Abstract
Background
Post-dural puncture headache (PDPH) is a major complication of neuraxial anesthesia. PDPH usually occurs after Caesarean section in obstetric patients. The efficacy of prophylactic pharmacological therapies remains controversial.
Methods
Seven pharmacological therapies (aminophylline (AMP), dexamethasone, gabapentin/pregabalin (GBP/PGB), hydrocortisone, magnesium, ondansetron (OND), and propofol (PPF)), were studied in this Bayesian network meta-analysis. The primary outcome was the cumulative incidence of PDPH within 7 days. Secondary outcomes included the incidence of PDPH at 24 and 48 h postoperatively, the severity of headache in PDPH patients (24, 48, and 72 h postoperatively), and postoperative nausea and vomiting (PONV).
Results
Twenty-two randomized controlled trials with 4,921 pregnant women (2,723 parturients received prophylactic pharmacological therapies) were included. The analyses demonstrated that PPF, OND, and AMP were efficient in decreasing the cumulative incidence of PDPH during the follow-up period compared to the placebo group (OR = 0.19, 95% CI: 0.05 to 0.70; OR = 0.37, 95% CI: 0.16 to 0.87; OR = 0.40, 95% CI: 0.18 to 0.84, respectively). PPF and OND had the lower incidence of PONV compared to the placebo group (OR = 0.07, 95% CI: 0.01 to 0.30; and OR = 0.12, 95% CI: 0.02 to 0.63). No significant difference in other outcomes was found among different therapies.
Conclusions
Based on available data, PPF, OND, and AMP may have better efficacy in decreasing the incidence of PDPH compared to the placebo group. No significant side effects were revealed. Better-designed studies are requested to verify these conclusions.
Similar content being viewed by others
Introduction
Post-dural puncture headache (PDPH) is a serious complication of the neuraxial blockade that may arise after spinal anesthesia or epidural analgesia with an accidental dural puncture [1]. PDPH was first described in 1899 [2]. Previous studies demonstrated that PDPH had a wide range of incidences: 1.5% to 36% after spinal anesthesia [3,4,5]. A recent meta-analysis revealed that the incidence of PDPH was 23.47% in a total of 175,652 parturients who underwent Caesarean section with spinal anesthesia [6].
In spite of developments in needle design and puncture techniques, PDPH remains the most common complication of neuraxial blockade due to the popularity of neuraxial blockade in obstetric anesthesia [7]. Pregnancy, female sex, and young age are all established risk factors for PDPH, which are frequently observed in obstetric patients. PDPH symptoms often start within 48 to 72 h after the operation and resolve spontaneously within 1 week [8]. However, some PDPH can be delayed for months afterward. PDPH may develop as chronic postpartum headache [9,10,11,12], causing hearing loss [10, 13], backache [10,11,12], neckache [11], postpartum depression [11, 12], and decreased breastfeeding [12].
Several conservative treatments are recognized, including bed rest, hydration, and abdominal binder. However, bed rest may increase the risk of thromboembolic complications. Hydration and abdominal binder had insufficient evidence in the treatment of obstetric PDPH. Although there have been advances in the therapeutic epidural blood patch for PDPH treatment, it may cause chronic backache and a variety of neurological consequences [7, 14]. Given the foregoing, obstetric anesthetists are eager to explore effective prophylactic medicines.
Several pharmacological therapies for preventing PDPH have been developed in parturients, including aminophylline (AMP), dexamethasone (DXM), gabapentin/pregabalin (GBP/PGB), hydrocortisone (HCT), magnesium (Mg), ondansetron (OND), and propofol (PPF) [15,16,17]. However, the results were inconsistent. There have been few randomized controlled trials (RCTs) that directly compared these pharmacological therapies [18,19,20,21]. In this study, a network meta-analysis (NMA) was therefore conducted comparing these prophylactic pharmacological therapies among pregnant women during the perinatal period. This NMA aimed to provide a comprehensive overview of the efficacy of pharmacological therapies for preventing PDPH in obstetric patients in clinical practice.
Methods
This systematic review was preregistered (https://www.crd.york.ac.uk/prospero/, ID: CRD42022346544).
Search strategy
PubMed, MEDLINE, EMBASE, Scopus, ClinicalTrials.gov, Cochrane Library, and Google Scholar were searched (from database inception to July 25, 2022) to identify the available literature by two independent investigators (G.S. and G.Z.). The keywords were “post-dural puncture headache” and “pregnancy/Caesarean section” (Supplemental Table S1). In addition, citations of papers were examined to find other relevant literature.
Eligibility criteria
Original studies were eligible if the following criteria were met: (i) was an RCT study; (ii) full text available in English; (iii) all participants were pregnant women; and (iv) assessed the efficacy of pharmacological therapies for preventing PDPH in parturients.
Original studies were ineligible for the following reasons: (i) observational studies, conference abstracts, or case reports; (ii) studies involving invasive therapies (i.e., prophylactic epidural blood patch or prophylactic intrathecal/subarachnoid morphine/fentanyl); (iii) lacked data to determine odds ratios (ORs) and 95% confidence interval (CI) of the efficacy of pharmacological therapies or mean difference and 95% CI of the severity of PDPH; or (iv) research on laboratory animals.
Selection process and data extraction
Individual studies of NMA were first screened based on titles and abstracts. If a judgment could not be made based on titles and abstracts, we proceeded to read the full text. Both the screening process and data extraction were performed independently by two investigators (G.S. and G.Z.). Also, we used Cohen’s κ statistic for measuring inter-rater agreement. Senior investigators (J.L.) resolved discrepancies through discussions.
For each eligible MA, two independent investigators (G.Z. and J.L.) extracted data including: the first author, details of interventions, sample size, inclusion and exclusion criteria in each involved study, duration of follow-up, and outcomes. Data was obtained from the figures by using the GetData Graph Digitizer if they were not in the tables or full text [22].
Quality assessment
The quality of the selected studies and risk of bias were assessed by the two independent reviewers (G.S. and G.Z.) using Cochrane Collaboration's tool [23]. Any disagreements were resolved by the senior reviewers (J.L.) or through consensus-based discussion. The quality evaluation charts were generated using the “robvis” package of the R software.
Outcome definition
Cumulative incidence of PDPH within 7 days was the primary outcome. The incidence of PDPH at 24 and 48 h postoperatively, the severity of headache in PDPH patients (24, 48, and 72 h postoperatively), and side effects (such as postoperative nausea and vomiting (PONV)) were selected as secondary outcomes. Pain measures such as visual analogue scales represented the severity of PDPH. In this NMA, these pain measures were converted to an adjusted 0–10 point score ( “0” means no pain, and “10” means most serious pain) for further analysis [22].
Statistical analysis
OR and 95% CI were used to report the incidence of PDPH and PONV. The severity of headache at different time points was reported as mean difference and 95% CI. The efficacy of pharmacological therapies for preventing PDPH in parturients was evaluated using an NMA. Random-effects and consistency models were used in the analysis (four chains, 50,000 iterations, and 20,000 per chain). Inconsistencies were reported if the Bayesian P values were greater than 0.05 after using the node-splitting method. Each therapy was given a rank based on the surface under the cumulative ranking curve (SUCRA) (worst = 0%; best = 100%) [24].
The GRADE method was applied to evaluate the overall quality of each outcome. Comparison-adjusted funnel plots were used to evaluate possible publication bias. The R software 3.6.3 (R Foundation, Vienna, Austria) with the “gemtc” package and Stata version 17.0 (StataCorp, College Station, TX, USA) was adopted.
Results
Study selection and study characteristics
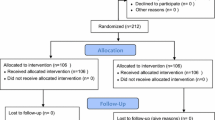
Using an extensive search method, about 2,000 possibly relevant papers were gathered. Finally, 22 RCTs were included in our final analysis (Fig. 1) [18,19,20,21, 25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. The inter-rater agreement was very good for titles/abstracts and full-text screening (κ = 0.87 and 0.93, respectively). These trials were conducted between 2012 and 2022. There were 4,921 patients involved in this NMA, including 2,723 patients who received prophylactic pharmacological therapies (Table 1). Seven pharmacological therapies were assessed in these studies, including AMP, DXM, GBP/PGB, HCT, Mg, OND, and PPF (Fig. 2). All trials involved spinal anesthesia, and all patients underwent Caesarean section. Eighteen trials were two-arm studies; the other four trials were a three-arm design. The duration of follow-up ranged from 2 to 7 days. In addition, a summary of bias risk assessment was provided (Supplemental Figures S1 & S2).
Network geometry. Circles represent the intervention as a node in the network. The size of the circle corresponds to the number of participants included in each comparison; lines represent direct comparisons using randomized controlled trials; and the thickness of the lines corresponds to the number of RCTs included in each comparison. AMP, Aminophylline; DXM, Dexamethasone; GBP/PGB, Gabapentin or pregabalin; HCT, Hydrocortisone; Mg, Magnesium; OND, Ondansetron; PDPH, Post-dural puncture headache; PPF, Propofol; PONV, Postoperative nausea and vomiting
Cumulative incidence of PDPH within 7 days
Twenty trials reported a difference in cumulative incidence of PDPH within 7 days among pharmacological therapies and placebo groups [18,19,20,21, 25, 27,28,29,30,31,32,33,34,35, 37,38,39,40,41,42]. Of these 4,697 pregnant women, 2,611 received pharmacological therapies, and 2,086 received the placebo treatment. The incidence of PDPH was 15.2% (398/2611) in the pharmacological groups and 22.6% (471/2,086) in the placebo group.
As shown in Figs. 3 and 4, the results of the NMA demonstrated that PPF, OND, and AMP were efficient in decreasing the incidence of PDPH compared to the placebo group (OR = 0.19, 95% CI: 0.05 to 0.70; OR = 0.37, 95% CI: 0.16 to 0.87; OR = 0.40, 95% CI: 0.18 to 0.84, respectively).
Forest plots of network meta-analysis of all outcomes. AMP, Aminophylline; DXM, Dexamethasone; GBP/PGB, Gabapentin or Pregabalin; HCT, Hydrocortisone; IV, Ontravenous; Mg, Magnesium; OND, Ondansetron; PDPH, Post-dural puncture headache; PPF, Propofol; PO, Oral; PONV, Postoperative nausea and vomiting
Head-to-head comparisons of incidence of post-dural puncture headache during the follow-up period. Highest probability of being the most efficient therapies (with high SUCRA values) and lowest probability of being the most efficient therapies (with low SUCRA values). AMP, Aminophylline; DXM, Dexamethasone; GBP/PGB, Gabapentin or Pregabalin; HCT, Hydrocortisone; Mg, Magnesium; OND, Ondansetron; PDPH, Post-dural puncture headache; PPF, Propofol; SUCRA, Surface under the cumulative ranking area curves
Comparison of secondary outcomes
GBP/PGB decreased the incidence of PDPH at 48 h after surgery compared to the placebo group (OR = 0.12, 95% CI: 0.02 to 0.82, Fig. 3, Supplemental Table S3). No difference in the incidence of PDPH at 24 h after surgery was found among different therapies (Supplemental Table S4). No difference in the severity of PDPH (pain scores) was found at any time point among different therapies (Supplemental Tables S5-S7).
Twelve studies reported side effects during the perioperative period [20, 27,28,29,30, 32,33,34,35, 39, 40, 42]. All twelve studies reported PONV [20, 27,28,29,30, 32,33,34,35, 39, 40, 42]. Meanwhile, the results demonstrated that PPF and OND had lower incidence of PONV compared to the placebo group (OR = 0.07, 95% CI: 0.01 to 0.30; and OR = 0.12, 95% CI: 0.02 to 0.63, Fig. 3, Supplemental Table S8). Sedation [28], diplopia [33], and tinnitus [33] were also mentioned in individual studies, but were not involved in the qualitative synthesis. Dexamethasone did not show any superiority to other pharmacological therapies or to placebo in any secondary outcomes.
Inconsistency, ranking, and publication bias
No inconsistency was found in any of the outcomes (all P > 0.05) (Supplemental Figures S3-S9). The ranking of each pharmacological therapy was performed and presented (Table 2, Fig. 4). There was no publication bias identified (Supplemental Table S9 and Supplemental Figure S10).
Discussion
This is the first NMA regarding the efficacy of pharmacological therapies for preventing PDPH in obstetric patients who underwent Caesarean sections. A large amount of evidence was pooled to make it possible to indirectly compare the efficacy of these seven medicines. Analysis demonstrated that PPF, OND, and AMP could decrease the incidence of PDPH. No obvious side effects were revealed in these analyses or in the involved studies. These are encouraging findings.
The pathophysiology of PDPH is uncertain. There are three hypothesized mechanisms: compensatory meningeal venodilation and blood volume increase induced by cerebrospinal fluid (CSF) leak hypotension, leading to acute intracranial dilatation and headaches [43, 44]; CSF leak hypotension causing brain tissue to sag and nerves to stretch, resulting in headaches [45, 46]; and spinal puncture changing craniospinal elasticity, resulting in increasing caudal compliance and acute intracranial dilatation [47].
PPF is a γ-aminobutyric acid receptor and ultra-short-acting anesthetic [48]. PPF has favorable pharmacokinetic and pharmacodynamic characteristics and has become one of the most commonly used intravenous anesthetics [49]. Previous studies have demonstrated the efficacy of PPF in treating migraine [48, 50,51,52]. Soleimanpour et al. performed an RCT and proved that intravenous PPF was a more effective and safer treatment than DXM for patients presenting with migraine headaches [52]. Later, Golfam [33], Refky [20], and their colleagues attempted to use PPF for preventing PDPH in obstetric patients. The mechanism of PPF in PDPH prevention still needs further study. Meanwhile, PPF decreased the risk of PONV, which was consistent with previous studies [53, 54].
OND, a specific 5-HT 3 receptor antagonist, is frequently used for the prevention and management of PONV [29]. Four studies focused on the prophylactic effect on PDPH [18, 19, 29, 35]. The following mechanism of OND in PDPH prevention has been proposed: by inhibiting 5-HT3 receptors, OND reduced acute intracranial dilatation and maintained mean arterial pressure, which prevented compensatory intracranial vasodilation through autoregulation of cerebral circulation [29]. This effect might reduce the incidence of PDPH in parturients. A very low-probability complication requires doctors to be vigilant: OND or palonosetron may induce migraine headaches among those parturients who have experienced migraines, according to findings from two case reports [55,56,57].
As a theophylline active metabolite, AMP is a well-known methylxanthine medication. Previous studies showed that theophylline [58] and caffeine [59] might prevent PDPH by adenosine antagonization and vasoconstriction. Therefore, some doctors have tried to explore its efficacy in preventing PDPH among women experiencing Caesarean sections [19,20,21, 26, 30, 37]. However, they obtained conflicting results. Three trials found positive results [26, 30, 37], and the other three had negative results [19,20,21]. A meta-analysis, published in 2021, revealed that AMP could not prevent PDPH, but decreased pain scores in individuals who underwent different surgeries under spinal anesthesia and developed PDPH [16]. The findings in this study are contrary to this meta-analysis, which has suggested that further large-scale studies are warranted to confirm our result.
Only a few trials have focused on the efficacy of GBP [28], PGB [34, 40], and Mg [41]. All these therapies need more raw data to draw solid conclusions. Finally, the results of this study revealed that DXM and HCT, as the two most common glucocorticoids, were unable to reduce the incidence and severity of PDPH in parturients, which was consistent with the recent meta-analysis [17].
Strengths and limitations
Considering all the above-described available options, the main objective of this study was to determine the best prophylactic pharmacological therapies for preventing PDPH after Caesarean section. The current meta-analysis had some limitations. First, there were only a few well-designed RCTs in this NMA. For example, some studies failed to reveal the details of allocation concealment. Second, needle type/size, the direction of bevel of the needle, angle of approach, and number of attempts may increase heterogeneity and affect the credibility of the conclusions. Third, the characteristics of parturients, such as maternal age, body mass index, and history of headache, were all underlying confounders. Fourth, variations in the dose of pharmacological therapies, type of placebo, and the duration of follow-up may bias the results. Fifth, the incidences of PDPH in the placebo group in the involved studies varied over a wide range. For a disease with a low incidence, it was difficult to find the difference in efficacy between two drugs in a small sample. Therefore, large sample-sized RCTs are needed in future to confirm our findings.
Conclusions
Based on available data, PPF, OND, and AMP may have better efficacy than other proposed treatments in decreasing the incidence of PDPH. No obvious side effects were revealed in the analyses or the involved studies. Better-designed RCTs are needed to validate the conclusions.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMP:
-
Aminophylline
- CI:
-
Confidence interval
- DXM:
-
Dexamethasone
- GBP/PGB:
-
Gabapentin/pregabalin
- HCT:
-
Hydrocortisone
- MD:
-
Mean difference
- Mg:
-
Magnesium
- NMA:
-
Network meta-analysis
- OND:
-
Ondansetron
- OR:
-
Odds ratio
- PDPH:
-
Post-dural puncture headache
- PONV:
-
Postoperative nausea and vomiting
- PPF:
-
Propofol
- RCT:
-
Randomized controlled trial
- SUCRA:
-
Surface under the cumulative ranking curve
References
Kwak KH. Postdural puncture headache. Korean J Anesthesiol. 2017;70(2):136–43.
Bier A. Versuche über cocainisirung des rückenmarkes. Deutsche Zeitschrift für Chirurgie. 1899;51(3):361–9.
Choi PT, Galinski SE, Takeuchi L, Lucas S, Tamayo C, Jadad AR. PDPH is a common complication of neuraxial blockade in parturients: a meta-analysis of obstetrical studies. Can J Anaesth. 2003;50(5):460–9.
Vallejo MC, Zakowski MI. Post-dural puncture headache diagnosis and management. Best Pract Res Clin Anaesthesiol. 2022;36(1):179–89.
Li H, Wang Y, Oprea AD, Li J. Postdural puncture headache-risks and current treatment. Curr Pain Headache Rep. 2022;26(6):441–52.
Chekol B, Yetneberk T, Teshome D. Prevalence and associated factors of post dural puncture headache among parturients who underwent cesarean section with spinal anesthesia: a systemic review and meta-analysis, 2021. Ann Med Surg (Lond). 2021;66: 102456.
Dabas R, Lim MJ, Sng BL. Postdural puncture headache in obstetric neuraxial anaesthesia: current evidence and therapy. Trends Anaesth Crit Care. 2019;25:4–11.
Plewa MC, McAllister RK. Postdural Puncture Headache. [Updated 2022 Aug 7]. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430925/.
Ansari JR, Barad M, Shafer S, Flood P. Chronic disabling postpartum headache after unintentional dural puncture during epidural anaesthesia: a prospective cohort study. Br J Anaesth. 2021;127(4):600–7.
Lacombe A, Downey K, Ye XY, Carvalho JCA. Long-term complications of unintentional dural puncture during labor epidural analgesia: a case-control study. Reg Anesth Pain Med. 2022;47(6):364–9.
Mims SC, Tan HS, Sun K, Pham T, Rubright S, Kaplan SJ, Habib AS. Long-term morbidities following unintentional dural puncture in obstetric patients: a systematic review and meta-analysis. J Clin Anesth. 2022;79: 110787.
Orbach-Zinger S, Eidelman LA, Livne MY, Matkovski O, Mangoubi E, Borovich A, Wazwaz SA, Ioscovich A, Zekry ZHB, Ariche K, et al. Long-term psychological and physical outcomes of women after postdural puncture headache: a retrospective, cohort study. Eur J Anaesthesiol. 2021;38(2):130–7.
Darvish B, Dahlgren G, Irestedt L, Magnuson A, Moller C, Gupta A. Auditory function following post-dural puncture headache treated with epidural blood patch. Long-term follow-up. Acta Anaesthesiol Scand. 2015;59(10):1340–54.
Russell R, Laxton C, Lucas DN, Niewiarowski J, Scrutton M, Stocks G. Treatment of obstetric post-dural puncture headache. Part 2: epidural blood patch. Int J Obstet Anesth. 2019;38:104–18.
Bradbury CL, Singh SI, Badder SR, Wakely LJ, Jones PM. Prevention of post-dural puncture headache in parturients: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2013;57(4):417–30.
Hung KC, Ho CN, Chen IW, Hung IY, Lin MC, Lin CM, Wang LK, Chen JY, Sun CK. The impact of aminophylline on incidence and severity of post-dural puncture headache: A meta-analysis of randomised controlled trials. Anaesth Crit Care Pain Med. 2021;40(4): 100920.
Fenta E, Kibret S, Hunie M, Teshome D. Dexamethasone and post-dural puncture headache in women who underwent cesarean delivery under spinal anesthesia: A systemic review and meta-analysis of randomized controlled trials. Ann Med Surg (Lond). 2021;62:104–13.
Shokrpour M, Homayuni S, Kamali A, Pazuki S. Comparing the prophylactic effect of ondansetron and dexamethasone in controlling headaches caused by spinal anesthesia among women candidated for caesarean a randomized controlled trial. Electron J Gen Med. 2018;15(4):6.
Dehghanpisheh L, Bayani S, Azemati S, Rakhshan M. The effect of intravenous administration of ondansetron compared to aminophylline on incidence and severity of post-dural puncture headache (PDPH) in cesarean section surgeries. Biomed Res. 2019;30(6):1–6.
Refky MA, Sayouh EF, Mohamed AG, Abdelnaby SM. The effect of intravenous infusion of Propofol or Aminophylline on incidence and severity of post-dural puncture headache in elective cesarean section. Egyptian J Hosp Med. 2021;84(1):2646–54.
Razavizadeh SM, Ebnenasir M, Sehat M, Pouramini A. Effects of aminophylline and dexamethasone prophylaxis on headache after spinal anesthesia in cesarean section: a randomized clinical trial. Braz J Anesthesiol. 2022;72(4):529–32.
Wang J, Zhao G, Song G, Liu J. The efficacy and safety of local anesthetic techniques for postoperative analgesia after cesarean section: a Bayesian network meta-analysis of randomized controlled trials. J Pain Res. 2021;14:1559–72.
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Huang P, Chen Y, Zhang H, Chen B, Zhao S, Feng Y, Lei S, Wu Q. Comparative efficacy of Chinese herbal injections for septic shock: a Bayesian network meta-analysis of randomized controlled trials. Front Pharmacol. 2022;13: 850221.
Hamzei A, Basiri-Moghadam M, Pasban-Noghabi S. Effect of dexamethasone on incidence of headache after spinal anesthesia in cesarean section. A single blind randomized controlled trial. Saudi Med J. 2012;33(9):948–53.
Sadeghi SE, Abdollahifard G, Nasabi NA, Mehrabi M, Safarpour AR. Effectiveness of single dose intravenous aminophylline administration on prevention of post dural puncture headache in patients who received spinal anesthesia for elective cesarean section. World J Med Sci. 2012;7(1):13–6.
Yousefshahi F, Dahmardeh AR, Khajavi M, Najafi A, Khashayar P, Barkhordari K. Effect of dexamethasone on the frequency of postdural puncture headache after spinal anesthesia for cesarean section: a double-blind randomized clinical trial. Acta Neurol Belg. 2012;112(4):345–50.
Nofal WH, Mahmoud MS, Al Alim AA. Does preoperative gabapentin affects the characteristics of post-dural puncture headache in parturients undergoing cesarean section with spinal anesthesia? Saudi J Anaesth. 2014;8(3):359–63.
Fattahi Z, Hadavi SM, Sahmeddini MA. Effect of ondansetron on Post-Dural Puncture Headache (PDPH) in parturients undergoing cesarean section: a double-blind randomized placebo-controlled study. J Anesth. 2015;29(5):702–7.
Ghanei M, Sahraei R, Zabetian H, Radmehr M, Jahromi AS, Ghobadifar MA, Eftekharian F. Intravenous aminophyline prevents post dural puncture headache in women undergoing cesarean section: a randomized placebo-controlled trial. Revista Kasmera. 2015;43:305–19.
Mahmoud MF, Ali HM, Hashim MM, Fahmy AAK. Dexamethasone preventing dural puncture headache. J Pain Manage. 2015;7(4):273–7.
Yang B, Li DL, Dong P, Zhang XY, Zhang L, Yu JG. Effect of dexamethasone on the incidence of post-dural puncture headache after spinal anesthesia: a randomized, double-blind, placebo-controlled trial and a meta-analysis. Acta Neurol Belg. 2015;115(1):59–67.
Golfam P, Yari M, Rezaei M, Farhadi K, Jafari RM, Lahoorpour A. The effect of intravenous propofol on the incidence of post-dural puncture headache following spinal anesthesia in cesarean section. J Kermanshah Univ Med Sci. 2016;20(2):51–5.
El-guoshy MM, Stohy EMMA, Sale HHK, Saleh AA, Seliem MMMA. Clinical Comparative study of the effect of preoperative pregabalin on reduction of the incidence of headache after spinal anesthesia in cesarean section. Egyptian J Hosp Med. 2018;73(4):6507–14.
Pazoki S, Modir H, Kamali A, Zamani A, Shahidani M. Ondansetron 8 mg and 4 mg with normal saline against post-operative headache and nausea/vomiting after spinal anesthesia: a randomized double-blind trial. Med Gas Res. 2018;8(2):48.
Shakhsemampour F, Allahyari E, Rajabpour-sanati A, Sabertanha A. Evaluation the effect of dexamethasone on post-dural puncture headache in cesarean surgery. J Surg Trauma. 2018;6(1):6–10.
Yang CJ, Chen T, Ni X, Yu WY, Wang W. Effect of pre-administration with aminophylline on the occurrence of post-dural puncture headache in women undergoing caesarean section by combined spinal-epidural anaesthesia. J Int Med Res. 2019;47(1):420–6.
Anbarlouei M, Bozorgan TJ, Naeiji Z, Malek S, Shekarriz-Foumani R, Etemad FS. Comparative study of the effect of intravenous dexamethasone and hydrocortisone on the incidence of headache after spinal anesthesia in patients after cesarean section. Arch Pharm Pract. 2020;1:143.
Ogunsiji AO, Osinaike BB, Amanor-Boadu SD, Obajimi GO. Evaluation of prophylactic intravenous hydrocortisone for the prevention of postdural puncture headache following spinal anesthesia for cesarean section. J Clin Sci. 2020;17(2):13.
Karami T, Hoshyar H, Jafari AF. The effect of pregabalin on postdural puncture headache among patients undergoing elective cesarean section: a randomized controlled trial. Ann Med Surg (Lond). 2021;64:102226.
Nikooseresht M, Hajian P, Moradi A, Sanatkar M. Evaluation of the effects of oral magnesium sachet on the prevention of spinal anesthesia-induced headache after cesarean section: a randomized clinical trial. Anesth Pain Med. 2022;12(1):e121834.
Okpala BC, Eleje GU, Ikechebelu JI, Ofojebe CJ, Ejikeme TB, Nwachukwu CE, Okpala AN. A double-blind placebo controlled trial on effectiveness of prophylactic dexamethasone for preventing post- dural puncture headache after spinal anesthesia for cesarean section. J Matern Fetal Neonatal Med. 2022;35(17):3407–12.
Hannerz J, Ericson K, Bro Skejo HP. MR imaging with gadolinium in patients with and without post-lumbar puncture headache. Acta Radiol. 1999;40(2):135–41.
Bakshi R, Mechtler LL, Kamran S, Gosy E, Bates VE, Kinkel PR, Kinkel WR. MRI findings in lumbar puncture headache syndrome: abnormal dural-meningeal and dural venous sinus enhancement. Clin Imaging. 1999;23(2):73–6.
Pannullo SC, Reich JB, Krol G, Deck MD, Posner JB. MRI changes in intracranial hypotension. Neurology. 1993;43(5):919–26.
Ljubisavljevic S, Trajkovic JZ, Ignjatovic A, Stojanov A. Parameters related to lumbar puncture do not affect occurrence of postdural puncture headache but might influence its clinical phenotype. World Neurosurg. 2020;133:e540–50.
Levine DN, Rapalino O. The pathophysiology of lumbar puncture headache. J Neurol Sci. 2001;192(1–2):1–8.
Mosier J, Roper G, Hays D, Guisto J. Sedative dosing of propofol for treatment of migraine headache in the emergency department: a case series. West J Emerg Med. 2013;14(6):646–9.
Sahinovic MM, Struys M, Absalom AR. Clinical pharmacokinetics and pharmacodynamics of Propofol. Clin Pharmacokinet. 2018;57(12):1539–58.
Krusz JC, Scott V, Belanger J. Intravenous propofol: unique effectiveness in treating intractable migraine. Headache. 2000;40(3):224–30.
Mohseni M, Fatehi F. Propofol alleviates intractable migraine headache: a case report. Anesth Pain Med. 2012;2(2):94–6.
Soleimanpour H, Ghafouri RR, Taheraghdam A, Aghamohammadi D, Negargar S, Golzari SE, Abbasnezhad M. Effectiveness of intravenous dexamethasone versus propofol for pain relief in the migraine headache: a prospective double blind randomized clinical trial. BMC Neurol. 2012;12:114.
Gan TJ, Ginsberg B, Grant AP, Glass PS. Double-blind, randomized comparison of ondansetron and intraoperative propofol to prevent postoperative nausea and vomiting. Anesthesiology. 1996;85(5):1036–42.
Borgeat A, Wilder-Smith OH, Saiah M, Rifat K. Subhypnotic doses of propofol possess direct antiemetic properties. Anesth Analg. 1992;74(4):539–41.
Sharma R, Panda A. Ondansetron-induced headache in a parturient mimicking postdural puncture headache. Can J Anaesth. 2010;57(2):187–8.
Singh V, Sinha A, Prakash N. Ondansetron-induced migraine-type headache. Can J Anaesth. 2010;57(9):872–3.
Jain A. Palonosetron-induced migraine-type headache. Can J Anaesth. 2011;58(2):230–1.
Ergun U, Say B, Ozer G, Tunc T, Sen M, Tufekcioglu S, Akin U, Ilhan MN, Inan L. Intravenous theophylline decreases post-dural puncture headaches. J Clin Neurosci. 2008;15(10):1102–4.
Yucel A, Ozyalcin S, Talu GK, Yucel EC, Erdine S. Intravenous administration of caffeine sodium benzoate for postdural puncture headache. Reg Anesth Pain Med. 1999;24(1):51–4.
Acknowledgements
We gratefully acknowledge the assistance of Jian Wang, Doctor of Department of Anesthesiology, First Hospital of China Medical University, for refining the manuscript.
Funding
This project was supported by the Liaoning Provincial Natural Science Foundation of China (2022-MS-202).
Author information
Authors and Affiliations
Contributions
G. Z. performed the data collection, data analysis, and manuscript writing; G. S. did the data collection and data analysis; J. L. was involved in the data collection, project development, and manuscript writing. All authors contributed intellectually to the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of China Medical University (NO. 2022435 on July 25, 2022). The IRB waived the need for informed consent because this was a meta-analysis study based on published data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA Network Meta-analysis Checklist.
Additional file 2:
Table S1. Strategy of this meta-analysis. Table S2. Inclusion and exclusion criteria in each involved study. Table S3. Head-to-head comparisons of incidence of post-dural puncture headache at 48 hours after surgery. Table S4. Head-to-head comparisons of incidence of post-dural puncture headache at 24 hours after surgery. Table S5. Head-to-head comparisons of severity of post-dural puncture headache at 24 hours after surgery. Table S6. Head-to-head comparisons of severity of post-dural puncture headache at 48 hours after surgery. Table S7. Head-to-head comparisons of severity of post-dural puncture headache at 72 hours after surgery. Table S8. Head-to-head comparisons of incidence of postoperative nausea and vomiting. Table S9. Assessment of publication bias for network meta-analysis. Figure S1. Risk of bias summary. Figure S2. Risk of bias graph. Figure S3. Inconsistency test of cumulative incidence of post-dural puncture headache within 7 days. Figure S4. Inconsistency test of incidence of post-dural puncture headache at 24 hours after surgery. Figure S5. Inconsistency test of incidence of post-dural puncture headache at 48 hours after surgery. Figure S6. Inconsistency test of severity of post-dural puncture headache at 24 hours after surgery. Figure S7. Inconsistency test of severity of post-dural puncture headache at 48 hours after surgery. Figure S8. Inconsistency test of severity of post-dural puncture headache at 72 hours after surgery. Figure S9. Inconsistency test of postoperative nausea and vomiting. Figure S10. Funnel plot of the outcomes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, G., Song, G. & Liu, J. Efficacy of pharmacological therapies for preventing post-dural puncture headaches in obstetric patients: a Bayesian network meta-analysis of randomized controlled trials. BMC Pregnancy Childbirth 23, 215 (2023). https://doi.org/10.1186/s12884-023-05531-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05531-7