWG between women with GDM and women with NGT. In a birth cohort of 3260 Finnish pregnant women, GDM individuals had a GWG of 9.4 kg through gestation as opposed to 12.6 kg in NGT individuals [31]. Also, in a prospective study of 212 Australian women (115 GDM and 97 NGT), GDM individuals gained more weight before OGTT (8.4 vs. 7.5 kg) but gained less thereafter, in comparison to NGT individuals (1.18 vs. 4.0 kg) [32]. Our data, along with those from previous studies, consistently indicated a substantial difference in weight gain profiles between GDM and NGT individuals. Additionally, we were able to compare the average change in weight progression based on serial weight measurements during pregnancy. We found a similar average rate of weight gain between GDM and NGT individuals before the OGTT, however, there was a substantial decrease in the average rate of weight gain after the test among GDM individuals in comparison to NGT individuals. Individuals affected by GDM are typically offered comprehensive health advice on achieving glycemic goals, including nutrition, physical activity, lifestyle and behavioral modification, and glucose monitoring [18]. Such structured interventions would result in a significant reduction in nutrients and energy, which may be the main explanation for the decreased weight gain in GDM individuals [31, 33, 34].
In this study, less than half of the pregnant women met the current weight gain targets, which was consistent with previous studies, reporting a compliance rate of 23–51% worldwide [35], including 25–35% in those with GDM [20, 36, 37]. However, as a result of less weight gain, GDM individuals were more prone to be labeled as having insufficient weight gain instead of being identified as having excessive weight gain in comparison to NGT individuals. Likewise, Zheng et al. found the percentage of women with GDM who gained weight below the recommendations doubled before and after the OGTT (21% vs. 41%) [8]. Komem et al. also reported a considerable increase in the percentage of women with insufficient GWG before and after GDM diagnosis (42.9% vs. 70%) [20]. From the perspective of weight management, women with insufficient weight gain require appropriate increases in nutrition and energy to achieve the established weight targets, however, this is in turn possible to hinder their later glycemic control interventions. This discrepancy between weight management and glycemic control points to the need to distinguish between NGT individuals and GDM individuals, who may require lower weight targets than they currently have. Gestational weight gain is significantly correlated with a decrease in insulin sensitivity and excessive GWG following GDM diagnosis may exaggerate insulin sensitivity and the progress of GDM to DM [38,39,40]. In this sense, failing to identify women with potential excessive weight gain may cause additional health risks. Recently, several studies found that GDM individuals with GWG below the weight recommendations were at a significantly decreased risk of fetal macrosomia, and those who lost weight during GDM management did not increase the risk of the small-for-gestational-age infant compared to those above IOM targets [8, 41,42,43,44,45], adding further evidence supporting the benefit of lower weight targets for women with GDM.
The main strength of this study was the thorough use of serial weight measurements. Given that GDM individuals were more likely to have a shorter length of gestation in comparison to NGT individuals, the less weight gain observed in individuals with GDM may be partly influenced by the residual bias of gestation length. Based on the serial weight measurements, we were able to evaluate the average change in weight progression within different gestation windows, which was independent of gestation length and may be more effective than the total GWG in weight management as a result. In addition, each woman undergoes scheduled obstetric care, and maternal weight was measured using a unified scale and captured timely by the EMR system, therefore, measurement bias and recall bias was largely avoided. Third, the large study population was also an important strength of this study, which enables us to clarify the group differences across different BMI classes. The proportion of overweight/obese BMI among GDM individuals was higher than that among NGT individuals, and pregnant women with overweight/obesity generally gained less weight during pregnancy, therefore, analysis stratified by BMI was possible to avoid the confounding bias by BMI. Our study also had several limitations. First, we do not have data on dietary patterns, nutrient intake, and physical activity, which might help to further elucidate the mediating or moderating effects of these factors. Second, there was about 10% of missing data with pre-pregnancy weight. While we used a multiple imputation method to assign the missing values, some degree of misclassification was possible for pre-pregnancy BMI. However, we repeated the main analysis excluding patients with missing data, and the results did not alter. Third, the population is dominated by the Han ethnicity living in Guangzhou, thus it may potentially limit the generalizability of the findings to other races/ethnicities.
Conclusions
Weight progression varied significantly between GDM and NGT individuals, resulting in a substantial difference in identifying insufficient and excessive weight gain between the two groups under the current gestational weight gain guidelines. These findings indicate the need for developing distinct weight gain recommendations for GDM and NGT populations in future clinical practice, and lower weight gain targets may be more appropriate for GDM individuals given the requirement for glucose management.
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to ethical concerns but are available from the corresponding author on reasonable request.
References
LifeCycle Project-Maternal Obesity Childhood Outcomes Study Group, Voerman E, Santos S, Inskip H, Amiano P, Barros H, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. 2019;321(17):1702–15.
Ali Z, Nilas L, Ulrik CS. Excessive gestational weight gain in first trimester is a risk factor for exacerbation of asthma during pregnancy: a prospective study of 1283 pregnancies. J Allergy Clin Immunol. 2018;141(2):761–7.
Champion ML, Harper LM. Gestational weight gain: update on outcomes and interventions. Curr Diab Rep. 2020;20(3):11.
Platner MH, Ackerman C, Howland RE, Xu X, Pettker CM, Illuzzi JL, et al. Gestational weight gain and severe maternal morbidity at delivery hospitalization. Obstet Gynecol. 2019;133(3):515–24.
Qi Y, Sun X, Tan J, Zhang G, Chen M, Xiong Y, et al. Excessive gestational weight gain in the first and second trimester is a risk factor for gestational diabetes mellitus among women pregnant with singletons: a repeated measures analysis. J Diabetes Investig. 2020;11(6):1651–60.
Brunner S, Stecher L, Ziebarth S, Nehring I, Rifas-Shiman SL, Sommer C, et al. Excessive gestational weight gain prior to glucose screening and the risk of gestational diabetes: a meta-analysis. Diabetologia. 2015;58(10):2229–37.
Mustaniemi S, Nikkinen H, Bloigu A, Pouta A, Kaaja R, Eriksson JG et al. Normal gestational weight gain protects from large-for-gestational-age birth among women with obesity and gestational diabetes. Front Public Health 2021;9(2296–2565 (Electronic)):550860.
Zheng W, Huang W, Liu C, Yan Q, Zhang L, Tian Z, et al. Weight gain after diagnosis of gestational diabetes mellitus and its association with adverse pregnancy outcomes: a cohort study. BMC Pregnancy Childbirth. 2021;21:1471–2393. (Electronic)):216.
Zhang S, Li N, Li W, Wang L, Liu E, Zhang T, et al. Increased gestational weight gain is associated with a higher risk of offspring adiposity before five years of age: a population-based cohort study. Diabetes Metab Syndr Obes. 2022;15:2353–63.
Mamun AA, Mannan M, Doi SA. Gestational weight gain in relation to offspring obesity over the life course: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2014;15(4):338–47.
Gaillard R, Steegers EA, Franco OH, Hofman A, Jaddoe VW. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int J Obes (Lond). 2015;39(Electronic):1476–5497.
Tam CHT, Ma RCW, Yuen LY, Ozaki R, Li AM, Hou Y, et al. The impact of maternal gestational weight gain on cardiometabolic risk factors in children. Diabetologia. 2018;61(12):2539–48.
American College of Obstetricians Gynecologists. ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol. 2013;121(1):210–2.
Chen F, Wang P, Wang J, Liao Z, Zong X, Chen Y, et al. Analysis and comparison of early childhood nutritional outcomes among offspring of chinese women under the chinese 2021 and us 2009 gestational weight gain guidelines. JAMA Netw Open. 2022;5(9):e2233250.
Griffith RJ, Alsweiler J, Moore AE, Brown S, Middleton P, Shepherd E, et al. Interventions to prevent women from developing gestational diabetes mellitus: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2020;6(6):Cd012394.
ACOG Practice Bulletin No. 190: gestational diabetes Mellitus. Obstet Gynecol. 2018;131(2):e49–e64.
Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, Fuglsang J. Diet and healthy lifestyle in the management of gestational diabetes mellitus. Nutrients. 2020;12(10):3050.
14. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):200–s10.
Atakora L, Poston L, Hayes L, Flynn AC, White SL. Influence of GDM diagnosis and treatment on weight gain, dietary intake and physical activity in pregnant women with obesity: secondary analysis of the upbeat study. Nutrients 2020;12(2).
Komem D, Salman L, Krispin E, Arbib N, Bardin R, Wiznitzer A, et al. Gestational weight gain and weight loss among women with gestational diabetes mellitus. Diabetes Res Clin Pract. 2018;141:88–97.
Chen Q, Wei J, Tong M, Yu L, Lee AC, Gao YF, et al. Associations between body mass index and maternal weight gain on the delivery of LGA infants in chinese women with gestational diabetes mellitus. J Diabetes Complications. 2015;29(8):1037–41.
Cosson E, Cussac-Pillegand C, Benbara A, Pharisien I, Nguyen MT, Chiheb S, et al. Pregnancy adverse outcomes related to pregravid body mass index and gestational weight gain, according to the presence or not of gestational diabetes mellitus: a retrospective observational study. Diabetes Metab. 2016;42(1):38–46.
Chiou YL, Hung CH, Liao HY. The impact of prepregnancy body mass index and gestational weight gain on perinatal outcomes for women with gestational diabetes mellitus. Worldviews Evid Based Nurs. 2018;15(4):313–22.
He S, Allen JC, Razali NS, Win NM, Zhang JJ, Ng MJ, et al. Are women in Singapore gaining weight appropriately during pregnancy: a prospective cohort study. BMC Pregnancy Childbirth. 2019;19(1):290.
Liang H, Tsui BY, Ni H, Valentim CCS, Baxter SL, Liu G, et al. Evaluation and accurate diagnoses of pediatric diseases using artificial intelligence. Nat Med. 2019;25(3):433–8.
Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International Association of Diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82.
Hu W, Hu H, Zhao W, Huang A, Yang Q, Di J. Current status of antenatal care of pregnant women-8 provinces in China, 2018. BMC Public Health. 2021;21(1):1135.
American College of Obstetricians. Gynecologists’ Committee on Practice, Bulletins-Obstetrics: ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstet Gynecol. 2019;133(1):e26–e50.
Zhou BF, Cooperative Meta-Analysis Group of the Working Group on Obesity in C. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in chinese adults–study on optimal cut-off points of body mass index and waist circumference in chinese adults. Biomed Environ Sci. 2002;15(1):83–96.
Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–9.
Salmenhaara M, Uusitalo L, Uusitalo U, Kronberg-Kippilä C, Sinkko H, Ahonen S, et al. Diet and weight gain characteristics of pregnant women with gestational diabetes. Eur J Clin Nutr. 2010;64(12):1433–40.
Stewart ZA, Wallace EM, Allan CA. Patterns of weight gain in pregnant women with and without gestational diabetes mellitus: an observational study. Aust N Z J Obstet Gynaecol. 2012;52(5):433–9.
Teede HJ, Bailey C, Moran LJ, Bahri Khomami M, Enticott J, Ranasinha S, et al. Association of antenatal diet and physical activity-based interventions with gestational weight gain and pregnancy outcomes: a systematic review and meta-analysis. JAMA Intern Med. 2022;182(2):106–14.
International Weight Management in Pregnancy, Collaborative G. Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ. 2017;358:j3119.
Martinez-Hortelano JA, Cavero-Redondo I, Alvarez-Bueno C, Garrido-Miguel M, Soriano-Cano A, Martinez-Vizcaino V. Monitoring gestational weight gain and prepregnancy BMI using the 2009 IOM guidelines in the global population: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2020;20(1):649.
Mastella LS, Weinert LS, Gnielka V, Hirakata VN, Oppermann MLR, Silveiro SP, et al. Influence of maternal weight gain on birth weight: a gestational diabetes cohort. Arch Endocrinol Metab. 2018;62(1):55–63.
Wong T, Barnes RA, Ross GP, Cheung NW, Flack JR. Are the Institute of Medicine weight gain targets applicable in women with gestational diabetes. mellitus? Diabetologia. 2017;60(3):416–23.
Bevier WC, Jovanovic L. Weight gain and gestational diabetes mellitus is a sensitive issue. Diabetes Care. 2008;31(1):e1.
Minschart C, Lammertyn A, Crombrugge PV, Moyson C, Verhaeghe J, Vandeginste S et al. Low gestational weight gain in women with gestational diabetes is safe with better metabolic profile postpartum. J Clin Endocrinol Metabolism 2022:dgac599.
Cade WT, Mittendorfer B, Patterson BW, Haire-Joshu D, Cahill AG, Stein RI, et al. Effect of excessive gestational weight gain on insulin sensitivity and insulin kinetics in women with overweight/obesity. Obes (Silver Spring). 2022;30(10):2014–22.
Bogdanet D, Mustafa M, Khattak A, Shea PMO, Dunne FP. Atlantic DIP: is weight gain less than that recommended by IOM safe in obese women with gestational diabetes mellitus? Int J Obes (Lond). 2021;45(5):1044–51.
Barnes RA, Flack JR, Wong T, Ross GP, Griffiths MM, Stephens M et al. Does weight management after gestational diabetes mellitus diagnosis improve pregnancy outcomes? A multi-ethnic cohort study. Diabet Med 2021:e14692.
Xie X, Liu J, Pujol I, López A, Martínez MJ, García-Patterson A, et al. Inadequate weight gain according to the Institute of Medicine 2009 guidelines in women with gestational diabetes: frequency, clinical predictors, and the association with pregnancy outcomes. J Clin Med. 2020;9(10):3343.
Xu Q, Ge Z, Hu J, Shen S, Bi Y, Zhu D. The association of gestational weight gain and adverse pregnancy outcomes in women with gestational diabetes mellitus. Endocr Pract. 2019;25(11):1137–50.
Kurtzhals LL, Norgaard SK, Secher AL, Nichum VL, Ronneby H, Tabor A, et al. The impact of restricted gestational weight gain by dietary intervention on fetal growth in women with gestational diabetes mellitus. Diabetologia. 2018;61(12):2528–38.
Acknowledgements
We acknowledge all women included in this cohort and clinicians in the department of obstetrics, and those clinicians in the department of clinical nutrition of Guangzhou Women and Children’s Medical Center for their contribution to data support.
Funding
This work was supported by Guangzhou Women and Children’s Medical Center [grant numbers 3001153-04].
Author information
Authors and Affiliations
Contributions
M.H. conceived the study. HM.X., F.L., Y.G., and KR. L. provided overall guidance. M.H. performed the statistical analysis and drafted the article. F.L., Z.Z., XJ.L., HM. C., KR. L., and XH. L. contributed greatly to the acquisition and validation of data. F.L., Z.Z., XJ.L., HM. C., Y.G., KR. L., XH. L., and HM.X made critical revisions to the manuscript. All authors have reviewed the final version and agreed to the published version of the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research methods involving human data were performed in accordance with the Declaration of Helsinki and have been approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center. Given all maternal and neonatal data were extracted from the hospital EMR system by a unique identifier with no participant involved in the design, the Ethics Committee of Guangzhou Women and Children’s Medical Center has waived the requirement of informed consent for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Miao, H., Liang, F., Zheng, Z. et al. Weight progression and adherence to weight gain target in women with vs. without gestational diabetes: a retrospective cohort study. BMC Pregnancy Childbirth 23, 513 (2023). https://doi.org/10.1186/s12884-023-05832-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05832-x