Abstract
Background
Maternal lipid levels in early pregnancy are associated with maternal health and foetal growth. It is however unclear if maternal lipids in early pregnancy can be used to predict childhood lipid levels. The aim of this study is to assess the association between maternal and offspring childhood lipid levels, and to investigate the influence of maternal BMI and diet on these associations.
Methods
This study included 2692 women participating in the Generation R study, an ongoing population-based prospective cohort study from early life onwards. Women with an expected delivery date between 2002 and 2006 living in Rotterdam, the Netherlands were included. Total cholesterol, triglycerides and high-density lipoprotein cholesterol (HDL-c) were measured in early pregnancy (median 13.2 weeks [90% range 10.6; 17.1]). Low-density lipoprotein cholesterol (LDL-c), remnant cholesterol and non-HDL-c were calculated. Corresponding lipid measurements were determined in 2692 children at the age of 6 (median 6.0 years [90% range 5.7; 7.5]) and 1673 children 10 years (median 9.7 years [90% range 9.5; 10.3]). Multivariate linear regression analysis was used to examine the association between maternal lipid levels in early pregnancy and the corresponding childhood lipid measurements at the ages of 6 and 10 years while adjusting for confounders.
Results
Maternal lipid levels in early pregnancy are positively associated with corresponding childhood lipid levels 6 and 10 years after pregnancy, independent of maternal body mass index and diet.
Conclusions
Maternal lipid levels in early pregnancy may provide an insight to the lipid profile of children years later. Gestational lipid levels may therefore be used as an early predictor of children’s long-term health. Monitoring of these gestational lipid levels may give a window-of-opportunity to start early interventions to decrease offspring’s lipid levels and possibly diminish their cardiovascular risk later in life. Future studies are warranted to investigate the genetic contribution on maternal lipid levels in pregnancy and lipid levels of their offspring years later.
Similar content being viewed by others
Background
In pregnancy the maternal metabolism undergoes adaptations to support maternal and foetal demands. Regarding lipoprotein metabolism these changes consist of increased levels of total cholesterol, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and in particular triglycerides during gestation [1]. The prevalence of hyperlipidaemia in pregnancy is still unknown. Maternal lipid levels are mainly determined by genetics and lifestyle factors such as diet and obesity. Maternal lipid levels in early pregnancy are associated with pregnancy complications such as pre-eclampsia and gestational diabetes [2,3,4]. Recently, gestational lipid levels were also found to be associated with metabolic syndrome many years after pregnancy, suggesting that the lipid profile during pregnancy may be used as an early marker of women’s cardiovascular health in later life [5]. The influence of maternal lipid levels in pregnancy on health markers in their children is less studied. High levels of triglycerides and remnant cholesterol in early pregnancy have been associated with adverse neonatal outcomes such as increased risk of a child born large-for-gestational age [6, 7]. In addition, foetuses and children of mothers with elevated cholesterol levels show more and larger fatty streak formation in their aortas, and a more rapid progression to atherosclerosis in early childhood [8, 9]. Balder et al. showed that the prevalence of hypercholesterolemia in children and adolescents of Caucasian descent in the Netherlands is 1:450 [10]. However, it is still unsure whether the maternal lipid profile in pregnancy may also provide a glimpse into long-term cardiovascular health of children. Therefore, the aim of this study was to assess the association between the lipid profile of women in early pregnancy with the lipid profile of children at the age of 6 and 10 years.
It is known that maternal lifestyle factors, such as an unhealthy diet and obesity, are associated with an increased cardiovascular risk in children [11, 12]. We therefore additionally examined the influence of body mass index and diet on the association of maternal lipid levels in pregnancy with corresponding lipid levels in children. Moreover, since lipid levels in children may be affected by foetal programming, we repeated all analyses in women without a placental syndrome (pre-eclampsia, a child born small-for-gestational age and spontaneous preterm birth) to minimize its effect [13].
Methods
Design and study population
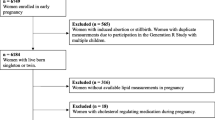
This study was embedded in the Generation R Study, an ongoing population-based prospective cohort study from early pregnancy onward in Rotterdam, the Netherlands [14]. Women enrolled between April 2002 and January 2006. All methods were performed in accordance with the relevant guidelines and regulations. For this study, we included women with a live born singleton and available information on lipid measurements in early pregnancy. We excluded women with a twin pregnancy, (gestational) diabetes mellitus, and women on glucose or lipid regulating medication at enrolment. Children without available lipid measurements at the different time points (6 and 10 years after pregnancy) were also excluded (Fig. 1). Additional file 2 contains a Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for the current study [15].
Ethics approval and consent to participate
The study has been approved by the Medical Ethical Committee of the Erasmus Medical Centre in Rotterdam (MEC-2007-413). For minors, written informed consent from a parent and/or legal guardian was obtained. From the rest of the participants written informed consent was obtained.
Exposure: maternal lipid levels
Non-fasting blood samples were obtained at enrolment in early pregnancy (median 13.2 weeks [90% range 10.6; 17.1]) by trained research nurses to determine the levels of total cholesterol, triglycerides, and high-density lipoprotein cholesterol (HDL-c) [16]. Total cholesterol, triglycerides and HDL-c were analysed using standard laboratory methods. The mean interassay coefficients of variation for total cholesterol, triglycerides, and HDL-c were, respectively, 4.2, 3.4, and 2.8%. Levels of LDL-c were calculated using the Friedewald equation [17]. Non-HDL-c was calculated by subtracting HDL-c from the total cholesterol and remnant cholesterol as ([total cholesterol – LDL-c] – HDL-c). Details of processing procedures and lipid calculations have been described previously [2, 7, 18].
Placental syndrome
Placental syndrome was defined as having pre-eclampsia, a child born small-for-gestational age (SGA) or a spontaneous preterm birth (sPTB) in the index pregnancy. We obtained information on clinically diagnosed pre-eclampsia from medical records that were cross-checked with the original hospital charts [19]. Pre-eclampsia was defined, using the ISSHP criteria that were in effect at the time of the study, as the development of systolic blood pressure (SBP) ≥ 140 mmHg and/or a diastolic blood pressure (DBP) ≥ 90 mmHg with new-onset proteinuria in a random urine sample and no evidence of a urinary tract infection [20]. Midwife and hospital registries provided information on gestational age at birth, birth weight, and child’s sex. SGA was defined as a child with a birth weight below the 10th percentile, adjusted for gestational age and sex of the child. We defined sPTB as the spontaneous onset of labour before 37 weeks of gestation.
Outcome: childhood lipid levels
Non-fasting venous blood samples were collected to measure total cholesterol, triglycerides and HDL-c in the children at the age of 6 (median 6.0 years [90% range 5.7; 7.5]), and 10 years (median 9.7 years [90% range 9.5; 10.3]). Blood samples were obtained, transported, and stored as described in detail previously [16]. Serum concentrations of total cholesterol, triglycerides and HDL-c were measured at the Erasmus Medical Centre with enzymatic methods (Cobas 8000, Roche, Almere, the Netherlands) [16, 21, 22]. Levels of LDL-c, remnant cholesterol and non-HDL-c were calculated [17].
Covariates
Information on maternal characteristics during pregnancy including age, ethnicity, educational level, parity, smoking, and the use of cholesterol or glucose regulating medication, and information on smoking and gravidity 6 and 10 years after pregnancy was obtained through questionnaires. We obtained information on pre-pregnancy weight through questionnaires at study enrolment. Maternal weight (kilograms) and height (centimetres) were measured at enrolment in early pregnancy and 6 and 10 years after pregnancy without shoes and heavy clothing, after which body mass index (BMI) was calculated (kilograms per square meter). Pre-pregnancy weight and measured weight at enrolment were highly correlated (Pearson’s correlation coefficient 0.97 [value of P < .001]), and therefore, pre-pregnancy weight was used to calculate BMI in the analyses. At the age of 6 and 10 years, weight and height were measured without shoes and heavy clothing. We calculated BMI, and categorized children into age- and sex specific groups; normal-weight, overweight and obese, using the definition of Cole et al. [23]. Women’s dietary intake in early pregnancy was assessed using a semi-quantitative 293-item food frequency questionnaire (FFQ) at enrolment. The FFQ included foods that were frequently consumed in the Dutch population and was modified for use during pregnancy. National dietary guidelines were used to develop a predefined diet quality score for pregnant women. The diet score included 15 components in which the scores for the individual components were summed, resulting in an overall score ranging from 0 to 15, with a higher score representing a healthier diet [24].
Statistical analyses
First, we describe pregnancy and follow-up characteristics for all women and children. The mean ± standard deviation is presented for data with a normal distribution and the median with 90% range for data with a skewed distribution. Second, we examined the distribution of early pregnancy lipid levels in women, and in their children at the age of 6 and 10 years. Third, we present the lipid distributions in children stratified on child’s sex. Paired sample t test and Wilcoxon signed rank test were used for comparison of paired samples. Independent samples were tested through Students t test and Mann-Whitney U test. Fourth, triglyceride and remnant cholesterol levels were log transformed to achieve a normal distribution. To enable comparison of effect estimates, we constructed SD-scores (SDS) for all lipid levels [7]. We tested whether the association of maternal lipid levels with corresponding childhood lipid levels was nonlinear. Fifth, since we found a linear relation, we further examined the association between maternal lipid levels in early pregnancy and the corresponding childhood lipid measurements at the age of 6 and 10 years using multiple linear regression models. These models were adjusted for maternal age at enrolment, gestational age at blood sampling, ethnicity, parity, educational level and pre-pregnancy BMI. Sixth, missing data of the covariates were imputed. We used the Markov Chain Monte Carlo multiple imputation procedures to reduce potential bias attributable to missing data [25]. In this study, 1.3% had missing information on ethnicity, 4.8% on educational level, 0.5% on parity, 9.6% on smoking in early pregnancy, 16.6% on pre-pregnancy BMI. For the regression analysis, we used the SDS for all lipid levels as exposure. Regression models adjusted for confounders were: basic model (maternal age at enrolment, gestational age at blood sampling, educational level, ethnicity, parity and smoking), BMI model (basic model additionally adjusted for pre-pregnancy BMI) and maternal diet model (BMI model additionally adjusted for diet score). These confounders were selected based on previous studies, and on their associations with the exposures and outcomes of interest. The effect estimates in Fig. 2 are regression coefficients from the BMI model and represent an increase in the outcome measure for each unit increase in the exposure. Seventh, we tested for unmeasured confounding through the E-value method (Additional file 1, Table S1) [26]. Eight, we tested whether there was effect modification in all associations by sex of the child, through inclusion of the interaction term (exposure * sex of child) in each regression model. Since we found no effect modification for the associations, we did not stratify the results on child’s sex. In addition, child’s sex did not contribute to our models, therefore we chose not to include child’s sex as a confounder. Ninth, in attempt to exclude the effect of placental syndromes, we performed the regression analyses in a subgroup of women without placental syndromes in their index pregnancy. Lastly, for clinical purposes we defined the 90th percentile for maternal gestational lipid levels (total cholesterol, triglycerides, LDL-c, remnant cholesterol and non-HDL-c), whereas for HDL-c we defined the 10th percentile. Thereafter we tested the association of these cut-off values with the corresponding lipid measurements in their children. Statistical analyses were performed with SPSS version 21.0 for Windows (SPSS INC, Chicago, IL, USA).
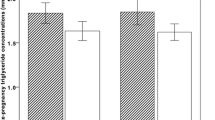
Association of maternal lipid profile in early pregnancy with childhood lipid levels 6 and 10 years after pregnancy. Abbreviations: BMI, body mass index; CI, confidence interval; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol. Data were adjusted for maternal age at enrolment, gestational age at blood sampling, ethnicity, parity, educational level and pre-pregnancy BMI. Values are linear regression coefficients (95% confidence interval)
Results
General characteristics
This study included 2692 mothers with lipids measured in early pregnancy and children with lipids measured at 6 years. Ten years after pregnancy, lipid measurements were available in 1673 children (Fig. 1). Women were on average 30.3 (SD 4.9) years at the start of pregnancy (Table 1). They were mostly European, highly educated and nulliparous. Their pre-pregnancy BMI was 23.7 (90% range 19.5; 32.9) kg/m2 and 26.6% of the women smoked during their pregnancy. At the age of 6 years (median 6.0 years, 90% range 5.7; 7.5) children’s BMI was 15.9 (90% range 14.0; 19.4) and at the age of 10 years (median 9.7 years, 90% range 9.5; 10.3) children’s BMI was 16.9 (90% range 14.4; 22.9).
Maternal and offspring lipid levels
Table 2 shows the lipid profile of women in early pregnancy and the lipid profile of children at the age of 6 and 10 years. BMI, total cholesterol, triglycerides, HDL-c, and remnant cholesterol levels are significantly higher, while LDL-c and non-HDL-c levels are significantly lower at the age of 10 years than at the age of 6 years. Additional file 1, Table S2 shows the lipid distribution in children stratified by child’s sex. At 6 and 10 years, girls have a more atherogenic lipid profile with higher levels of total cholesterol, triglycerides, LDL-c, remnant cholesterol, non-HDL-c, and lower levels of HDL-c than boys.
Association between maternal gestational and offspring lipid levels at 6 and 10 years
Maternal total cholesterol, triglyceride, LDL-c, HDL-c, remnant cholesterol and non-HDL-c levels are all positively associated with the corresponding measurements in children at the age of 6 and 10 years (Fig. 2). These results are independent of maternal pre-pregnancy BMI and diet (Fig. 2 and Table 3). In addition, the results of our regression analyses were not influenced by placental syndrome since the results were similar when the analyses were repeated in a subset of women without placental syndrome in their index pregnancy (n = 2143) (Additional file 1, Table S3). In Additional file 1, Table S4 we show the association of cut-off values of maternal lipid levels in early pregnancy with the corresponding childhood lipid measurements. We found that all levels were positively associated, independent of confounders.
Discussion
This study shows that maternal lipid levels in early pregnancy are positively associated with lipid levels of children at ages 6 and 10 years. These associations were independent of maternal pre-pregnancy BMI and maternal diet.
Previous studies on the associations of lipid levels measured in pregnancy with lipid levels in children are mostly limited to total cholesterol, LDL-c, HDL-c, and triglycerides. This study shows that all measured gestational lipid levels, including remnant cholesterol and non-HDL-c, are positively associated with lipid levels in children, independent of maternal pre-pregnancy BMI and diet. This is in agreement with findings from the Framingham Heart Study cohort demonstrating that elevated maternal LDL-c before pregnancy was correlated to offspring LDL-c in young adulthood (mean age 26 years); while interestingly, paternal LDL-c was not associated with the offspring LDL-c [27]. A study from the Rhea pregnancy cohort also showed a positive association in 348 mother/child pairs between gestational total cholesterol and LDL-c with total cholesterol of children at the age of four, independent of maternal pre-pregnancy BMI [28]. Similarly, a smaller study by Christensen et al. that included women in early pregnancy (gestational week 14 to 16) showed that 27 women with LDL-c levels in the upper percentiles during early gestation had offspring with significantly higher LDL-c levels (0.4 mmol/L) at the age of 6–13 years, compared to 34 women with LDL-c in the lower percentiles [29]. A study by Juhola et al. found a strong relationship between childhood lipid levels and lipid levels measured in middle age [30], which underlines the importance of early markers for the cardiovascular disease risk of children in childhood and thereafter.
Gestational lipids in early pregnancy may be associated with childhood lipids through four potential pathways. First, we hypothesized that lifestyle factors would largely explain the association between gestational lipid levels and lipid levels of children years later. However, the associations between maternal and offspring total cholesterol, triglycerides, LDL-c, HDL-c, remnant cholesterol and non-HDL-c remained significant after adjustment for maternal pre-pregnancy BMI and diet. This suggests that lipid levels may have a certain level of stability; independent of these factors. Therefore, the second pathway we hypothesized on was that genetic inheritance is an important contributor to the association of gestational lipid levels with lipid levels in childhood. In healthy women, LDL-c levels above the 99th percentile have been found to be caused by unfavorable genotypes or mutations causing familiar hypercholesterolemia [31]. In addition, several studies have found specific genes affecting lipid profiles [32,33,34]. A study of Kathiresan et al. found that almost 50% of childhood total cholesterol, triglycerides, LDL-c, and HDL-c levels can be explained by genetic inheritance [35]. Unfortunately, in this study we were not able to test genetic inheritance. However, although our results point towards genetic inheritance as an important contributor, the possible effects of lifestyle cannot be entirely ruled out. As a third pathway, the prenatal environment including nutritional exposures, may also have a strong impact on the epigenome through DNA methylation, which may result in phenotypic consequences in the offspring as shown in animal models and humans [36,37,38,39]. As a fourth pathway, we hypothesized that our findings may be the result of intrauterine programming of the fetus [13], which might be partially mediated by epigenetics. In women with a placental syndrome, insufficient foetal growth and development may occur, resulting in an increased risk of cardiovascular disease later in life [40, 41]. Therefore, we performed the same analyses in a subset of mothers without placental syndromes to see if intrauterine programming may explain our associations, however this did not change our results (Additional file 1, Table S2). We therefore hypothesize that our results may be explained by genetic inheritance and to a lesser extent by lifestyle or pregnancy-related factors.
This study found that girls had a more atherogenic lipid profile than boys, which is in agreement with a large population-based cohort providing reference levels for lipids in children [42]. Notably, in this study the girls had lower levels of HDL-c than boys at the age of 6 and 10 years. A study by Dathan-Stumpf et al. of 2571 children showed a continuous increase in serum HDL-c for both sexes up to the age of 8 years [43]. In addition, boys have a decrease in their HDL-c levels by 10–12 years of age, resulting in higher HDL-c levels in girls than boys from 12 years of age onwards [10]. Interestingly, the sex difference in lipid levels is already found in cord blood since girls have higher levels of total cholesterol and HDL-c in cord blood than boys [44], which may possibly be explained by hormonal influence [45]. Another reason may be a difference in body composition, as we found a higher BMI in girls than boys at the age of 10 years. Girls tend to have more fat mass than boys, which is associated with higher lipid levels [46, 47]. Also, ethnicity could be a possible reason for lipid profile variation between boys and girls, however we found no differences in the distribution of sexes among European and non-European children (data not shown).
Increased levels of total cholesterol, triglycerides, LDL-c, non-HDL-c, and low HDL-c levels are associated with cardiovascular disease and mortality later in life [48]. In order to reduce the risk of cardiovascular disease and its clinical consequences in later life, a low lifetime risk must be achieved by preventing an unfavourable lipid profile and the development of other risk factors from early life onwards [48, 49]. Dietary and lifestyle modifications could bring multiple benefits, including an improved lipid profile [50]. In a large randomized trial, lipid levels of low-risk pregnant women were safely modified through dietary changes from gestational week 17–20 until birth [51]. The PREDIMED trial including 7447 participants (55–80 years of age, without cardiovascular disease) has also shown that following a Mediterranean diet supplemented with consumption of healthy fats from extra-virgin olive oil or nuts, reduces the relative risk of CVD with 30%, compared to a low-fat diet [52]. As dyslipidaemia and obesity often co-exist, dietary interventions during pregnancy may also be beneficial in reducing excessive gestational weight gain [53].
Current guidelines of the American Heart Association and guidelines of the European Heart Association do not recommend to measure lipid levels in early pregnancy [54, 55]. However, based on this study, we suggest that in addition to routine pregnancy care glucose measurements it may be meaningful to measure lipid levels in order to initiate dietary changes if necessary. It may be even more interesting to measure lipid levels preconceptional since the preconceptional period gives opportunities to prevent later risks. This may be beneficial for timely intervention, especially since women who attend preconception care and pregnant women are willing to improve their lifestyle [56, 57]. Lipid levels measured in early pregnancy and subsequent beneficial lifestyle changes may be seen as a window-of-opportunity since lifestyle changes may not only affect pregnancy outcomes, but also future health of women and children.
Strengths and limitations
We had a prospective data collection from early pregnancy onwards and a large sample of 2695 women and children with lipid measurements 6 years after pregnancy, and additional lipid measurements of 1673 children at the age of 10 years. Having two time points of lipid measurements during childhood is unique. In addition to the traditional lipid levels, we also assessed non-HDL-c and remnant cholesterol measures in children, since remnant cholesterol and non-HDL-c have already been proven important risk factors of cardiovascular disease in adults [58, 59]. Blood samples were collected with a minimum fasting time of 30 minutes and are therefore non-fasting. Changes in lipid and lipoprotein levels are considered minimal in response to normal food intake [60, 61]. In addition, lipid levels will also differ between fasting measurements in the same individual [60]. Non-fasting lipid measurements may also be more applicable in clinics since it may be more difficult to measure pregnant women in a fasting state. Regarding dietary assessment, data from the FFQs are self-reported and memory based, and the diet quality score ranging from 0 to 15 is rather rough. However, FFQs are still widely used as the primary dietary assessment tool in epidemiological studies. We tested unmeasured confounding for the regression models, and found that our models are considered rather robust. However, physical activity prior to or in early pregnancy may be considered as a potential confounder. Unfortunately, we did not have information on this variable and therefore did not include this potential confounder. Generalizability of this study may be comprised due to non-response at follow-up and since the women in this study had a relatively low BMI, which indicates selection towards a healthy population. Although we included many lifestyle factors, residual confounding may still be present. Future studies are warranted to investigate the genetic contribution on maternal lipid levels in pregnancy and lipid levels of their offspring years later.
Conclusion
The gestational lipid profile in early pregnancy is associated with the lipid profile of children years after pregnancy, independent of maternal BMI and diet. Monitoring of these gestational lipid levels may give a window-of-opportunity to start early interventions to decrease offspring’s lipid levels and possibly diminish their cardiovascular risk later in life.
Availability of data and materials
Raw data were generated at Generation R. Derived data supporting the findings of this study are available from the secretary of Generation R by sending an email to secretariaat.genr@erasmusmc.nl.
Abbreviations
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular Disease
- DBP:
-
Diastolic blood pressure
- DNA:
-
Deoxyribonucleic Acid
- FFQ:
-
Food frequency questionnaire
- HDL-c:
-
High-density lipoprotein-cholesterol
- LDL-c:
-
Low-density lipoprotein-cholesterol
- PREDIMED trial:
-
Prevención con Dieta Mediterránea trial
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
- SDS:
-
Standard deviation scores
- SGA:
-
Small-for-gestational age
- sPTB:
-
Spontaneous preterm birth
References
Wiznitzer A, Mayer A, Novack V, Sheiner E, Gilutz H, Malhotra A, et al. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol. 2009;201(5):482.e481–8.
Adank MC, Benschop L, Peterbroers KR, Smak Gregoor AM, Kors AW, Mulder MT, et al. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long term postpartum? Am J Obstet Gynecol. 2019;221(2):150.e151–13.
Enquobahrie DA, Williams MA, Butler CL, Frederick IO, Miller RS, Luthy DA. Maternal plasma lipid concentrations in early pregnancy and risk of preeclampsia. Am J Hypertens. 2004;17(7):574–81.
Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG. 2015;122(5):643–51.
Adank MC, Benschop L, van Streun SP, Smak Gregoor AM, Mulder MT, Steegers EAP, et al. Gestational lipid profile as an early marker of metabolic syndrome in later life: a population-based prospective cohort study. BMC Med. 2020;18(1):394.
Vrijkotte TG, Krukziener N, Hutten BA, Vollebregt KC, van Eijsden M, Twickler MB. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J Clin Endocrinol Metab. 2012;97(11):3917–25.
Adank MC, Benschop L, Kors AW, Peterbroers KR, Smak Gregoor AM, Mulder MT, et al. Maternal lipid profile in early pregnancy is associated with foetal growth and the risk of a child born large-for-gestational age: a population-based prospective cohort study : maternal lipid profile in early pregnancy and foetal growth. BMC Med. 2020;18(1):276.
Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100(11):2680–90.
Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999;354(9186):1234–41.
Balder JW, Lansberg PJ, Hof MH, Wiegman A, Hutten BA, Kuivenhoven JA. Pediatric lipid reference values in the general population: the Dutch lifelines cohort study. J Clin Lipidol. 2018;12(5):1208–16.
Lahti-Pulkkinen M, Bhattacharya S, Wild SH, Lindsay RS, Räikkönen K, Norman JE, et al. Consequences of being overweight or obese during pregnancy on diabetes in the offspring: a record linkage study in Aberdeen, Scotland. Diabetologia. 2019;62(8):1412–9.
Estampador AC, Franks PW. Genetic and epigenetic catalysts in early-life programming of adult cardiometabolic disorders. Diabetes Metab Syndr Obes. 2014;7:575–86.
Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93(446):26–33.
Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243–64.
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9.
Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CCW, et al. The Generation R Study: Biobank update 2015. Eur J Epidemiol. 2014;29(12):911–27.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
Benschop L, Bergen NE, Schalekamp-Timmermans S, Jaddoe VWV, Mulder MT, Steegers EAP, et al. Maternal lipid profile 6 years after a gestational hypertensive disorder. J Clin Lipidol. 2018;12(2):428–436 e424.
Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H, Steegers EA. Medical record validation of maternally reported history of preeclampsia. J Clin Epidemiol. 2010;63(8):932–7.
Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20(1):IX–XIV.
Gishti O, Gaillard R, Durmus B, Abrahamse M, van der Beek EM, Hofman A, et al. BMI, total and abdominal fat distribution, and cardiovascular risk factors in school-age children. Pediatr Res. 2015;77(5):710–8.
Erkamp JS, Jaddoe VWV, Mulders AGMGJ, Steegers EAP, Reiss IKM, Duijts L, et al. Customized versus population birth weight charts for identification of newborns at risk of long-term adverse cardio-metabolic and respiratory outcomes: a population-based prospective cohort study. BMC Med. 2019;17(1):186.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240–3.
Nguyen AN, de Barse LM, Tiemeier H, Jaddoe VWV, Franco OH, Jansen PW, et al. Maternal history of eating disorders: diet quality during pregnancy and infant feeding. Appetite. 2017;109:108–14.
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–74.
Mendelson MM, Lyass A, O’Donnell CJ, D’Agostino RB Sr, Levy D. Association of maternal prepregnancy dyslipidemia with adult offspring dyslipidemia in excess of anthropometric, lifestyle, and genetic factors in the Framingham Heart Study. JAMA Cardiol. 2016;1(1):26–35.
Daraki V, Georgiou V, Papavasiliou S, Chalkiadaki G, Karahaliou M, Koinaki S, et al. Metabolic profile in early pregnancy is associated with offspring adiposity at 4 years of age: the Rhea pregnancy cohort Crete, Greece. PLoS One. 2015;10(5):e0126327.
Christensen JJ, Retterstol K, Godang K, Roland MC, Qvigstad E, Bollerslev J, et al. LDL cholesterol in early pregnancy and offspring cardiovascular disease risk factors. J Clin Lipidol. 2016;10(6):1369–1378 e1367.
Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159(4):584–90.
Balder JW, Rimbert A, Zhang X, Viel M, Kanninga R, van Dijk F, et al. Genetics, lifestyle, and low-density lipoprotein cholesterol in young and apparently healthy women. Circulation. 2018;137(8):820–31.
Hamsten A, Iselius L, Dahlen G, de Faire U. Genetic and cultural inheritance of serum lipids, low and high density lipoprotein cholesterol and serum apolipoproteins A-I, A-II and B. Atherosclerosis. 1986;60(3):199–208.
Fuentes RM, Notkola IL, Shemeikka S, Tuomilehto J, Nissinen A. Familial aggregation of serum total cholesterol: a population-based family study in eastern Finland. Prev Med. 2000;31(5):603–7.
Kathiresan S, Musunuru K, Orho-Melander M. Defining the spectrum of alleles that contribute to blood lipid concentrations in humans. Curr Opin Lipidol. 2008;19(2):122–7.
Kathiresan S, Manning AK, Demissie S, D'Agostino RB, Surti A, Guiducci C, et al. A genome-wide association study for blood lipid phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S17.
van Dijk SJ, Tellam RL, Morrison JL, Muhlhausler BS, Molloy PL. Recent developments on the role of epigenetics in obesity and metabolic disease. Clin Epigenetics. 2015;7:66.
Bogdarina I, Haase A, Langley-Evans S, Clark AJ. Glucocorticoid effects on the programming of AT1b angiotensin receptor gene methylation and expression in the rat. PLoS One. 2010;5(2):e9237.
Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping F, et al. A maternal high-fat diet induces DNA methylation changes that contribute to glucose intolerance in offspring. Front Endocrinol (Lausanne). 2019;10:871.
Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun. 2014;5:5592.
Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–68.
Rinaudo PF, Lamb J. Fetal origins of perinatal morbidity and/or adult disease. Semin Reprod Med. 2008;26(5):436–45.
De Henauw S, Michels N, Vyncke K, Hebestreit A, Russo P, Intemann T, et al. Blood lipids among young children in Europe: results from the European IDEFICS study. Int J Obes. 2014;38:S67–75.
Dathan-Stumpf A, Vogel M, Hiemisch A, Thiery J, Burkhardt R, Kratzsch J, et al. Pediatric reference data of serum lipids and prevalence of dyslipidemia: results from a population-based cohort in Germany. Clin Biochem. 2016;49(10–11):740–9.
Kelishadi R, Badiee Z, Adeli K. Cord blood lipid profile and associated factors: baseline data of a birth cohort study. Paediatr Perinat Epidemiol. 2007;21(6):518–24.
Mumford SL, Dasharathy S, Pollack AZ, Schisterman EF. Variations in lipid levels according to menstrual cycle phase: clinical implications. Clin Lipidol. 2011;6(2):225–34.
Choi JW, Pai SH, Kim SK. Associations between total body fat and serum lipid concentrations in obese human adolescents. Ann Clin Lab Sci. 2002;32(3):271–8.
Taylor RW, Gold E, Manning P, Goulding A. Gender differences in body fat content are present well before puberty. Int J Obes. 1997;21(11):1082–4.
Duncan MS, Vasan RS, Xanthakis V. Trajectories of blood lipid concentrations over the adult life course and risk of cardiovascular disease and all-cause mortality: observations from the Framingham study over 35 years. J Am Heart Assoc. 2019;8(11):e011433.
Magnussen CG, Niinikoski H, Juonala M, Kivimäki M, Rönnemaa T, Viikari JS, et al. When and how to start prevention of atherosclerosis? Lessons from the cardiovascular risk in the young Finns study and the special Turku coronary risk factor intervention project. Pediatr Nephrol. 2012;27(9):1441–52.
Mannu GS, Zaman MJ, Gupta A, Rehman HU, Myint PK. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr Cardiol Rev. 2013;9(1):2–14.
Khoury J, Henriksen T, Christophersen B, Tonstad S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomized clinical trial. Am J Obstet Gynecol. 2005;193(4):1292–301.
Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34.
Spencer L, Rollo M, Hauck Y, MacDonald-Wicks L, Wood L, Hutchesson M, et al. The effect of weight management interventions that include a diet component on weight-related outcomes in pregnant and postpartum women: a systematic review protocol. JBI Database System Rev Implement Rep. 2015;13(1):88–98.
Authors/Task Force M, Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 2016;23(11):NP1–NP96.
Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–88.
Lindqvist M, Lindkvist M, Eurenius E, Persson M, Mogren I. Change of lifestyle habits - motivation and ability reported by pregnant women in northern Sweden. Sex Reprod Healthc. 2017;13:83–90.
van der Zee B, de Beaufort I, Temel S, de Wert G, Denktas S, Steegers E. Preconception care: an essential preventive strategy to improve children’s and women’s health. J Public Health Policy. 2011;32(3):367–79.
Robinson JG, Wang S, Jacobson TA. Meta-analysis of comparison of effectiveness of lowering apolipoprotein B versus low-density lipoprotein cholesterol and nonhigh-density lipoprotein cholesterol for cardiovascular risk reduction in randomized trials. Am J Cardiol. 2012;110(10):1468–76.
Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther. 2014;141(3):358–67.
Nordestgaard BG, Langsted A, Mora S, Kolovou G, Baum H, Bruckert E, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37(25):1944–58.
Langsted A, Nordestgaard BG. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology. 2019;51(2):131–41.
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Centre in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR). We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Funding
The financial support for the generation R study was made possible from the Erasmus Medical Centre, Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development, the Netherlands Organization for Scientific Research, the Ministry of Health, Welfare and Sport, and the Ministry of Youth and Families. AK Johansen was funded by the South-Eastern Regional Health Authority, Oslo, Norway; LKL Øyri and KB Holven are funded by the University of Oslo, Norway.
Author information
Authors and Affiliations
Contributions
MC Adank and AK Johansen analysed the data and wrote the article. L Benschop, SP van Streun, AM Smak Gregoor, and LKL Øyri contributed to the design of the paper, analyses and assisted with writing of the article. MT Mulder, EAP Steegers, KB Holven and JE Roeters van Lennep contributed to the design of the paper, writing the article, interpretation of the data, revisions and gave input at all stages of the study. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been approved by the Medical Ethical Committee of the Erasmus Medical Centre in Rotterdam (MEC-2007-413). All methods were performed in accordance with the relevant guidelines and regulations. For minors, written informed consent from a parent and/or legal guardian was obtained. From the rest of the participants written informed consent was obtained.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
E-value of the association of maternal lipid profile in early pregnancy with their corresponding offspring lipid level at the age of 6 and 10 years. Table S2. Lipid profile in boys and girls 6 (n = 2692) and 10 years after pregnancy (n = 1673). Table S3. Association of maternal lipid profile in early pregnancy with their corresponding offspring lipid level at the age of 6 and 10 years in women without a placental syndrome in their index pregnancy. Table S4. Association of cut-off values of the maternal lipid profile in early pregnancy with their corresponding offspring lipid level at the age of 6 and 10 years.
Additional file 2.
STROBE statement.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Adank, M.C., Johansen, A.K., Benschop, L. et al. Maternal lipid levels in early pregnancy as a predictor of childhood lipid levels: a prospective cohort study. BMC Pregnancy Childbirth 22, 588 (2022). https://doi.org/10.1186/s12884-022-04905-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-022-04905-7