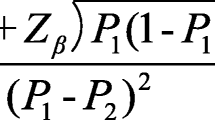Abstract
Background
Breastfeeding and maternal health play crucial roles in improving newborn health, which is closely related to the development of families and society. Early essential newborn care, which emphasizes early exclusive breastfeeding and skin-to-skin contact, is recommended by the World Health Organization. This study aimed to explore the association of early essential newborn care with breastfeeding and maternal outcomes.
Methods
A nonrandomized controlled study was carried out from May 2020 to January 2021 in a tertiary hospital in Chengdu city, China. Pregnant women were recruited from the maternity ward before they gave birth. Early essential newborn care was performed for 91 mother-newborn pairs after birth in the intervention group, while routine birth care was performed for 91 mother-newborn pairs in the control group. Data on breastfeeding and maternal outcomes were collected pre-test and post-test and were recorded by trained data collectors and retrieved from hospital case record files.
Results
Compared with the control group, the intervention group had a higher incidence of early breastfeeding initiation, an earlier initiation and longer duration for the first breastfeeding, a higher incidence of successful first breastfeeding, more exclusive breastfeeding at hospital discharge, higher maternal breastfeeding self-efficacy, a shorter duration of the third stage of labour, lower postpartum blood loss, and lower scores of maternal pain and anxiety postpartum; the differences were statistically significant (p < 0.05).
Conclusion
The implementation of high-quality early essential newborn care can help mothers initiate early breastfeeding, improve exclusive breastfeeding rates at hospital discharge, enhance breastfeeding self-efficacy, promote the woman’s recovery from labour, and reduce maternal anxiety and pain in the postpartum period. High-quality early essential newborn care is recommended to policymakers and medical professionals to improve breastfeeding and maternal outcomes.
Trial registration
Chinese Clinical Trial Registry, Retrospective Registration (27/7/2021), registration number: ChiCTR2100049231.
Similar content being viewed by others
Background
Breastfeeding is the ideal method for infant feeding. It is estimated that if the breastfeeding rate were to increase to 50% worldwide, the deaths of approximately 823,000 under-five children can be avoided every year [1]. Early postnatal breastfeeding behaviour is associated with long-term breastfeeding [2]. To improve the breastfeeding rate, the World Health Organization (WHO) has recommended skin-to-skin contact between mothers and newborn infants immediately after birth and breastfeeding during the first hour after birth [3]. Studies have shown that breastfeeding within the first hour after birth can improve exclusive breastfeeding rates at 6 weeks, 10 weeks, and 6 months postpartum [4,5,6] and that mothers who breastfeed early have a higher acceptance of exclusive breastfeeding [7]. Compared with newborn infants who initiated breastfeeding at 2–23 h and 24–96 h after birth, newborn infants who initiated breastfeeding within the first hour after birth had lower neonatal mortality [8].
Previous studies indicated that many medical professionals, especially in the West Pacific region, often implemented outdated and harmful practices during and after birth, such as unnecessary suctioning, delayed early skin-to-skin contact between the mother and the newborn infant, as well as umbilical cord cutting immediately after birth [9]. These outdated practices lead to an increase in the risk of neonatal morbidity and mortality [10]. To improve the quality of newborn care, the Action Plan for Healthy Newborn Infants in the Western Pacific Region (2014–2020) was issued by the WHO Western Pacific Regional Office (WHO/WPRO) [11]. This plan aimed to give every newborn a healthy start and implement early essential newborn care (EENC) for all newborn infants. EENC contains evidence-based interventions that are simple, that are low-cost and that do not require expensive technologies. The central element of EENC is immediate skin-to-skin contact between the mother and newborn infant after birth for at least 90 min and initiation of exclusive breastfeeding when cues occur (such as drooling, tonguing, rooting, and hand biting). Additionally, midwives should appropriately delay clamping and cutting of the cord and other routine care. These practices can ensure that most newborn infants complete the first breastfeeding during the period of skin-to-skin contact and improve the early breastfeeding initiation rate, as well as strengthen the rooting reflex of the newborn infant [12]. Furthermore, implementing EENC may also have positive effects on mothers because skin-to-skin contact between mothers and newborn infants can reduce maternal pain, depression and anxiety, accelerate placental detachment, reduce postpartum haemorrhage, and promote uterine involution by promoting the secretion of oxytocin [13].
EENC was introduced to China in 2016 and had been implemented in 112 medical institutions by 2017 [14]. Yang et al. surveyed the medical institutions of four provinces that implemented EENC in China and showed that only 36.2% of the newborn infants had skin-to-skin contact with their mothers, the rate of the duration of skin-to-skin contact over 90 min was 19.7%, and the breastfeeding rate and exclusive breastfeeding rate before discharge were 76.5% and 32.2%, respectively [15]. The findings of the study by Yang et al. indicated that EENC was not fully implemented in line with the WHO guidelines in these medical institutions. Xu et al. pointed out that there were many obstacles to implementing EENC in China hospital policies, including insufficient awareness of medical professionals, shortage of human resources, and little clinical evidence about EENC in China [16]. Previous studies have explored the benefits of skin-to-skin contact and timed clamping for newborn infants separately [13, 17]. However, the EENC is an intervention package; thus, the effect of EENC should be regarded as a general effect on mothers and newborn infants. In addition, most published studies have focused on the effect of implementing EENC on improving newborn outcomes, while few studies have explored the benefits of EENC for breastfeeding and maternal outcomes. This study aimed to fill this research gap, explore the effect of implementing high-quality EENC on breastfeeding and maternal outcomes, and provide more clinical evidence for improving the health of newborn infants and mothers in the West Pacific Region.
The definitions of certain terminology used in this paper are as follows: early breastfeeding initiation, defined as the initiation of first breastfeeding within the first hour after birth; successful first breastfeeding, defined as the score of first breastfeeding assessed by the 4-item Infant Breast Feeding Assessment Tool (IBFAT) [18] is between 10 and 12; exclusive breastfeeding, defined as only breast milk given to the newborn infant without any liquid or solid food; mixed feeding, defined as breastfeeding combined with artificial feeding of the newborn infant; and artificial feeding, defined as feeding newborn infants with foods other than breast milk, such as formula milk.
Methods
Study design and setting
This study was a nonrandomized controlled study and was carried out from May 2020 to January 2021 in a tertiary hospital in Chengdu city, Sichuan Province, China. This hospital is one of the largest women and children’s hospitals in Sichuan Province and has two labour wards with identical health facilities and similar human resources in different hospital areas. These two wards had not implemented EENC or received any EENC coaching before this study, and they were assigned randomly to be the intervention group and control group. Each pregnant woman chose the labour ward in which she preferred to give birth at her antenatal visit in the hospital, and her selection depended entirely on her preferences. However, we began participant recruitment when the woman was awaiting delivery in the maternity ward; thus, the participants were not assigned to each group randomly.
The current study was a part of a larger trial. Because of the limitations to the length of an article, this paper focuses only on breastfeeding and maternal outcomes.
Participants
Participants in this study comprised women and their newborn infants. Pregnant women were recruited from the maternity ward when they were admitted to hospitals for await delivery and with no signs of labour. Pregnant women who met the following inclusion criteria were considered eligible and were invited by the researchers to participate in this study: (1) aged over 18 years, (2) gestational age between 37 and 42 weeks, (3) singleton pregnancy, (4) vaginal delivery, (5) no severe pregnancy complications and/or underlying disease, and (6) no medical indications against breastfeeding. If the woman was transferred from vaginal delivery to caesarean section or the newborn infant had an abnormal birthweight (< 2500 g or > 4000 g), deformities or needed to be transferred to the neonatal intensive care unit (NICU) immediately after birth, the mother and infant were excluded from the study. Written informed consent was obtained from all participants. Ethical approval was received from the hospital ethics review board.
Interventions
Participants in the intervention group received the EENC interventions after birth from midwives in the intervention group, while participants in the control group received routine birth care from midwives in the control group. These interventions delivered in the delivery room. The midwives in the intervention group received 5-month training sessions from national and provincial facilitators, following the guidelines formulated by the WHO [19]. After 5-month training sessions, a pilot study on 18 mother-newborn pairs was conducted in October 2020 to ensure that every midwife could implement the EENC correctly. The formal interventions were performed from November 2020 to January 2021.
EENC interventions include (1) drying the newborn infant immediately and thoroughly within five seconds after birth, (2) immediate skin-to-skin contact within the first minute and lasting for at least 90 min, (3) exclusive breastfeeding, (4) appropriately timed clamping and cutting of the cord, and (5) other routine care – eye care, vitamin K, immunizations, weighing and examinations [11]. The duration of implementing EENC was between 90 to 120 min.
The sequence of routine birth care in this hospital was (1) drying of the newborn infant, (2) placement of the newborn infant on a heated table to keep warm for 20 min, during which the umbilical cord is clamped and the weight and length are measured, (3) vaccination, (4) skin-to-skin contact between the mother and newborn infant, and (5) exclusive breastfeeding after the third stage of labour. The duration of implementing routine birth care was between 90 to 120 min.
For both groups, the same postnatal care and education were delivered to mothers by midwives, including the contents of breastfeeding, diet, physical activity, safety, urine output, and stool output.
Objectives
This study aimed to explore the effect of implementing EENC on breastfeeding and maternal outcomes. We hypothesized that implementing EENC could improve the breastfeeding outcomes and help mothers recover from delivery, especially for the incidence of early breastfeeding initiation.
Measures and data collection
Variables collected at baseline for women included age, educational level, height, weight, gestational age, previous obstetric history, anxiety, and nipple pattern. Variables for newborn infants included sex, length, and birthweight. Among these variables, the anxiety of women was assessed by the Chinese version of the strait form of the State-Trait Anxiety Inventory (STAI-S), which was developed by Spielberger in 1970 and was introduced to China in 1988 [20]. The STAI-S has 20 self-report items and items are scored on a four-point Likert scale of 1 (not at all) to 4 (severe), with the scores summated to derive a total score ranging from 20 to 40 points. The Cronbach’s α of the Chinese version of STAI-S was 0.91. Higher STAI-S scores indicate severer anxiety. In addition, the nipple pattern was classified into three types, namely, normal, flat, and inverted patterns, and assessed by two female data collectors.
The primary outcome of the current study was the incidence of early breastfeeding initiation. If the first breastfeeding was initiated successfully within the first hour after birth, the early breastfeeding initiation was considered and would be recorded by the data collectors.
The second outcome of this study consisted of some breastfeeding-related outcomes and maternal outcomes; namely, the time of rooting reflex occurrence, the initiation time and duration of first breastfeeding, the number of successful first breastfeeding, the time when formula milk is first served, the total amount of formula milk given before discharge, the number of breastfeeding within the first day after birth, the feeding pattern before discharge, the duration of the third stage of labour, the postpartum blood loss within 2 h after birth, and the pain and anxiety of the woman after birth. Data on the duration of the third stage of labour and the postpartum blood loss within 2 h after birth were retrieved from hospital case record files. The woman’s pain was evaluated by means of the Visual Analogue Scale (VAS) [21] at 30 min, 60 min, and 120 min after birth. Anxiety was evaluated by the state form of the State-Trait Anxiety Inventory (STAI-S) [22] at 120 min after birth. Other variables were recorded by data collectors. Additionally, the 4-item Infant Breast Feeding Assessment Tool (IBFAT) [18] was used to assess the success of the first breastfeeding by data collectors. The total score of IBFAT ranges from 0 to 12, with 10–12 being the scores for vigorous and effective breastfeeding. The Breastfeeding Self-efficacy Scale Short Form (BSES-SF) [23] was used to assess the confidence of women to breastfeed before discharge from the hospital, with a higher BSES-SE score indicating stronger breastfeeding self-efficacy.
The data collectors were all women with medical educational background. Before the study, data collectors received the methods for collecting data by researchers. They were permitted to enter the ward to collect data by both the participants and the ethnic committee of hospitals.
Sample size
PASS version 15.0 was used to calculate the sample size. We estimated the sample size based on the primary outcome of this study, which is the incidence of early breastfeeding initiation. The results of the pilot study showed that the incidence of early breastfeeding initiation were 77.8% and 44.4% in the intervention group and the control group, respectively. Hence, a sample size of 100 participants would be required (α = 0.05, β = 0.1) [10]. Considering that the drop-out rate was 10%, the minimum sample size needed was 110 participants, with 55 participants in each group. To reduce sampling error [24], we include all pregnant women who met the inclusion criteria in the study during the recruitment phase.
Blinding
The current study was a single-blinded trial. It was impossible to blind the midwives and data collectors in the delivery rooms because midwives were responsible for implementing EENC or routine birth care and data collectors were responsible for assessing and recording. Hence, only participants were blinded.
Statistical methods
SPSS version 25.0 was used to analyse the data. The smallest unit that is being analyzed to assess intervention effects was the group. The mean ± standard deviation (SD) and median (interquartile range, IQR) were used to describe continuous data, and t test and Mann–Whitney U test were used to identify the differences. The number (n) and percentage (%) were used to describe categorical data, and the chi-square test and Fisher’s exact test were used to identify differences.
Results
Baseline information of participants
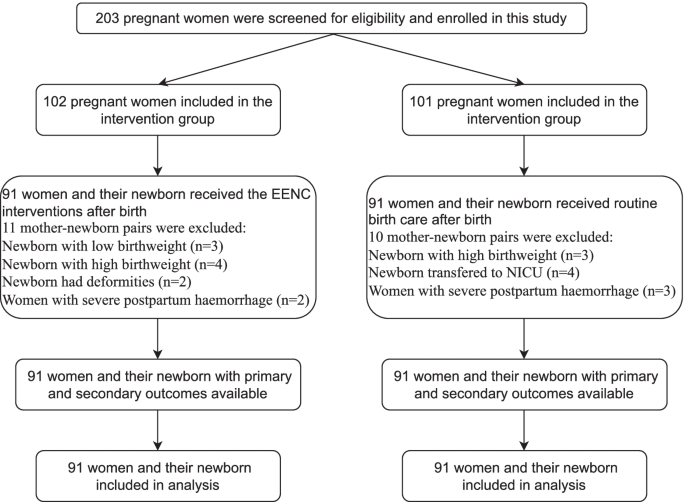
In total, 203 pregnant women were recruited for this study from November 2020 to January 2021, with 102 included in the intervention group and 101 in the control group. Figure 1 shows the flow of participants through each stage of the study. Ultimately, there were 91 mother-newborn pairs in the intervention group and 91 mother-newborn pairs in the control group. Table 1 shows the basic information of all participants. There were no significant differences between the two groups regarding the baseline information.
Breastfeeding within 2 h after birth in the two groups
The incidence of early breastfeeding initiation in the intervention group was higher than that in the control group (n = 69 vs. n = 39, p < 0.001). The first breastfeeding in the intervention group started earlier (48.02 ± 16.30 min vs. 66.97 ± 35.41 min, p < 0.001) and lasted longer (34.98 ± 15.02 min vs. 22.30 ± 11.70 min, p < 0.001) than that in the control group. Additionally, the mean IBFAT scores of the first breastfeeding were higher (10.05 ± 2.17 vs. 8.68 ± 2.04, p < 0.001). Furthermore, more successful first breastfeeding (n = 83 vs. n = 68, p = 0.003) were observed in the intervention group. However, there was no significant difference in the time of rooting reflex occurrence. (Table 2).
Breastfeeding before discharge in the two groups
The median time at which the formula milk was first served in the intervention group was later than that in the control group (4 h vs. 2 h, p < 0.001), and the median amount of formula milk given to babies before discharge was higher in the control group than in the intervention group (70 ml vs. 90 ml, p < 0.001). The number of breastfeeding within 24 h after birth in the intervention group was greater than that in the control group (n = 7 vs. n = 5, p < 0.001). Regarding the feeding pattern, the number of exclusive breastfeeding in the intervention group was greater than that in the control group (n = 67 vs. n = 40), with less mixed breastfeeding (n = 24 vs. n = 47) and artificial breastfeeding (n = 0 vs. n = 4). The women in the intervention group had higher breastfeeding self-efficacy assessed by the BSES-SF (55.78 ± 8.96 vs. 46.74 ± 10.08, p = 0.024). (Table 3).
Duration of third stage of labour, postpartum blood loss, pain and anxiety of women
Compared to those in the control group, the duration of the third stage of labour was shorter (5.25 ± 5.66 min vs. 6.10 ± 2.92 min, p < 0.001), and the amount of postpartum blood loss within 2 h after birth was lower (234.64 ± 63.65 ml vs. 281.37 ± 72.29 ml, p < 0.001) in the intervention group. The mean VAS (at 30 min, 1 h, and 2 h) and STAI-S scores in the control group were higher than those in the intervention group, which indicated that pain and anxiety were more severe in the control group (Table 4).
Discussion
This study compared the effect of EENC and routine birth care on breastfeeding and maternal outcomes in a tertiary hospital in China. The results showed that EENC can improve the early breastfeeding initiation, establish correct breastfeeding behaviour, increase the self-efficacy in breastfeeding among mothers and help them recover from childbirth.
Although there were more primiparous women in the intervention group, our results showed that the breastfeeding outcomes in the intervention group were better than that in the control group. Some studies showed that women who have breastfed previously have better breastfeeding outcomes than primiparous women [25, 26]. However, the study by Anette et al. showed that parity cannot affect the duration of exclusive breastfeeding or any breastfeeding, but early first breastfeeding can lead to a positive impact [27]. Similarly, previous studies also pointed out that although inverted or flat nipples would hinder breastfeeding [28], if the babies can be breastfed early, they are more likely to attach and can be fed well in the postnatal period. In this study, although more women with inverted and flat nipples were in the intervention group, the breastfeeding outcomes were still better than that in the control group. For primiparous women and women with flatted or inverted nipples, EENC may therefore also be recommended as it can improve breastfeeding outcomes.
The findings of this study indicated that the intervention group had a higher incidence of early breastfeeding initiation, earlier initiation and longer duration of first breastfeeding, and a higher IBFAT score for first breastfeeding. Similar findings have been reported in studies by Aiping G et al. and Min et al. [12, 29]. Early breastfeeding is an important factor for constructing correct breastfeeding behaviour. The WHO proposed Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services in 2017 [30], which emphasized that medical institutions should provide all feasible support to help women initiate early breastfeeding. Previous studies also showed that women who initiated breastfeeding within the first hour after birth had a higher acceptance of breastfeeding, which is especially important for improving the exclusive breastfeeding rates up to 6 months postpartum [5, 6]. EENC interventions contain a long duration of skin-to-skin contact, which is a key factor in ensuring the success of early breastfeeding. The study by Mahmood et al. showed that newborn infants with successful skin-to-skin contact can initiate first breastfeeding 62 min earlier than newborn infants with routine birth care, and the success rate of first breastfeeding increased by 26.3% [31]. In addition, EENC recommends that midwives assist women in initiating first breastfeeding when the newborn infants experience a rooting reflex and active breast-seeking action, which is in line with newborn infants’ instincts and can avoid excessive intervention in breastfeeding. Therefore, implementing EENC could increase the rate of early breastfeeding initiation and successful first breastfeeding.
In the current study, the rates of exclusive breastfeeding and the breastfeeding self-efficacy of women at hospital discharge in the intervention group were higher than those in the control group, which were impacted mainly by the early skin-to-skin contact between mothers and newborn infants and successful first breastfeeding. The study by Almqvist et al. showed that the issues of breastfeeding encountered by women in the early postpartum period were the main reason they gave up exclusive breastfeeding [32]. Success in the first breastfeeding means that the issues in the process of breastfeeding will be partly solved with the help of health professionals. Therefore, the women in the intervention group could gain confidence and skills from the experience of success in the first breastfeeding, which would in turn motivate them to perform exclusive breastfeeding [33]. The WHO recommends that unless there are medical indications, the staff of medical institutions should dissuade women and their families from providing any food other than breast milk to their infants. However, in the clinical practice setting, the phenomenon of mothers or family members feeding infants with formula milk or other food is unavoidable even though breast milk is sufficient because midwives and nurses cannot help every woman solve the problems encountered in breastfeeding due to the demands of their work. The study by Raghavan et al. showed that formula milk given to babies on the first day emerged as the only independent predictor of failure to continue exclusive breastfeeding at 6 weeks after birth (OR 2.96; 95% CI 1.09–8.06) [5]. In this study, newborn infants in the intervention group were given formula milk for the first time approximately 2 h later than those in the control group, and the total amount of formula milk added before discharge was also lower, which indicated that the construction of correct breastfeeding behaviour within the first hour after birth can reduce the use of unnecessary formula milk to some extent. Additionally, babies in the intervention group had more breastfeeding times within 24 h postpartum on the first day postpartum than those in the control group, indicating that the implementation of EENC can help women breastfeed correctly and have higher breastfeeding self-efficacy, which is conducive to the growth and development of newborn infants [34].
Our findings also showed that the EENC can help women recover from labour. The women in the intervention group had a shorter duration of the third stage of labour and lower postpartum blood loss, which is in line with the study by Yuan et al. [35]. During skin-to-skin contact, sucking from newborn infants can stimulate the nerve endings of the maternal nipple and then promote the synthesis and secretion of oxytocin [36]. Oxytocin can stimulate uterine contraction directly, reduce the interference of oxidative stress on uterine contraction, and finally reduce postpartum blood loss [37, 38]. In addition, placing the newborn infant on the mother’s breast and abdomen plays a similar role to massage, which can also promote the contraction of the uterus [13]. Furthermore, lower levels of postnatal anxiety and pain among mothers were observed in the intervention group, which may be related to the secretion of oxytocin and the joy of successful breastfeeding. Previous studies indicated that oxytocin can increase the threshold of maternal pain perception [39] and alleviate maternal anxiety [40, 41].
This study systematically explored the effects of EENC on breastfeeding and maternal outcomes and provided more evidence for the implementation of EENC in the future. However, this study also has some shortcomings. First, the design of this study is quasi-experimental. Due to hospital policies and funding limits, the participants could not be randomly assigned to two groups. However, because the intervention and control measures are implemented in two wards of the same hospital, which have similar human resources and facilities and are far away from each other, contamination and bias were excluded as much as possible. Second, the results of pain and anxiety were self-reported variables, so self-report bias cannot be avoided. Third, follow-up in this study lasted until the mother was discharged from the hospital, so a longer-term follow-up study to clarify the long-term effect can be considered in the future. Last, although the sample size had been previously calculated, this study was conducted only in a tertiary hospital, so the generalization of the results is limited. Large-sample and multicentre randomized controlled trials are necessary to further clarify the effect of EENC.
Conclusion
The implementation of EENC is associated with better breastfeeding and maternal outcomes, which can not only improve the early initiation of breastfeeding and exclusive breastfeeding rate but also relieve the anxiety and pain of the mother and increase her confidence in breastfeeding at hospital discharge. Hence, it is strongly recommended that policymakers and medical professionals implement EENC in clinical practice to improve the outcomes of both women and infants.
Availability of data and materials
All raw data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- EENC:
-
Early Essential Newborn Care
- IQR:
-
Inter Quartile Range
- WHO:
-
World Health Organization
- SD:
-
Standard deviation
- NICU:
-
Neonatal intensive care unit
- WPRO:
-
Western Pacific Regional Office
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- IBFAT:
-
Infant Breast Feeding Assessment Tool
- BSES-SF:
-
Breastfeeding Self-efficacy Scale Short Form
- VAS:
-
Visual Analogue Scale
- STAI-S:
-
The state form of State-Trait Anxiety Inventory
References
Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. The Lancet. 2016;387(10017):475–90.
Kronborg H, Vaeth M, Olsen J, Iversen L, Harder I. Effect of early postnatal breastfeeding support: a cluster-randomized community based trial. Acta Paediatr. 2007;96(7):1064–70.
World Health Organization: Implementation guidance: protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services: the revised baby-friendly hospital initiative. 2018.
Arusei RJ, Ettyang GA, Esamai F. Feeding patterns and growth of term infants in Eldoret. Kenya Food nutrition bulletin. 2011;32(4):307–14.
Raghavan V, Bharti B, Kumar P, Mukhopadhyay K, Dhaliwal L. First hour initiation of breastfeeding and exclusive breastfeeding at six weeks: prevalence and predictors in a tertiary care setting. The Indian Journal of Pediatrics. 2014;81(8):743–50.
Meshram I, Laxmaiah A, Venkaiah K, Brahmam G. Impact of feeding and breastfeeding practices on the nutritional status of infants in a district of Andhra Pradesh, India. Natl Med J India. 2012;25(4):201.
Kavle JA, LaCroix E, Dau H, Engmann C. Addressing barriers to exclusive breast-feeding in low-and middle-income countries: a systematic review and programmatic implications. Public Health Nutr. 2017;20(17):3120–34.
Group NS. Timing of initiation, patterns of breastfeeding, and infant survival: prospective analysis of pooled data from three randomised trials. Lancet Glob Health. 2016;4(4):e266–75.
Sobel HL, Silvestre MAA, Mantaring JBV III, Oliveros YE, Nyunt-U S. Immediate newborn care practices delay thermoregulation and breastfeeding initiation. Acta Paediatr. 2011;100(8):1127–33.
Cohen J. A power primer. Psychol Bull. 1992;112(1):155.
World Health Organization. Regional Office for the Western Pacific: Action plan for healthy newborn infants in the Western Pacific region (2014–2020). Geneva: World Health Organization; 2014.
Min C, Zhenfang L, Aihua W, Xiaolian Z, Yuanyuan Z, Xuelian W: Effects of early essential newborn care on the onset of lactogenesis among primiparaes. Chinese Journal of Child Health Care 2019.
Moore ER, Bergman N, Anderson GC, Medley N: Early skin‐to‐skin contact for mothers and their healthy newborn infants. Cochrane database of systematic Reviews 2016(11).
Wang CR, Li XY, Zhang L, Wu LM, Tan L, Yuan F, Guo Y, Williams S, Xu T. Early essential newborn care is associated with increased breastfeeding: a quasi-experimental study from Sichuan Province of Western China. Int Breastfeed J. 2020;15(1):99.
Jin Y, Xing L, Xiayun L, Yan W, Yue X, Yingpeng Q, Liwei S, Lai W, Hongyu L, Min Z. Current status of early basic neonatal health care services in 4 provinces and county-level medical and health care institutions in western my country. Maternal and Child Health Care of China. 2019;34(10):2178–82.
Tao X. Early essential newborn care: priority interventions to end preventable neonatal death. Chinese Journal of Preventive Medicine. 2021;54(5):498–502.
Rabe H, Reynolds GJ, Diaz‐Rosello JL: Early versus delayed umbilical cord clamping in preterm infants. Cochrane Database of Systematic Reviews 2004(4).
Matthews MK. Developing an instrument to assess infant breastfeeding behaviour in the early neonatal period. Midwifery. 1988;4(4):154–65.
WHO Regional Office for the Western Pacific. Coaching for the First Embrace: Facilitator’s Guide (Early Essential Newborn Care) Module 2 (A WPRO Publication). Geneva: World Health Organization; 2016.
Spielberger CD: State‐Trait anxiety inventory. The Corsini encyclopedia of psychology 2010:1–1.
Crichton N. Visual analogue scale (VAS). J Clin Nurs. 2001;10(5):706–706.
Spielberger CD: State-trait anxiety inventory for adults. 1983.
Dennis CL. The breastfeeding self-efficacy scale: Psychometric assessment of the short form. Journal of Obstetric, Gynecologic, Neonatal Nursing. 2003;32(6):734–44.
Sedgwick P: What is sampling error? BMJ 2012, 344.
Hackman NM, Schaefer EW, Beiler JS, Rose CM, Paul IM. Breastfeeding outcome comparison by parity. Breastfeed Med. 2015;10(3):156–62.
Koskinen KS, Aho AL, Hannula L, Kaunonen MJM. Maternity hospital practices and breast feeding self-efficacy in Finnish primiparous and multiparous women during the immediate postpartum period. 2014;30(4):464–70.
Ekström A, Widström A-M, Nissen E. Duration of Breastfeeding in Swedish Primiparous and Multiparous Women. J Hum Lact. 2003;19(2):172–8.
Chakrabarti K, Basu S. Management of Flat or Inverted Nipples with Simple Rubber Bands. Breastfeed Med. 2011;6(4):215–9.
Aiping G, Jiejing T, Xiaodan C, Yeping W, Hehe W, Yangyang L. Effects of early basic health care for newborns on newborns and their mothers. Chinese Journal Woman and Child Health Research. 2019;30(5):4.
World Health Organization: Guideline: protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services: World Health Organization; 2017.
Mahmood I, Jamal M, Khan N. Effect of mother-infant early skin-to-skin contact on breastfeeding status: a randomized controlled trial. J Coll Physicians Surg Pak. 2011;21(10):601–5.
Almqvist-Tangen G, Bergman S, Dahlgren J, Roswall J, Alm B. Factors associated with discontinuation of breastfeeding before 1 month of age. Acta Paediatr. 2012;101(1):55–60.
Araban M, Karimian Z, Kakolaki ZK, McQueen KA, Dennis C-L. Randomized controlled trial of a prenatal breastfeeding self-efficacy intervention in primiparous women in Iran. J Obstet Gynecol Neonatal Nurs. 2018;47(2):173–83.
Chipojola R, Chiu H-Y, Huda MH, Lin Y-M, Kuo S-Y. Effectiveness of theory-based educational interventions on breastfeeding self-efficacy and exclusive breastfeeding: A systematic review and meta-analysis. Int J Nurs Stud. 2020;109: 103675.
Li Y, Lin Z, Ling Y, Yunyun C: Effect of the early essential newborn care on the early prognosis of the newborn and the rehabilitation of the parturient. Maternal and Child Health Care of China 2020.
Saxton A, Fahy K, Hastie C. Effects of skin-to-skin contact and breastfeeding at birth on the incidence of PPH: a physiologically based theory. Women and Birth. 2014;27(4):250–3.
Marín Gabriel M, Llana Martín I, López Escobar A, Fernández Villalba E, Romero Blanco I, Touza Pol P. Randomized controlled trial of early skin-to-skin contact: effects on the mother and the newborn. Acta Paediatr. 2010;99(11):1630–4.
Velandia M: Parent-infant skin-to-skin contact studies: Parent-infant interaction and oxytocin levels during skin-to-skin contact after Cesarean section and mother-infant skin-to-skin contact as treatment for breastfeeding problems: Inst för kvinnors och barns hälsa/Dept of Women's and Children's Health; 2012.
Tracy LM, Georgiou-Karistianis N, Gibson SJ, Giummarra MJ. Oxytocin and the modulation of pain experience: implications for chronic pain management. Neuroscience Biobehavioral Reviews. 2015;55:53–67.
Handlin L, Jonas W, Petersson M, Ejdebäck M, Ransjö-Arvidson A-B, Nissen E, Uvnäs-Moberg K. Effects of sucking and skin-to-skin contact on maternal ACTH and cortisol levels during the second day postpartum—influence of epidural analgesia and oxytocin in the perinatal period. Breastfeed Med. 2009;4(4):207–20.
Uvnäs-Moberg K, Arn I, Magnusson D. The psychobiology of emotion: the role of the oxytocinergic system. Int J Behav Med. 2005;12(2):59–65.
Acknowledgements
The authors would like to express their sincere appreciation to all the midwives and participants who contributed to this study.
Funding
None.
Author information
Authors and Affiliations
Contributions
HCY, WYH and LBR designed and conducted the research study. HCY and HL wrote the original manuscript and conceptualized the analysis. HCY and LBR performed the analysis. HL, WYH, LBR and HL, reviewed and contributed to the final draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was part of a larger research trail that was conducted in Chengdu, Sichuan, China. This study was approved by the Ethics Committee of West China Second University Hospital, Sichuan University, and the ethics approval number is 2020 (144). The date of approval was 21 September 2020. All methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, C., Hu, L., Wang, Y. et al. Effectiveness of early essential newborn care on breastfeeding and maternal outcomes: a nonrandomized controlled study. BMC Pregnancy Childbirth 22, 707 (2022). https://doi.org/10.1186/s12884-022-05037-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-022-05037-8