Abstract
Background
Endovascular therapy for acute ischemic stroke has been shown to be highly effective in selected patients. However, the ideal criteria for patient selection are still debated. It is well known that collateral flow is an important factor, but the assessment is often subjective and time-consuming. Relative cerebral blood volume (rCBV) is a putative indicator of collateral capacity and can be quickly and easily determined by automated quantitative analysis. We investigated the relationship between rCBV of the affected region and clinical outcome in patients with acute ischemic stroke after endovascular therapy.
Methods
We conducted a retrospective study on consecutive patients between January 2017 and May 2019. Patients with acute ischemic stroke of the anterior circulation who underwent imaging including computed tomography perfusion and were treated with mechanical thrombectomy (MT) were eligible for inclusion. rCBV was calculated automatically with RAPID software by dividing the average cerebral blood volume (CBV) of the affected region (time-to-maximum (Tmax) > 6 s) by the CBV of the unaffected contralateral side. The primary outcome was determined by the modified Rankin Scale (mRS) after 90 days. Good clinical outcome was defined as mRS ≤ 2. We compared means, performed mono- and multivariate logistical regression and calculated a receiver operating characteristic (ROC)-analysis to determine the ideal cutoff value to predict clinical outcomes.
Results
155 patients were enrolled in this study. 66 patients (42.58%) had good clinical outcomes. Higher rCBV was associated with good clinical outcome (p < 0.001), even after adjustment for the patients’ status according to mRS and National Institute of Health Stroke Scale (NIHSS) age and Alberta stroke program early computed tomography score (ASPECTS) at baseline (p = 0.006). ROC-analysis revealed 0.650 (confidence interval: 0.616–0.778) as the optimal cutoff value.
Conclusion
Higher rCBV at baseline is associated with good clinical long-term outcomes in patients with acute ischemic stroke treated by MT. In this study we provide the biggest collective so far that gives evidence that rCBV can be a valuable tool to identify patients who might benefit from MT and are able give a threshold to help to offer patients MT in borderline cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Acute ischemic stroke is one of the most frequent diseases leading to death or permanent functional deficits in industrialized countries and imposes a high economic burden [1, 2]. Because clinical outcomes are highly dependent on the time window between the initial stroke onset and treatment despite the time of onset being frequently unknown, several methods have been established to identify patients who could benefit from treatment [3].
Final infarct extent can be estimated using the Alberta stroke program early computed tomography score (ASPECTS) at time of presentation in non-contrast cranial computed tomography (NCCT) when optimized reconstruction algorithms are used, but it remains more challenging than approaches combining NCCT with contrast media based imaging techniques [4,5,6]. An option based on computed tomography angiography (CTA) is to use visual collateralization scores for which subjective grading must be performed [7, 8]. Another approach is to identify the penumbra and the ischemic core in patients with a large vessel occlusion (LVO) who may benefit from mechanical thrombectomy (MT) using CT perfusion (CTP) [9,10,11,12,13,14,15,16,17,18,19]. CTP can identify acutely hypoperfused regions that appear normal on NCCT, and it is therefore recommended in current guidelines for patients with a prolonged time window or unknown time of symptom onset [20, 21]. CTP as a diagnostic tool to identify salvable tissue is not limited to MT but can also be used for intravenous recombinant tissue plasminogen activator (rt-PA) and therefore has a pivotal role in deciding further treatment at time of presentation [22, 23].
The applicability of CTP is based on its ability to objectively depict local collateralization, thus predicting infarct growth in late presenting patients [24,25,26,27]. Early CTP mismatch volumes depend on the grade of collateralization, exemplified by occlusions of the internal carotid artery being associated with large absolute mismatch volumes [28]. On the other hand, CTP also has a relevant margin of error, possibly due to a lack of discrimination between penumbra and oligemia making it relevant to optimize the use of its parameters [29,30,31,32,33,34]. Several CTP parameters have been identified that can be used to estimate local collateralization and whether local brain tissue can still be rescued [23, 35,36,37,38]. The most relevant to date are cerebral blood volume (CBV), the hypoperfusion intensity ratio (HIR), relative cerebral blood flow (CBF) < 38% of the unaffected contralateral side and relative CBV (rCBV). HIR, defined as a brain volume with a time-to-maximum longer than 10 s over the brain volume with a time-to-maximum longer than 6 s, correlates well with macrovascular occlusions, low blood pressure, deep tissue infarction and functional outcomes [37, 39, 40]. Relative CBF < 38% is a recently discovered indicator of collateral supply, but the data concerning its benefits and limitation is still scarce [37]. CBV is well established and correlates strongly with final infarct volume [15, 23, 24, 41].
rCBV is defined as the average CBV in the time-to-maximum (Tmax) > 6 s region compared to the average CBV in the normal brain, like CBV it strongly correlates with local collateralization and can be measured objectively [23,24,25,26,27,28, 37, 42]. Although CTP can provide information about of the affected brain parenchyma, the ability to correctly identify patients with a good chance of functional improvement is even more important than the correct prediction of infarct volume. rCBV has been shown to predict functional outcomes in the posterior and anterior circulation in relatively small studies [25,26,27, 43, 44]. Because the underlying data are still scarce and therefore the viability of this potentially helpful and objective parameter is unclear, we aim to determine whether rCBV can correctly predict functional outcomes according to the modified Rankin Scale (mRS) after 3 months. Additionally we aim to provide a cutoff value that identifies patients potentially benefitting from MT.
Methods
Study design
This study is designed as a retrospective cohort study and adheres to the respective checklist guidelines for strengthening the reporting of observational studies in epidemiology (STROBE) [45]. This study was approved by the ethics committee of the Technical University Munich in accordance with local and regional law. Written informed consent was waived by the ethics committee of the Technical University Munich due to the retrospective design. Patients with good clinical outcomes were defined as having a score ≤ 2 on the modified Rankin Scale (mRS) after 90 days. The scale ranges from no symptoms (score of 0) to severe disability and death (score of 5 or 6) and a score ≤ 2 means that the patient is still able to look after himself without assistance from others [46, 47]. Patients with poor clinical outcomes were defined as having a mRS score ≥ 3 after 90 days.
Inclusion criteria
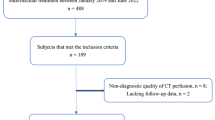
All consecutive patients who were admitted in the university hospital of the Technical University Munich between January 2017 and May 2019 where eligible for study entry if they met all the following inclusion criteria: 1) The patient suffered from acute ischemic stroke. 2) A LVO of the anterior circulation was diagnosed. 3) A baseline CT with CTP was performed. 4) The baseline mRS at presentation in the university hospital was acquired. 5) Treatment was performed via MT including stroke unit care with or without intra-venous thrombolysis (IVT).
Exclusion criteria
Patients were excluded from this study when they did not respond to mRS follow-up after 90 days or if rCBV could not be calculated. Other missing data points did not lead to an exclusion but the missing of the data point was noted. Whenever possible we tried to attain the missing values retrospectively.
Clinical data
The following demographic and clinical data were gathered from our electronic medical records: Biological sex, age and the risk factors for stroke hypertension, hyperlipidemia, diabetes, atrial fibrillation and whether the patient was a current smoker or had previously suffered from a stroke or a transient ischemic attack were collected. Additionally, we noted current treatment with anticoagulants, platelet aggregation inhibitors or statins at the time of presentation and whether the time of the stroke’s onset was known. mRS at presentation and initial severity of the neurological deficit as defined by the National Institute of Health Stroke Scale (NIHSS), a 42 point scale testing for 11 neurological deficits were collected by experienced board-certified neurologists [48]. mRS 3 months after the initial presentation was collected by doctors and nurses from the neurology department who were certified to evaluate mRS score. This was done either during a scheduled visit at the university hospital or by phone interview either by speaking directly to the patient or, if that was not possible, to his or her legal representative. After 3 months routine follow-up ended. Furthermore, CRP and leukocytes at time of presentation were gathered from the laboratory reports. We also gathered whether complications arose during or after treatment, additional intravenous thrombolysis was administered, how many passes were performed during MT and which result according to the modified treatment in cerebral ischemia (TICI) score was achieved [49, 50]. All data points were gathered in a fixed manner in order to reduce selection bias.
Imaging analysis
ASPECTS at baseline, a parameter for initial infarct extension, was scored according to the respective guideline by judging whether the 10 regions of interest (caudate nucleus, putamen, internal capsule, insular cortex and 6 cortical zones in the hypoperfused media territory) where already showing signs of infarction [6]. ASPECTS scoring was performed by board certified neuroradiologists. LVO location was determined based on our CTA at presentation. CTP parameters were calculated using RAPID Software (iSchemaView, Inc., Menlo Park, California). The ischemic territory was identified by the area showing an increased Tmax of more than 6 s. CBV within the ischemic and nonischemic areas were subsequently calculated. In the next step the mean CBV of the affected territory was divided by the mean CBV of the unaffected territory resulting in the rCBV. HIR was calculated as the ratio of brain volume with time-to-maximum > 10 s over brain volume with time-to-maximum > 6 s. Additionally, the volumes of the infarcted core and the penumbra were gathered.
Statistical analysis
All parametric and nonparametric tests were calculated using SPSS version 29. Quantitative data were tested for normality by Shapiro–Wilk and Kolmogorov–Smirnov tests and the variables were expressed as mean ± standard deviation (SD) or median ± interquartile range (IQR). Chi-square tests were used to find or exclude differences for categorial variables. Groupwide analyses were performed using the Mann–Whitney U tests or t-tests when applicable. The limit for significance was set to 0.05 a priori. We correlated clinical outcomes as measured by mRS after 90 days in a multivariate analysis with backward elimination. All confounders potentially affecting the outcome available at the time of presentation with a p-value lower than 0.1 were included and corrected for. The odds ratio (OR) and confidence intervals (CI) were given when applicable. Receiver operating characteristic (ROC)-analysis was performed for the rCBV to provide the cutoff value with the best sensitivity and specificity for good clinical outcomes after 3 months based on mRS.
Results
Basic characteristics of the study population
A total of 230 patients were eligible for study entry. 26 patients did not respond to mRS follow-up after 90 days and were excluded. For 49 patients rCBV could not be determined. Altogether, 155 patients could be included. See Table 1 for demographic, treatment, and outcome data for the groups with good (mRS ≤ 2) or poor (mRS ≥ 3) outcome after 3 months.
The mean age was 75.8 (12.1) year, and 84 (54.2%) were female. Preexisting medical conditions were hypertension in 112 (72.3%), diabetes mellitus in 38 (24.5%), hyperlipidemia in 49 (31.6%), atrial fibrillation in 78 (50.3%) and continued smoking in 15 (9.7%) patients. 24 (15.5%) patients had a history of stroke or a transient ischemic attack. 35 (22.6%) patients were under treatment with anticoagulants, 45 (29.0%) received platelet aggregation inhibitors and 40 (25.8%) were under treatment with statins. Initial INR was 1.10 (± 0.03).
At presentation, the median NIHSS score was 12 (IQR: 12), and the median mRS score was 4 (IQR: 2). The time of symptom onset was unknown in 61 (39.4%) patients. The median baseline ASPECTS was 9 (IQR: 3). The infarcted core measured 21.71 (± 3.04) ml and the penumbra measured 121.38 (± 7.27) ml. rCBV and HIR were 0.72 (± 0.01) and 0.37 (± 0.02) respectively. While mean C-reactive protein (CRP) at presentation was increased to 1.54 (± 0.26) mg/dl, leukocyte counts were normal at 9.4 (± 0.32) per nl. Additional intravenous treatment was administered in 57 (36.8%) patients. Altogether, 137 (88.4%) patients were successfully (mTICI score ≥ 2b) recanalized in a mean of 2.32 (± 0.16) passes. Complications during the interventional treatment occurred in 10 (6.5%) patients. Of those 4 (2.6%) were embolisms in new territories, 2 (1.3%) were larger postinterventional hemorrhages at arterial puncture, 1 (0.6%) was a self-limited subarachnoid bleeding, 1 (0.6%) was a dissection of the distal internal carotid artery, 1 (0.6%) was a sudden cardiac arrest during treatment followed by an unsuccessful resuscitation and 1 (0.6%) was a tearing of the stent retriever resulting in unsuccessful attempts to remove the device. 66 (42.6%) patients had favorable outcomes. 89 (57.4%) patients had poor clinical outcomes and 18 (11.6%) had died during their hospital stay.
Outcome analysis
The groups of patients with good and poor clinical outcomes showed no significant differences for sex (p = 0.219). Medical history of preexisting hypertension (p = 0.068), diabetes mellitus (p = 0.223), atrial fibrillation (p = 0.090), continued smoking (p = 0.339) and a history of stroke (p = 0.132) was not more prevalent in either group. Leukocytes at presentation were not significantly different (p = 0.075). The international normalized ratio (INR) at time of presentation was 1.1 (0.3) for both groups (p = 0.104). There was no significant difference between the groups in terms of the administration of additional intravenous rt-PA (p = 0.104), with 29 (43.9%) and 28 (31.5%) respectively. Treatment with antiplatelet agents (p = 0.488) or statins (p = 0.681) had similar prevalences in both groups. There were no significant differences regarding complications (p = 0.405).
Worse outcome was associated with older age (p < 0.001), current treatment with anticoagulants (p = 0.015), higher CRP levels (p < 0.001) and a need for more passes until successful recanalization (p = 0.017).
In the group with good outcomes, 64 (97.0%) patients were successfully recanalized while the other group had 74 (83.1%) successful recanalization attempts (p < 0.012). Patients with good outcomes were more likely to have a lower NIHSS score at presentation (p < 0.001), a lower mRS score at presentation (p < 0.001), a higher ASPECTS (p = 0.02), a higher rCBV (p < 0.001, see Fig. 1), a smaller infarcted core volume (p < 0.001), a smaller penumbra (p = 0.002) and a lower HIR (p = 0.019).
Comparison of boxplots of mean rCBV between patients with mRS ≤ 2 after 90 days and patients with mRS ≥ 3 after 90 days revealed significant differences (p < 0.001). For mRS scores ≥ 3 are defined as poor outcome. mRS ≥ 3 is interpreted as not being able to look after oneself without the assistance of others. mRS scores ≤ 2 are interpreted as not having to rely on the help of others though deficits may be present, they are defined as good outcomes. rCBV, relative cerebral blood volume; mRS, modified Rankin Scale
Multivariate regression analysis
The multivariate regression analysis adjusted for the potential confounders age, NIHSS, mRS and ASPECTS at time of presentation revealed that higher rCBV was associated with a higher likelihood of a favorable outcome, as indicated by a lower mRS score after 90 days (OR: 0.212; CI: 0.93–0.481, p = 0.006). The calculated odds ratio suggests that a patient with a rCBV of 0.65 and above is almost 5 times more likely to have a good clinical outcome after 3 months than a patient with rCBV below that value.
ROC-analysis
The ROC-analysis yielded 0.650 (CI: 0.616–0.778) as the ideal cutoff value for good clinical outcomes after 3 months (Fig. 2). With this threshold a sensitivity of 86.4% and a specificity of 42.7% was calculated. The ROC-analysis found rCBV to predict mRS at 90 days after LVO very well with an area under the curve of 0.697 (SD: 0.041, p < 0.001).
ROC-curve for rCBV values predicting clinical outcomes according to mRS after 3 months. The rCBV at presentation shows a cutoff value of 0.650 (CI: 0.616–0.778) for good clinical outcome after 3 months. AUC: 0.697 (SD: 0.041, p < 0.001,). The closer AUC is to 1 the better it functions as a classifier. ROC, receiver operating characteristic; mRS, modified Rankin Scale; rCBV, relative blood volume; CI, confidence interval; SD, standard deviation
Discussion
In this study we were able to show that rCBV at the time of presentation can identify patients with good clinical outcomes (mRS ≤ 2) after 3 months and we identified a cutoff value of 0.65.
In addition to the main findings, other factors potentially influencing the outcome were analyzed. We found several parameters not derived from the initial CT associated with worse outcome. Similarly to previous studies older age was linked to poor outcomes, likely due to higher prevalence of comorbidities [51]. We also found the previously published association between having suffered a stroke or a transient ischemic attack prior to the current LVO with remaining dependent on help from others [52]. Receiving treatment with anticoagulants before presentation was also associated with worse outcomes this might be because of underlying preexisting vascular diseases. Patients with increased CRP levels at time of presentation were more commonly seen in patients with worse outcomes. This could be due to negative effects due to inflammatory reaction and is in line with similar findings in the past [53]. Worse functional characteristics at baseline measured by mRS and NIHSS were more likely to depend on help by others after 3 months as expected from previous studies [51, 54]. NIHSS of 5 and below is generally considered to be a minor stroke, median NIHSS for both groups were above that threshold with 7 and 16 points respectively as is expected in LVO [48]. We could confirm that patients in whom a higher degree of recanalization according to the mTICI score was achieved with a lower number of passes are more likely to have favorable outcomes after MT [50, 55].
Other parameters seemingly did not influence the outcome after 3 months. Biological sex had no influence in our collective while its overall influence is still debated [56]. We found no impact of medical history concerning previously diagnosed diabetes mellitus, hyperlipidemia or being a smoker. Having a known history of hypertension or atrial fibrillation were not significantly associated with poor outcomes but showed a trend towards worse mRS after 3 months. Neither receiving treatment with platelet aggregation inhibitors nor with statins before the LVO were associated with outcome. We could not show an influence of additional intravenous rt-PA which is potentially caused by the relatively small number of patients, 43% in the group with favorable outcomes and 32% in the group with poor outcomes, that received rt-PA. This falls in line with studies that did not show inferiority of MT alone against a combined treatment approach [57, 58].
All imaging parameters that were included in our study were associated with clinical outcomes. As expected higher ASPECTS in the initial CT scan, which translates to lower volumes of infarcted brain tissue, was linked to favorable outcome [59]. The CTP derived parameters were linked to outcome in the same way. Larger infarcted core volume, identifying tissue that was not salvable even at the time of the initial CTP, was associated with worse outcomes while larger penumbra, meaning the tissue at risk for infarction that could still recover after blood circulation was restored, was linked to favorable outcome as is consistent with current clinical guidelines [20].
The CTP derived parameters that are considered to depict the collateralization found better local perfusion to be associated with better outcomes. HIR, defined as the volume brain with extremely slow time-to-maximum longer than 10 s compared to the brain volume with a time-to-maximum longer than 6 s, showed the expected inverse correlation [37, 39, 40]. While HIR can identify patients who might benefit from MT it is based solely on relative time to maximum. Consequentially, patients with slower cardiac output or a reduced venous outflow capacity might distort this parameter [26].
Other parameters, not applied in this study, that have been used previously to stratify good and poor collateralization and linked them to outcome can be categorized by their respective way of acquisition: The CTP derived parameters CBV, also combining CBV with ASPECTS, and CBF < 30% of the contralateral side are well established and have shown good predictive performance for final infarct volume and clinical outcomes [10, 13, 14, 16, 18, 32, 35, 41, 60]. A relatively new and promising parameter is CBF < 38% of the unaffected contralateral side which performed better than other CTP derived parameters in identifying good collateral flow in a retrospective study but there is still a paucity of data on whether it also is linked to clinical outcomes [37]. CTA-derived collateral scores can be used as indirect measurements for local collateralization. They can be divided between mono- and multiphase CTA. Several monophase CTA scores have been proposed and correlated to clinical outcomes, but they all share the necessity to visually grade the CTA costing some additional time and being at risk of interrater disagreement [61,62,63,64,65,66]. Multiphase CTA-based scoring is better at predicting final outcomes, but still requires visual grading of the phases while requiring additional scan time and radiation exposure [67, 68]. In the last step before MT is performed angiographic grading systems such as the one proposed by the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) or local time to peak can depict the collateralization, but since they require invasive angiography the decision whether MT is attempted should be made before they can be applied [69,70,71].
rCBV, which was the main parameter of interest in this retrospective study, showed a strong correlation with outcome even after correction for potential confounders. We identified 0.65 as the ideal cutoff value by ROC-analysis. This is consistent with previously published data that proposed similar cutoff values and found higher rCBV to correlate with favorable outcomes and imaging based surrogate parameters [24,25,26,27, 37, 44].
Substantial infarct growth was previously found to be expected with a rCBV of 0.74 and lower while good collateral scores were associated with a rCBV of 0.8 and higher [24, 37]. Additionally, in similar study setups using magnetic resonance imaging-based perfusion at baseline rCBV of 0.8, or 0.85, and higher were associated with good clinical outcome and correlated with blood flow in subsequent digital subtraction angiography [72, 73]. We found that patients with a rCBV of at least 0.65 were almost five times more likely to have a mRS score between 0 and 2 after 3 months, identifying them as very good candidates for MT. Our threshold has a sensitivity of 86.4% and a specificity of 42.7% which means that it is very likely that patients benefitting from MT are likely to be included. The relatively bad specificity can only be tolerated since MT in LVO is a relatively safe treatment [74,75,76,77].
This should encourage treating those patients in situations where there has been no clear recommendation for MT. Considering the overarching trend toward attempting MT in borderline cases with patients having lower ASPECTS or lower NIHSS at presentation while being admitted in prolonged intervals between symptom onset and treatment, rCBV could be applied to simplify and objectify the decision regarding MT in those difficult scenarios [9, 78,79,80,81,82,83]. While in our collective rCBV above a cutoff value of 0.65 was able to predict good clinical outcomes, there is still a lack of data concerning the question whether patients with lower rCBV values may also benefit from MT in a more granular way than mRS after 3 months is able to identify, and consequentially further research is needed limiting the generalizability of this study.
Our study is also limited by its retrospective and monocentric design, making it susceptible to undetected selection bias and an inherent difficulty to control all confounding variables [84]. While we are confident in the overall finding that higher rCBV is associated with good clinical outcome after 3 months a prospective study is needed to prove the applicability of the identified threshold. Another limitation is that 26 patients did not respond to our attempts to determine mRS after 90 days, which could be due to poor outcomes potentially confounding the results. Also, the mRS prior to the current stroke was not acquired systematically which could influence the data by potentially incorrectly associating poor recovery with lower rCBV due to preexisting conditions. In addition, the total group size, albeit larger than in similar studies, is still relatively small which enhances the risk of relatively few data points influencing the overall outcome and potentially changing the results of our ROC-analysis. Another limitation is that perfusion parameters can only depict the small timeframe of during which the scans are performed. Consequently, patients with undulating cerebral perfusion due to arrhythmia or low blood pressure could theoretically influence the results without us having a means to detect them. Thus far this does not seem to be a relevant influence on the CTP.
A strength of the monocentric study design is that all data were acquired within one university hospital therefore we were able to have a highly standardized data acquisition process and homogenous treatment pathways reducing potential bias. Another strength is the relatively large number of patients that could be included in comparison to similar studies which reduces the likelihood of undetected bias. We were able to use the mRS after 90 days as clinical outcome parameter minimizing the influence of short-term effects which can heavily influence the patient's disability in the early post stroke phase [85, 86].
A positive feature of the additional perfusion-derived parameters such as rCBV, especially compared to scores requiring visual grading, is that they can be acquired without causing any additional delays, while also being rater-independent and leading to a clear metric result.
Conclusions
rCBV can identify patients with favorable treatment outcomes after 90 days at the time of initial presentation in the hospital. Therefore, when established clinical and imaging parameters do not provide a clear recommendation towards mechanical recanalization, rCBV can be a valuable tool to aid in the decision-making process to offer MT in otherwise border line cases.
Availability of data and materials
Datasets used and/or analyzed during the current study may be obtained from corresponding authors upon reasonable request.
Abbreviations
- ASITN/SIR:
-
American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology
- ASPECTS:
-
Alberta stroke program early CT score
- CBV:
-
Cerebral blood volume
- CBF:
-
Cerebral blood flow
- CI:
-
Confidence interval
- CTA:
-
Computed tomography angiography
- CTP:
-
Computed tomography perfusion
- HIR:
-
Hypoperfusion intensity ratio
- LVO:
-
Large vessel occlusion
- mRS:
-
Modified Rankin Scale
- MT:
-
Mechanical thrombectomy
- mTICI score:
-
Modified treatment in cerebral infarction score
- NCCT:
-
Non-contrast cranial computed tomography
- NIHSS:
-
National Institute of Health Stroke Scale
- OR:
-
Odds ratio
- rCBV:
-
Relative cerebral blood volume
- rt-PA:
-
Recombinant tissue plasminogen activator
- ROC:
-
Receiver operating characteristic
- STROBE:
-
Strengthening the reporting of observational studies in epidemiology
- Tmax:
-
Time-to-maximum
References
Luengo-Fernandez R, Violato M, Candio P, Leal J. Economic burden of stroke across Europe: A population-based cost analysis. Eur Stroke J. 2020;5(1):17–25.
Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820.
Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CB, Dippel DW, Campbell BC, Nogueira RG, Demchuk AM, Tomasello A, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316(12):1279–88.
Dissaux B. Cheddad El Aouni M, Ognard J, Gentric JC: Model-Based Iterative Reconstruction (MBIR) for ASPECT Scoring in Acute Stroke Patients Selection: Comparison to rCBV and Follow-Up Imaging. Tomography. 2022;8(3):1260–9.
Hopyan J, Ciarallo A, Dowlatshahi D, Howard P, John V, Yeung R, Zhang L, Kim J, MacFarlane G, Lee T-Y, et al. Certainty of Stroke Diagnosis: Incremental Benefit with CT Perfusion over Noncontrast CT and CT Angiography. Radiology. 2010;255(1):142–53.
Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355(9216):1670–4.
Sallustio F, Motta C, Pizzuto S, Diomedi M, Giordano A, D’Agostino VC, Sama D, Mangiafico S, Saia V, Legramante JM, et al. CT angiography-based collateral flow and time to reperfusion are strong predictors of outcome in endovascular treatment of patients with stroke. J Neurointerv Surg. 2017;9(10):940–3.
Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Cloft HJ, Chimowitz MI. Warfarin-Aspirin Symptomatic Intracranial Disease I: Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab. 2011;31(5):1293–301.
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018;378(8):708–18.
Tsogkas I, Knauth M, Schregel K, Behme D, Wasser K, Maier I, Liman J, Psychogios MN. Added value of CT perfusion compared to CT angiography in predicting clinical outcomes of stroke patients treated with mechanical thrombectomy. Eur Radiol. 2016;26(11):4213–9.
Di Giuliano F, Picchi E, Sallustio F, Ferrazzoli V, Alemseged F, Greco L, Minosse S, Da Ros V, Diomedi M, Garaci F, et al. Accuracy of advanced CT imaging in prediction of functional outcome after endovascular treatment in patients with large-vessel occlusion. Neuroradiol J. 2019;32(1):62–70.
Schaefer PW, Roccatagliata L, Ledezma C, Hoh B, Schwamm LH, Koroshetz W, Gonzalez RG, Lev MH. First-pass quantitative CT perfusion identifies thresholds for salvageable penumbra in acute stroke patients treated with intra-arterial therapy. Am J Neuroradiol. 2006;27(1):20–5.
Parsons MW, Pepper EM, Chan V, Siddique S, Rajaratnam S, Bateman GA, Levi CR. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol. 2005;58(5):672–9.
Yoshie T, Ueda T, Takada T, Nogoshi S, Miyashita F, Takaishi S, Fukano T, Tokuura D, Hasegawa Y. Effects of Pretreatment Cerebral Blood Volume and Time to Recanalization on Clinical Outcomes in Endovascular Thrombectomy for Acute Ischemic Stroke. J Stroke Cerebrovasc Dis. 2018;27(7):1802–9.
Murayama K, Katada K, Toyama H, Hayakawa M. Quantitative evaluation of the penumbra and ischemic core in acute cerebral infarction using whole-brain CT perfusion. Neuroradiol J. 2011;24(1):48–58.
Naito Y, Tanaka S, Inoue Y, Ota S, Sakaki S, Kitagaki H. Hyperacute stroke patients and catheter thrombolysis therapy: correlation between computed tomography perfusion maps and final infarction. Radiat Med. 2008;26(4):227–36.
Naylor J, Churilov L, Chen Z, Koome M, Rane N, Campbell BCV. Reliability, Reproducibility and Prognostic Accuracy of the Alberta Stroke Program Early CT Score on CT Perfusion and Non-Contrast CT in Hyperacute Stroke. Cerebrovasc Dis. 2017;44(3–4):195–202.
Mokin M, Morr S, Fanous AA, Shallwani H, Natarajan SK, Levy EI, Snyder KV, Siddiqui AH. Correlation between cerebral blood volume values and outcomes in endovascular therapy for acute ischemic stroke. J Neurointerv Surg. 2015;7(10):705–8.
Rao V, Christensen S, Yennu A, Mlynash M, Zaharchuk G, Heit J, Marks MP, Lansberg MG, Albers GW. Ischemic Core and Hypoperfusion Volumes Correlate With Infarct Size 24 Hours After Randomization in DEFUSE 3. Stroke. 2019;50(3):626–31.
Ringleb P. KM, Jansen O., et al.: Akuttherapie des ischämischen Schlaganfalls, S2e-Leitlinie. Deutsche Gesellschaft für Neurologie 2021.
Parsons MWP. EM; Bateman, GA; Wang, Y; Levi, CR: Identification of the penumbra and infarct core on hyperacute noncontrast and perfusion CT. Neurology. 2007;68(10):730–6.
Silvennoinen HM, Hamberg LM, Lindsberg PJ, Valanne L, Hunter GJ. CT perfusion identifies increased salvage of tissue in patients receiving intravenous recombinant tissue plasminogen activator within 3 hours of stroke onset. AJNR Am J Neuroradiol. 2008;29(6):1118–23.
Rusanen H, Saarinen JT, Sillanpaa N. Collateral Circulation Predicts the Size of the Infarct Core and the Proportion of Salvageable Penumbra in Hyperacute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. Cerebrovasc Dis. 2015;40(3–4):182–90.
MacLellan A, Mlynash M, Kemp S, Ortega-Gutierrez S, Heit JJ, Marks MP, Lansberg MG, Albers GW. DEFUSE 3 Investigator: Perfusion Imaging Collateral Scores Predict Infarct Growth in Non-Reperfused DEFUSE 3 Patients. J Stroke Cerebrovasc Dis. 2022;31(1):106208.
Hirai S, Tanaka Y, Sato H, Kato K, Kim Y, Yamamura T, Sumita K, Arai T. Quantitative collateral assessment evaluated by cerebral blood volume measured by CT perfusion in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2021;30(7): 105797.
Arenillas JF, Cortijo E, Garcia-Bermejo P, Levy EI, Jahan R, Liebeskind D, Goyal M, Saver JL, Albers GW. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J Cereb Blood Flow Metab. 2018;38(10):1839–47.
Rao VL, Mlynash M, Christensen S, Yennu A, Kemp S, Zaharchuk G, Heit JJ, Marks MP, Lansberg MG, Albers GW. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cereb Blood Flow Metab. 2020;40(10):1966–74.
von Baumgarten L, Thierfelder KM, Beyer SE, Baumann AB, Bollwein C, Janssen H, Reiser MF, Straube A, Sommer WH. Early CT perfusion mismatch in acute stroke is not time-dependent but relies on collateralization grade. Neuroradiol. 2016;58(4):357–65.
Geuskens RR, Borst J, Lucas M, Boers AM, Berkhemer OA, Roos YB, van Walderveen MA, Jenniskens SF, van Zwam WH, Dippel DW, et al. Characteristics of Misclassified CT Perfusion Ischemic Core in Patients with Acute Ischemic Stroke. PLoS ONE. 2015;10(11): e0141571.
Alves JE, Carneiro A, Xavier J. Reliability of CT perfusion in the evaluation of the ischaemic penumbra. Neuroradiol J. 2014;27(1):91–5.
Kremenova K, Lukavsky J, Holesta M, Peisker T, Lauer D, Weichet J, Malikova H. CT brain perfusion in the prediction of final infarct volume: a prospective study of different software settings for acute ischemic Core calculation. Diagnostics. 2022;12(10):2290.
Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, Parsons MW. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42(12):3435–40.
Angermaier A, Khaw AV, Kirsch M, Kessler C, Langner S. Influence of Recanalization and Time of Cerebral Ischemia on Tissue Outcome after Endovascular Stroke Treatment on Computed Tomography Perfusion. J Stroke Cerebrovasc Dis. 2015;24(10):2306–12.
Kamalian SK, Konstas AA, Maas MB, Payabvash S, Pomerantz SR, Schaefer PW, Furie KL, González RG, Lev MH. CT perfusion mean transit time maps optimally distinguish benign oligemia from true “at-risk” ischemic penumbra, but thresholds vary by postprocessing technique. Am J Neuroradiol. 2012;33(3):545–9.
Zhang WY, Xiang SF, Yang SJ, Wu YP, Li JT, Liu GK, Li JF, Wang WW. The Application of Computed Tomography Perfusion in the Alberta Stroke Program Early Computed Tomography Score for Endovascular Treatment of Acute Ischemic Stroke in the Anterior Circulation. Int J Gen Med. 2021;14:1865–71.
Haupt W, Meyer L, Wagner M, McDonough R, Elsayed S, Bechstein M, Schön G, Kniep H, Kemmling A, Fiehler J, Hanning U. Assessment of irreversible tissue injury in extensive ischemic stroke—potential of quantitative cerebral perfusion. Translat Stroke Res. 2023;14(4):562–71.
Potreck A, Scheidecker E, Weyland CS, Neuberger U, Herweh C, Möhlenbruch MA, Chen M, Nagel S, Bendszus M, Seker F. RAPID CT perfusion–based relative CBF identifies good collateral status better than hypoperfusion intensity ratio, CBV-index, and time-to-maximum in anterior circulation stroke. Am J Neuroradiol. 2022;43(7):960–5.
Lee JY, Kim SH, Lee MS, Park SH, Lee SS. Prediction of clinical outcome with baseline and 24-hour perfusion CT in acute middle cerebral artery territory ischemic stroke treated with intravenous recanalization therapy. Neuroradiology. 2008;50(5):391–6.
Olivot JM, Mlynash M, Inoue M, Marks MP, Wheeler HM, Kemp S, Straka M, Zaharchuk G, Bammer R, Lansberg MG, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke. 2014;45(4):1018–23.
Lyndon D, van den Broek M, Niu B, Yip S, Rohr A, Settecase F. Hypoperfusion Intensity Ratio Correlates with CTA Collateral Status in Large-Vessel Occlusion Acute Ischemic Stroke. AJNR Am J Neuroradiol. 2021;42(8):1380–6.
Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37(7):1771–7.
Mlynash M, Lansberg MG, Kemp S, Christensen S, Yennu A, Heit JJ, Marks MP, Albers GW. Abstract WP79: Combination of Tmax and Relative CBV Perfusion Parameters More Accurately Predicts CTA Collaterals Than a Single Perfusion Parameter in DEFUSE 3. Stroke. 2019;50(Suppl_1):AWP79.
Karamchandani RS. D; Rhoten, JB; Prasad, T; Selig, J; Defilipp, G; Asimos, AW: Cerebral blood volume index as a predictor of functional independence after basilar artery thrombectomy. J Neuroimaging. 2021;32(1):171–8.
Li BH, Wang JH, Yang S, Wang DZ, Zhang Q, Cheng XD, Yu NW, Guo FQ. Cerebral blood volume index may be a predictor of independent outcome of thrombectomy in stroke patients with low ASPECTS. J Clin Neurosci. 2022;103:188–92.
Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35–53.
Rankin J. Cerebral Vascular Accidents in Patients over the Age of 60: II. Prognosis Scottish Medical Journal. 1957;2(5):200–15.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–7.
Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70.
Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, Marks MP, Prabhakaran S, Kallmes DF, Fitzsimmons BF, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650–63.
Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, Mueller-Kronast N, English JD, Dabus G, Malisch TW, et al. First Pass Effect: A New Measure for Stroke Thrombectomy Devices. Stroke. 2018;49(3):660–6.
Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. German Stroke Study C: Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35(1):158–62.
Kolmos M, Christoffersen L, Kruuse C. Recurrent Ischemic Stroke - A Systematic Review and Meta-Analysis. J Stroke Cerebrovasc Diseases. 2021;30(8)105935.
den Hertog HM, van Rossum JA, van der Worp HB, van Gemert HM, de Jonge R, Koudstaal PJ, Dippel DW. investigators P: C-reactive protein in the very early phase of acute ischemic stroke: association with poor outcome and death. J Neurol. 2009;256(12):2003–8.
Quinn TJ, Taylor-Rowan M, Coyte A, Clark AB, Musgrave SD, Metcalf AK, Day DJ, Bachmann MO, Warburton EA, Potter JF, et al. Pre-Stroke Modified Rankin Scale: Evaluation of Validity, Prognostic Accuracy, and Association with Treatment. Front Neurol. 2017;8:275.
Zaidat O, Suarez JI, Sunshine JL, Tarr RW, Alexander MJ, Smith TP, Enterline DS, Selman WR, Landis DMD. Thrombolytic Therapy of Acute Ischemic Stroke Correlation of Angiographic Recanalization with Clinical Outcom. AJNR Am J Neuroradiol. 2005;26(4):880–4.
Rexrode KM, Madsen TE, Yu AYX, Carcel C, Lichtman JH, Miller EC. The Impact of Sex and Gender on Stroke. Circ Res. 2022;130(4):512–28.
Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, Peng Y, Han H, Wang J, Wang S, et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N Engl J Med. 2020;382(21):1981–93.
Tong X, Wang Y, Fiehler J, Bauer CT, Jia B, Zhang X, Huo X, Luo G, Wang A, Pan Y, et al. Thrombectomy Versus Combined Thrombolysis and Thrombectomy in Patients With Acute Stroke. Stroke. 2021;52(5):1589–600.
Donnelly J, Hong JB, Yong VT, Diprose WK, Caldwell JR, Lee SS, McGuinness BJ, Brew S, Barber PA. Bridging thrombolysis and ASPECTS in patients with stroke treated with endovascular thrombectomy. Stroke Vascular Intervent Neurol. 2023;3(3):e000665.
Lin K, Rapalino O, Lee B, Do KG, Sussmann AR, Law M, Pramanik BK. Correlation of volumetric mismatch and mismatch of Alberta Stroke Program Early CT Scores on CT perfusion maps. Neuroradiology. 2009;51(1):17–23.
Regenhardt RW, González RG, He J, Lev MH, Singhal AB. Symmetric CTA Collaterals Identify Patients with Slow-progressing Stroke Likely to Benefit from Late Thrombectomy. Radiology. 2022;302(2):400–7.
Souza LCS, Yoo AJ, Chaudhry ZA, Payabvash S, Kemmling A, Schaefer PW, Hirsch JA, Furie KL, González RG, Nogueira RG, et al. Malignant CTA Collateral Profile Is Highly Specific for Large Admission DWI Infarct Core and Poor Outcome in Acute Stroke. Am J Neuroradiol. 2012;33(7):1331–6.
Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, Harris GJ, Halpern E, Kemmling A, Koroshetz WJ, et al. Collateral Vessels on CT Angiography Predict Outcome in Acute Ischemic Stroke. Stroke. 2009;40(9):3001–5.
Tan IYL, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, Martin M, Symons SP, Fox AJ, Aviv RI. CT Angiography Clot Burden Score and Collateral Score: Correlation with Clinical and Radiologic Outcomes in Acute Middle Cerebral Artery Infarct. Am J Neuroradiol. 2009;30(3):525–31.
Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-CT and CT-angiography in acute stroke patients. Ann Neurol. 2007;61(6):533–43.
Seker F, Potreck A, Möhlenbruch M, Bendszus M, Pham M. Comparison of four different collateral scores in acute ischemic stroke by CT angiography. J Neurointerv Surg. 2016;8(11):1116–8.
García-Tornel A, Carvalho V, Boned S, Flores A, Rodríguez-Luna D, Pagola J, Muchada M, Sanjuan E, Coscojuela P, Juega J, et al. Improving the Evaluation of Collateral Circulation by Multiphase Computed Tomography Angiography in Acute Stroke Patients Treated with Endovascular Reperfusion Therapies. Interventional Neurology. 2016;5(3–4):209–17.
Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, Goyal M. Multiphase CT Angiography: A New Tool for the Imaging Triage of Patients with Acute Ischemic Stroke. Radiology. 2015;275(2):510–20.
Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, Dillon W, Warach S, Broderick J, Tilley B, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109–137.
Liu L, Ding J, Leng X, Pu Y, Huang LA, Xu A, Wong KSL, Wang X, Wang Y. Guidelines for evaluation and management of cerebral collateral circulation in ischaemic stroke 2017. Stroke Vasc Neurol. 2018;3(3):117–30.
Ji Y, Shi B, Yuan Q, Wu K, Fang J, Wang H, Miao Z, Sun Y, Huang X, Zhou Z. Effect of prolonged microcirculation time after thrombectomy on the outcome of acute stroke. J Neurointerv Surg. 2023;15(11):1078–83.
Park H-I, Cha J-K, Kang M-J, Kim D-H, Yoo N-T, Choi J-H, Huh J-T. Reduced rCBV Ratio in Perfusion-Weighted MR Images Predicts Poor Outcome after Thrombolysis in Acute Ischemic Stroke. Eur Neurol. 2011;65(5):257–63.
Park HS, Cha JK, Kim DH, Kang MJ, Choi JH, Huh JT. The rCBV ratio on perfusion-weighted imaging reveals the extent of blood flow on conventional angiography after acute ischemic stroke. Clin neurol neurosurg. 2014;122:54–8.
Oliveira AJ, Viana SM, Santos AS. Mechanical thrombectomy for acute ischemic stroke: systematic review and meta-analysis. Einstein (São Paulo). 2022;20:eRW6642.
Rodriguez-Calienes A, Galecio-Castillo M, Farooqui M, Hassan AE, Jumaa MA, Divani AA, Ribo M, Abraham M, Petersen NH, Fifi J, et al. Safety Outcomes of Mechanical Thrombectomy Versus Combined Thrombectomy and Intravenous Thrombolysis in Tandem Lesions. Stroke. 2023;54(10):2522–33.
Prajapati C, Huded V, Mahajan N, Kulkarni A. Mechanical Thrombectomy: Review. Ann Indian Acad Neurol. 2022;25(4):606–15.
Amuluru K, Nguyen J, Al-Mufti F, Denardo A, Scott J, Yavagal D, et al. Safety and effectiveness of mechanical thrombectomy for acute ischemic stroke using single plane angiography. J Stroke Cerebrovasc Diseases. 2022;31(8):106553.
Saver JL, Chapot R, Agid R, Hassan A, Jadhav AP, Liebeskind DS, Lobotesis K, Meila D, Meyer L, Raphaeli G, et al. Thrombectomy for Distal, Medium Vessel Occlusions: A Consensus Statement on Present Knowledge and Promising Directions. Stroke. 2020;51(9):2872–84.
Kaesmacher J, Chaloulos-Iakovidis P, Panos L, Mordasini P, Michel P, Hajdu SD, Ribo M, Requena M, Maegerlein C, Friedrich B, et al. Mechanical Thrombectomy in Ischemic Stroke Patients With Alberta Stroke Program Early Computed Tomography Score 0–5. Stroke. 2019;50(4):880–8.
Sarraj A, Hassan AE, Savitz S, Sitton C, Grotta J, Chen P, Cai C, Cutter G, Imam B, Reddy S, et al. Outcomes of Endovascular Thrombectomy vs Medical Management Alone in Patients With Large Ischemic Cores: A Secondary Analysis of the Optimizing Patient’s Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) Study. JAMA Neurol. 2019;76(10):1147–56.
Abbas R, Herial NA, El Naamani K, Sweid A, Weinberg JH, Habashy KJ, Tjoumakaris S, Gooch MR, Rosenwasser RH, Jabbour P. Mechanical thrombectomy in patients presenting with NIHSS score < 6: a safety and efficacy analysis. J Stroke Cerebrovasc Diseases. 2022;31(3):106282.
Volny O, Zerna C, Tomek A, Bar M, Rocek M, Padr R, Cihlar F, Nevsimalova M, Jurak L, Havlicek R, et al. Thrombectomy vs medical management in low NIHSS acute anterior circulation stroke. Neurology. 2020;95(24):e3364–72.
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378(1):11–21.
De Sanctis V, Soliman AT, Daar S, Tzoulis P, Fiscina B, Kattamis C. International Network Of Clinicians For Endocrinopathies In T, Adolescence Medicine Icet A: Retrospective observational studies: Lights and shadows for medical writers. Acta Biomed. 2022;93(5):e2022319.
ElHabr AK, Katz JM, Wang J, Bastani M, Martinez G, Gribko M, Hughes DR, Sanelli P. Predicting 90-day modified Rankin Scale score with discharge information in acute ischaemic stroke patients following treatment. BMJ Neurol Open. 2021;3(1): e000177.
de Havenon A, Tirschwell DL, Heitsch L, Cramer SC, Braun R, Cole J, Reddy V, Majersik JJ, Lindgren A, Worrall BB. Variability of the Modified Rankin Scale Score Between Day 90 and 1 Year After Ischemic Stroke. Neurol Clin Pract. 2021;11(3):e239–44.
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
D. S. created the study concept and design. Data collection was done by M. B., S. W., T. B. and C. M. Data analysis and interpretation were performed by D. S. and M. S. M. S. drafted the manuscript. D. S. and C. Z. revised the article. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of the Technical University Munich in accordance with local and regional law. Written informed consent was waived by the ethics committee of the Technical University Munich due to the retrospective design. The retrospective data collection and all procedures were approved by the ethics committee of the Technical University Munich. All methods were carried out in accordance with relevant guidelines and regulations and the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Strinitz, M., Zimmer, C., Berndt, M. et al. High relative cerebral blood volume is associated with good long term clinical outcomes in acute ischemic stroke: a retrospective cohort study. BMC Neurol 24, 294 (2024). https://doi.org/10.1186/s12883-024-03806-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-024-03806-w