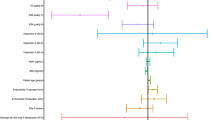
Abstract
Background
To compare the aneuploidy rate in spontaneous abortion chorionic villus (SA-CV) after D5 and D6 thawed-frozen blastocyst transfer(TBT).
Methods
This retrospective cohort study recruited 522 patients with early spontaneous abortion from March 2012 to January 2020 in the our center. The aneuploidy rate of SA-CV was compared according to the blastocyst development stage: D5 group (n = 398) and D6 group (n = 124).
Results
Patients’ characteristics, including age, body mass index, follicle-stimulating hormone, fertilization methods, type of infertility, infertility duration, and gestational age when abortion, did not differ between the two groups (all P > 0.05). Although the mean number of embryos was significantly higher in D6 than in the D5 group (P < 0.001), the mean number of high-quality embryos was similar (P = 0.773). In the D5 group, 46.5% of SA-CV showed aneuploidy, which was comparable to 41.1% in the D6 group (P = 0.296). After further grouping according to age (> 35 years or ≤ 35 years), the difference between the D5 and D6 groups remained not statistically significant (P = 0.247 and P = 0.690). Multivariate logistic analysis showed that women’s age was independently associated with the aneuploidy rate (OR = 0.891; 95% CI: [0.854–0.930]; P < 0.001). The rate of chromosomal aneuploidy was significantly higher in the age > 35 years group than in the age ≤ 35 years group (61.0% vs. 39.4%, P < 0.001). Other factors, including blastocyst formation speed, were not significant predictors of aneuploidy rate.
Conclusions
The rate of chromosomal aneuploidy in SB-CV after D6 TBT was comparable to that after D5 TBT. Chromosomal aneuploidy may not be a main factor contributing to the high prevalence early pregnancy loss at D6 group.
Similar content being viewed by others
Background
Blastocyst culture and transfer are effective in selecting optimal-quality embryos, improving endometrial synchronization, increasing cumulative pregnancy rates, and reducing multiple pregnancy rates. Normally, human embryos develop to the blastocyst stage on day 5(D5), however, some growth-retarded embryos require extended culture until day 6(D6)or day 7(D7) [1,2,3,4]. Meta-analysis showed that the speed of blastocyst development is related to blastocyst developmental potential and pregnancy outcomes in thawed blastocyst transfer (TBT) cycles, as evidenced by significantly lower clinical pregnancy and live birth rates and significantly higher miscarriage rates after D6 TBT compared to D5 TBT [1, 5]. These may be related to chromosomal abnormalities. A 10% increase in chromosomal aneuploidy rate has been reported in D6 blastocysts compared to D5 ones [6]. However, a few studies showed different findings in high-quality D5 and D6 blastocysts, with no significant difference in aneuploidy rates [7], and delayed blastocyst formation was not associated with chromosome abnormalities [8]. Thus, the opinion on the relationship between the speed of blastocyst formation and embryonic chromosomes is controversial.
To the best of our knowledge, all the above researches were based on studies of pre-implantation blastocysts. No data comparing the aneuploidy features in early spontaneous abortion chorionic villus(SA-CV) after D5 or D6 TBT, which is the direct evidence to confirm whether chromosomal abnormalities increase the risk of miscarriage after transfer of D6 blastocyst. Therefore, in this study, we compared the aneuploidy rate in abortuses between Day 5 and Day 6 groups, strengthening the understanding of the relationship between blastocyst development and chromosomal status.
Methods
Patients
This was a hospital-based retrospective study. The clinical data of patients who experienced pregnancy failure in the first trimester at the Sixth Affiliated Hospital of Sun Yat-sen University between March 2012 and January 2020 were analyzed. Pregnancy failure in the first trimester occurred at gestational age < 12 weeks and was diagnosed as follows: (1) Crown-rump length of ≥ 7 mm and no heartbeat, (2) mean sac diameter of ≥ 25 mm and no embryo, (3) absence of embryo with heartbeat ≥ 2 weeks after a scan that showed a gestational sac without a yolk sac, and (4) absence of embryo with heartbeat ≥ 11 days after a scan that showed a gestational sac with a yolk sac [9]. In this study, only TBT cycles with hormone replacement treatment (HRT) were included. They were divided into two groups according to the blastocyst developmental stage, D5 and D6. Exclusion criteria included twin or triplet pregnancies, chromosomal abnormalities in one or both partners, a history of miscarriages or repeated implantation failure, and reproductive tract abnormalities. The study population is shown in a flow chart (Fig. 1).Finally, a total of 522 patients were recruited, including 398 patients in the D5 group and 124 in the D6 group. The research protocol was approved by the ethics committee of the Sixth Affiliated Hospital of Sun Yat-sen University (2017ZSLYEC-016S). All patients signed an informed consent form before IVF treatment, allowing the use of their clinical information for scientific research.
Controlled Ovarian Stimulation(COS),fertilization and embryos culture
GnRH agonist or GnRH antagonist regimen was chosen depending on the patients’ age, ovarian reserve, and previous COS response. The process has been described in detail in our previous study [10]. Conventional in vitro fertilization ( IVF) or intracytoplasmic sperm injection (ICSI) was performed according to sperm characteristics. The presence of two pronuclei was considered normal fertilization. The zygotes were cultured for 3 days and scored according to Cummins criteria [11]. Available D3 embryos (i.e. embryos with no less than 4 blastomeres and scored as level I-II)were cultured in a commercial sequential culture medium (G5 series; Vitrolife AB Company, Gothenburg, Sweden) to blastocyst stage and graded according to the criteria of Gardner and Schoolcraft [12]. If embryos on day 5 were at the stage of morula or early blastocyst, they would be cultured one more day. Only blastocysts at stages 3–6 with at least either inner cell mass or trophectoderm scored as grade B were vitrified for future transfer.
Blastocysts vitrification, thawing and transfer
The vitrification of blastocyst was performed with a vitrification kit (Kitazato Company, Japan) according to the instruction manual. In TBT cycles, estradiol valerate (Bayer Pharmaceuticals, Germany) or Estradiol Gel (Oestrogel, Besins, Belgium) was administered on days 2–3 of menstruation. When the endometrial thickness was thicker than 8 mm and the duration of administration was at least 10 days, progesterone soft capsules (Utrogestan, Besins Healthcare, Belgium) were given vaginally for endometrium transforming, 1–2 blastocysts are thawed and transferred 5 days later.
Chorionic villus culture and chromosome karyotyping
For patients diagnosed with pregnancy failure, vacuum curettage was performed, and chorionic villus samples were collected under sterile conditions. Villi cells were cultured in AmnioMAXTM-C100 basal medium with supplement (GIBCO, Grand Island, New York) and recovered and fixed when grown to appropriate density, then digested with trypsin solution and stained with Giemsa. Chromosome karyotype results were interpreted with reference to the international system of human cytogenetic nomenclature. At least 20 cells per sample were analyzed by an experienced cytogenetics technician. Karyotypes were analyzed for 50 cells if chromosomal aneuploidy was detected, and for 100 cells if mosaic karyotypes were detected.
Outcomes
Aneuploidy was defined as a loss or gain of the genetic material of chromosome(s), including monosomies, trisomies, polyploidies, segmental aneuploidies, and complex chromosome aneuploidies. In this study, aneuploidy rate of of SA-CV was compered between D5 group and D6 group. Other factors such as age, type of infertility, diagnosed with PCOS were assessed to see if they affected chromosomal results.
Statistical analysis
Statistical analyses were performed using IBM SPSS statistical software version 22 for Windows (IBM Corp., Armonk, NY, USA). The normality of continuous variables was examined by the Shapiro–Wilk test. The differences were statistically significant for the age at oocyte pick-up (OPU), body mass index (BMI), basal follicle-stimulating hormone (FSH), infertility duration, and gestational age when abortion. However, since the histograms and P-P plots indicated that the data distributions were not overly skewed, they were still expressed as mean (standard deviation,SD), and Student t-tests were performed for group comparisons. Other nonnormally distributed continuous variables were expressed as median(Q1-Q3) and Mann–Whitney U test was performed for group comparisons. Categorical variables were presented as percentages, and chi-square tests with Fisher’s exact test were used for frequency comparisons. Univariate regression analyses were performed for all variables to identify candidate factors. Variables showing a tendency to be associated with aneuploidy rate (P < 0.10) in the univariate analysis and literature-based confounders were selected for the multivariate model. P < 0.05 was considered statistically different.
Results
Patient characteristics are shown in Table 1. There are no significant differences in age when OPU, BMI, FSH, type of infertility, fertilization methods, and gestation age when abortion between group D5 and D6 (all P > 0.05). The number of embryos transferred in the D5 group is significantly lower than that in the D6 group (P < 0.001), however, the difference is not significant in the number of high-quality embryos (P = 0.773).
The chorionic chromosome aneuploidy rate in the D5 group is 46.5% (185/398), which is not significantly different from 41.1% (51/124) in the D6 group (P = 0.296) (Table 2). After excluding potential confounding factors such as age when OPU, infertility type, diagnosed with PCOS, gestational age, the difference in the rate of chromosomal aneuploidy between the D5 and D6 groups is still not significant (OR = 0.756; 95% CI: [0.429–1.220]; P = 0.225) (Table 3).
Age when OPU significantly relate with chromosomal aneuploidy rates (OR = 0.891; 95% CI: [0.854–0.930]; P < 0.001). The rate of chromosomal aneuploidy is significantly higher in the age > 35 years group than in the age ≤ 35 years group (61.0% vs. 39.4%, P < 0.001). However, there is no significant difference in chromosomal aneuploidy rates between the D5 and D6 groups in both age > 35 years and age ≤ 35 years (P = 0.247 and P = 0.690) (Table 2).
Discussion
The relationship between the pace of blastocyst development and chromosomal aberrations in villi has not been previously reported. Our results showed (Table 2) that the chromosome aneuploidy rate of SA-CV in the D6 group was comparable to that of the D5 group. This is inconsistent with the studies of pre-implantation blastocysts, which showed that the aneuploidy rate of D6 blastocysts was significantly higher than that of D5 blastocysts [6, 13, 14]. Possible reasons included maybe as following.
Chromosomal aneuploidy between blastocyst TE and SA-CV manifest different characteristics
Although chromosomal aneuploidy is widely accepted as the most significant cause of spontaneous abortion in humans, different types of abnormality have different fates, manifesting with different characteristics from the blastocyst stage through early implantation to their ultimate fate. The main chromosomal abnormalities in oocytes and embryos were monosomy and trisomy [15, 16]. Large numbers of genes missing caused by monosomy are likely to lead to a lethal change in cellular pathways, such as massive methylation, transcriptomic, and proteomic changes. They may experience growth retardation or developmental failure and loss early due to severe developmental defects, as evidenced by failure in blastocysts forming and implantation or cessation of development after implantation (biochemical pregnancy) [17, 18]. In contrast, gaining a chromosome has relatively less impact than losing a chromosome [19]. Some particular trisomies are not lethal for blastocysts, they can continue to develop and successfully implant but still, abort at a certain stage. Only a few aneuploidies can result in live births, such as trisomy 21 and 45XO. The most notable change from blastocyst to SA-CV was an increased rate of trisomies, whereas the incidence of autosomes monosomies decreased by 30-fold [15]. Embryos with different chromosomal aneuploids arrest at different stages, therefore, we speculate that the chromosomal abnormalities affecting the speed of blastocyst development may be more severe, these embryos lost before or shortly after implantation, resulting in a lower clinical pregnancy rate (CPR) in the D6 group. However, there is no literature comparing the different distribution of chromosomal aneuploidy between D5 and D6 blastocysts. Further studies are needed to determine whether D6 blastocysts are with a higher rate of monosomy and other severe abnormalities than D5 blastocysts.
Non-chromosomal factors interfering with pregnancy outcomes after D6 TBT
Lower CPR and LBR of euploidy D6 blastocysts than euploidy D5 blastocysts [20,21,22], indicated other factors that affect the blastocyst development and the reproductive potential. Such as the metabolic or epigenetic or organelle abnormalities of the embryo. The study by Wu et al. provided evidence that D5 blastocysts contained significantly greater mitochondrial DNA quantity than D6 blastocysts [23]. Hashimoto et al. have reported that spindle abnormalities are significantly higher in D6 blastocysts, which block cell mitosis and reduce cell number and development retardation [24].
Maternal age is an independent factor affecting pregnancy outcomes in both spontaneous and IVF pregnancies. LBR decreases with age increasing and is less than 1% in those older than 44 years [25].While the miscarriage rate shows the opposite trend and exceeds 50% in those older than 40 years. Chromosomal abnormalities are the main cause of poor age-related pregnancy outcomes. Oocytes/embryos aneuploidy rates are significantly higher in women of advanced age [26]. Older women transferring euploidy embryos have similar pregnancy outcomes to younger women [26]. Our results showed the same conclusion, with 61.0% of miscarriages caused by aneuploidy in women over 35 years of age, compared to a value of 39.4% in women under 35 years of age. The great majority of aneuploidy is the consequence of abnormal meiotic chromosome segregation during oocyte formation [16]. However, the rate of oocyte aneuploidy was not linearly related to age, but rather showed a U-shaped curve distribution, consistent with an inverse U-curve in humans' natural fertility, where young females (13–20 years) and women of advancing maternal age (AMA, the mid-30 s and above) show reduced fertility rates. Meiosis I nondisjunction (MI NDJ), in which gain or loss of a whole chromosome, decreased with female age. Precocious separation of sister chromatids (PSSC) or predivision, sister chromatids of one homolog separating in meiosis I, and reverse segregation (RS), when both homologous chromosomes split their two sister chromatids already at meiosis I, increased with female age. This combination of distinct age-dependent error types forms the U-curve of aneuploidy in human oocytes [27]. In our study, the minimum age of the patients was 23 years, so it was not possible to analyze the distribution of SA-CV aneuploidy in young females and women of AMA.
Value of conventional karyotyping in early pregnancy loss
Aneuploidy is the most common numerical abnormality defined as a loss or gain of the genetic material of chromosome(s), the major types include monosomies, trisomies, polyploidies, segmental aneuploidies, and complex chromosome aneuploidies. It was reported that these abnormalities account for more than 87 ~ 93% of chromosomal abnormalities in SA-CV [28, 29]. Although copy number variants(CNVs) less than 3 Mb in euploid POCs distribute in all chromosomes, the clinical significance of these variants is unclear [30]. Therefore, karyotyping analysis is the main method of assessing chromosomal results in SA-CV.
Conventional karyotyping analysis can detect aneuploidy, balanced/unbalanced translocations, and inversions and is the first-line method for the diagnosis of chromosomal aberrations. However, the requirement for a large number of cells, long culture times and high failure rates, low resolution (> 5–10 Mb) limit its efficiency. Chromosomal microarray analysis (CMA) was shown to be superior to cytogenetics due to a significant increase in the test success rate [31]. It also could detect chromosomal duplication/deletion more than 200 Kb, uniparental disomy, loss of heterozygosity, and unbalanced translocation [31]. Nevertheless, the chromosomal abnormalities detected by CMA in early abortion are mainly numerical abnormalities and macro-segmental abnormalities(> 5 MB) [15, 28, 29], which can also be detected by conventional karyotyping. A meta-analysis also showed only a mean increment yield of 1% (95% CI, 1–2%) in CMA over conventional karyotyping in patients without recurrent pregnancy loss [31].
As a retrospective study, the present work has some limitations. However, we took measures to improve its credibility. (1) Only thaw-frozen cycles using HRT were included to minimize the influence of endometrial factors. (2) The underlying conditions of the included patients were controlled, such as excluding patients with miscarriage history or repeated implantation failure and excluding patients with chromosomal abnormalities. (3) All data in this study were from the same center. Experienced and fixed staffs were in charge of chorionic villus chromosome testing to reduce bias related to the operator. (4) A total of 522 patients were included in this work. The sample size was large enough to reflect the actual condition. Therefore, the results are credible to provide reference information on clinical treatment and provide guidance for patient counseling.
In conclusion, the rate of chromosomal aneuploidy in SB-CV after D6 TBT was comparable to that after D5 TBT, suggesting that chromosomal abnormalities may not be a factor contributing to the high prevalence early pregnancy loss in the D6 group. More studies are needed regarding the poorer pregnancy outcome in D6 than in D5 groups.
Availability of data and materials
The datasets generated during the current study are not publicly available since our institution prohibits public data sharing. However, the datasets are available from the corresponding author on reasonable request.
Abbreviations
- OPU:
-
Oocyte pick up
- IVF:
-
In vitro Fertilization
- ICSI:
-
Intracytoplasmic sperm injection
- SA-CV:
-
Spontaneous abortion chorionic villus
- TBT:
-
Thawed-frozen blastocyst transfer
- COS:
-
Controlled ovarian stimulation
- BMI:
-
Body mass index
- PCOS:
-
Polycystic ovary syndrome
References
Li YX, Wang J, Sun TZ, et al. Pregnancy outcomes after day 5 versus day 6 blastocyst-stage embryo transfer: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2020;46(4):595–605.
Yerushalmi GM, Shavit T, Avraham S, et al. Day 5 vitrified blastocyst transfer versus day 6 vitrified blastocyst transfer in oocyte donation program. Sci Rep. 2021;11(1):10715.
Corti L, Cermisoni GC, Alteri A, et al. Clinical outcomes deriving from transfer of blastocysts developed in day 7: a systematic review and meta-analysis of frozen-thawed IVF cycles. Reprod Sci. 2022;29(1):43–53.
Poulsen V, Ingerslev HJ, Kirkegaard K. Elective embryo transfers on day 6 reduce implantation compared with transfers on Day 5. Hum Reprod. 2017;32(6):1238–43.
Bourdon M, Pocate-Cheriet K, de FinetBantel A, et al. Day 5 versus day 6 blastocyst transfers: a systematic review and meta-analysis of clinical outcomes. Hum Reprod. 2019;34(10):1948–64.
Taylor TH, Patrick JL, Gitlin SA, et al. Comparison of aneuploidy, pregnancy and live birth rates between day 5 and day 6 blastocysts. Reprod Biomed Online. 2014;29(3):305–10.
Yang H, Yang Q, Dai S, et al. Comparison of differences in development potentials between frozen-thawed D5 and D6 blastocysts and their relationship with pregnancy outcomes. J Assist Reprod Genet. 2016;33(7):865–72.
Alfarawati S, Fragouli E, Colls P, et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95(2):520–4.
Doubilet PM, Benson CB, Bourne T, et al. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443–51.
Zhao WE, Li YJ, Ou JP, et al. Predictive value of initial serum human chorionic gonadotropin levels for pregnancies after single fresh and frozen blastocyst transfer. J Huazhong Univ Sci Technolog Med Sci. 2017;37(3):395–400.
Cummins JM, Breen TM, Harrison KL, et al. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. 1986;3(5):284–95.
Gardner DK, Lane M, Stevens J, et al. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8.
Huang TT, Huang DH, Ahn HJ, et al. Early blastocyst expansion in euploid and aneuploid human embryos: evidence for a non-invasive and quantitative marker for embryo selection. Reprod Biomed Online. 2019;39(1):27–39.
McDaniel KE, Awadalla MS, McGinnis LK, et al. Transfer the best and biopsy the rest? Blastocyst euploidy rates differ by morphology and day of biopsy. Arch Gynecol Obstet. 2021;303(1):249–58.
Zhang X, Wang Y, Zhao N, et al. Variations in chromosomal aneuploidy rates in IVF blastocysts and early spontaneous abortion chorionic villi. J Assist Reprod Genet. 2020;37(3):527–37.
Fragouli E, Alfarawati S, Spath K, et al. The origin and impact of embryonic aneuploidy. Hum Genet. 2013;132(9):1001–13.
McCallie BR, Parks JC, Patton AL, Griffin DK, Schoolcraft WB, Katz-Jaffe MG, et al. Hypomethylation and genetic instability in monosomy blastocysts may contribute to decreased implantation potential. PLoS ONE. 2016;11(7):e159507.
Halloran KH, Breg WR. Mahoney MJ.21 monosomy in a retarded female infant. J Med Genet. 1974;11(4):386–9.
Licciardi F, Lhakhang T, Kramer YG, et al. Human blastocysts of normal and abnormal karyotypes display distinct transcriptome profiles. Sci Rep. 2018;8(1):14906.
Boynukalin FK, Gultomruk M, Cavkaytar S, et al. Parameters impacting the live birth rate per transfer after frozen single euploid blastocyst transfer. PLoS ONE. 2020;15(1):e0227619.
Irani M, O’Neill C, Palermo GD, et al. Blastocyst development rate influences implantation and live birth rates of similarly graded euploid blastocysts. Fertil Steril. 2018;110(1):95–102.
Barash OO, Ivani KA, Willman SP, et al. Association between growth dynamics, morphological parameters, the chromosomal status of the blastocysts, and clinical outcomes in IVF PGS cycles with single embryo transfer. J Assist Reprod Genet. 2017;34:1007–16.
Hashimoto S, Amo A, Hama S, et al. Growth retardation in human blastocysts increases the incidence of abnormal spindles and decreases implantation potential after vitrification. Hum Reprod. 2013;28(6):1528–34.
Wu FS, Weng SP, Shen MS, et al. Suboptimal trophectoderm mitochondrial DNA level is associated with delayed blastocyst development. J Assist Reprod Genet. 2021;38(3):587–94.
Center for Disease Control and Prevention.Available at: https://www.cdc.gov/art/artdata/index.html.Accessed 12 Jan, 2018.
Irani M, Zaninovic N, Rosenwaks Z, et al. Does maternal age at retrieval influence the implantation potential of euploid blastocysts? Am J Obstet Gynecol. 2019;220(4):e1–7.
Gruhn JR, Zielinska AP, Shukla V, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science. 2019;365(6460):1–9.
Schilit S, Studwell C, Flatley P, et al. Chromosomal microarray analysis in pregnancy loss: Is it time for a consensus approach? Prenat Diagn. 2022;42(12):1545–53.
Xu Q, Chan Y, Feng Y, et al. Factors associated with fetal karyotype in spontaneous abortion: a case-case study. BMC Pregnancy Childbirth. 2022;22(1):320.
Gu C, Gao H, Li K, et al. Copy Number Variation Analysis of Euploid Pregnancy Loss. Front Genet. 2022;13:766492.
Pauta M, Grande M, Rodriguez-Revenga L, et al. Added value of chromosomal microarray analysis over karyotyping in early pregnancy loss: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(4):453–62.
Acknowledgements
Not applicable
Funding
This study was supported by the National Key Research and Development Program of China (2021YFC2700400).
Author information
Authors and Affiliations
Contributions
Among the authors in the list, Weie Zhao plays a guiding role in data collection and curation. Panyu Chen is responsible for the preparing of the original draft. Xiaoping Liu is responsible for the presentation of the published work, especially the data presentation. Yujie Li take charge of paper reviewing. Xiaoyan Liang is responsible for funding acquisition, subject validation. Jingjie Li takes charge of the design of the project, editing of the paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate.
The research protocol was approved by the ethics committee of the Sixth Affiliated Hospital of Sun Yat-sen University (approved number: 2017ZSLYEC-016S).All patients signed informed consent forms before IVF treatment, allowing the use of their clinical information for scientific research. All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no conflicts of interest to this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, W., Chen, P., Liu, X. et al. Comparison of aneuploidy rate in spontaneous abortion chorionic villus between D6 and D5 thawed-frozen blastocyst transfer. BMC Pregnancy Childbirth 23, 130 (2023). https://doi.org/10.1186/s12884-023-05452-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05452-5