Abstract
Background
Edaravone dexborneol has been reported as an effective neuroprotective agent in the treatment of acute ischemic stroke (AIS). This study aimed at investigating the impact of edaravone dexborneol on functional outcomes and systematic inflammatory response in AIS patient.
Methods
All participants were recruited from the AISRNA study (registered 21/11/2019, NCT04175691 [ClinicalTrials.gov]) between January 2022 and December 2022. The AIS patients were divided into two groups based on whether they received the treatment of edaravone dexborneol (37.5 mg/12 hours, IV) within 48 h after stroke onset. Inflammatory response was determined by detecting levels of cytokines (interleukin-2 [IL-2], IL-4, IL-5, IL-8, IL-6, IL-10, IL-12p70, IL-17, tumor necrosis factor-α [TNF-α], interferon-γ [IFN-γ], IFN-α, and IL-1β) within 14 days after stroke onset.
Results
Eighty-five AIS patients were included from the AISRNA study. Patients treated with edaravone dexborneol showed a significantly higher proportion of modified Rankin Scale score < 2 compared to those who did not receive this treatment (70.7% versus 47.8%; P = 0.031). Furthermore, individuals receiving edaravone dexborneol injection exhibited lower expression levels of interleukin (IL)-1β, IL-6, and IL-17, along with higher levels of IL-4 and IL-10 expression during the acute phase of ischemic stroke (P < 0.05). These trends were not observed for IL-2, IL-5, IL-8, IL-12p70, tumor necrosis factor-α, interferon-γ [IFN-γ], and IFN-α (P > 0.05).
Conclusions
Treatment with edaravone dexborneol resulted in a favorable functional outcome at 90 days post-stroke onset when compared to patients without this intervention; it also suppressed proinflammatory factors expression while increasing anti-inflammatory factors levels.
Trial registration
ClinicalTrials.gov NCT04175691. Registered November 21, 2019, https://www.clinicaltrials.gov/ct2/show/NCT04175691.
Similar content being viewed by others
Background
Stroke is a major cause of acquired adult disability and mortality worldwide [1]. Intravenous thrombolysis and mechanical thrombectomy are currently the two most efficacious therapeutic approaches for acute ischemic stroke (AIS). Despite significant advancements in reperfusion treatment for ischemic stroke, the rate of clinically ineffective reperfusion remains around 50% in AIS patients [2]. Moreover, numerous clinical trials investigating neuroprotective agents have failed to demonstrate any clinical benefits [3]. The SAINT I and II trials suggested that neuroprotective agent NXY-059 was ineffective for AIS treatment within 6 h after stroke onset [4]. Nerinetide was demonstrated to fail to improve functional outcomes after endovascular therapy [5]. The ALIAS trials also showed that 25% albumin (2 g/kg, IV) did not improve 90-day clinical outcomes and increased the incidence of pulmonary edema and intracerebral hemorrhage [6]. Another neuroprotective agent (magnesium sulfate) within 2 h after stroke onset also failed to improve functional outcomes at 90 days [7]. Therefore, there is an urgent need to identify an effective neuroprotective agent that can reduce disability and mortality rates in AIS management.
A novel neuroprotective agent of Edaravone dexborneol could protect against ischemic damage by multifunctional cytoprotective pathways including inflammatory, excitotoxic, oxidative and apoptotic insults [8]. The Treatment of Acute Ischemic Stroke with Edaravone Dexborneol (TASTE) trial, a phase III, randomized, double-blind, parallel, comparative study, enrolled 1200 participants with AIS, which has reported an effective neuroprotective agent of edaravone dexborneol in the improvement of 90-day functional outcomes [9]. Furthermore, the following TASTE-SL trial also showed sublingual edaravone dexborneol achieved a favorable outcome at 90 days in patients with AIS within 48 h [10]. Previous studies have highlighted its involvement in inhibiting pro-inflammatory factors and enhancing blood-brain barrier permeability following ischemic stroke [11, 12]. Additionally, edaravone dexborneol promotes microglial activation towards the M2 phenotype by modulating aryl hydrocarbon receptor expression, thereby exerting anti-inflammatory effects [13]. Therefore, edaravone dexborneol regulated inflammatory response to exert a neuroprotective effect. However, the systemic inflammatory factors (interleukin-2 [IL-2], IL-4, IL-5, IL-8, IL-6, IL-10, IL-12p70, IL-17, tumor necrosis factor-α [TNF-α], interferon-γ [IFN-γ], IFN-α, and IL-1β) associated with edaravone dexborneol treatment in AIS management remains unclear.
In this study, we aimed to investigate the expression profile of systemic inflammatory factors and clinical benefits following edaravone dexborneol treatment in the acute phase of AIS patients.
Materials and methods
Study population
The informed consent was obtained from all individuals. The study protocol adhered to the Declaration of Helsinki and received approval from the Ethics Committee of Nanjing First Hospital, Nanjing Medical University. A total of 85 patients with AIS were prospectively enrolled in an observational study of the AISRNA study (www.clinicaltrials.gov, NCT04175691). All participants were recruited from the Department of Neurology at Nanjing First Hospital, Nanjing Medical University. Inclusion criteria for enrollment included: [1] confirmation of anterior circulation cerebral infarction through non-contrast computer tomography (NCCT) or magnetic resonance imaging (MRI); [2] treatment initiation with edaravone dexborneol within 48 h after stroke onset, or absence of treatment with edaravone dexborneol; [3] age between 18 and 80 years old; and [4] National Institute of Health Stroke Scale (NIHSS) score ranging from 4 to 24. Exclusion criteria consisted of: [1] received the treatment with intravenous thrombolysis or mechanical thrombectomy after stroke onset; [2] presence of infectious diseases upon admission; [3] modified Rankin Scale score > 2 before stroke onset; [4] immunosuppressive therapy or antibiotic treatment within the past four weeks; [5] concurrent malignant tumors or severe organ failure including kidney, liver, and heart failure; [6] dysphagia; [7] lack of informed consent.
Procedures
All patients underwent standardized treatment according to AIS guidelines [14]. The standardized group included antiplatelet aggregation or anticoagulant therapy, statin therapy, and control of risk factors regarding AIS. The treatment group received intravenous infusion of edaravone dexborneol at a dose of 37.5 mg administered by neurological nurses every 12 h for 14 days or hospital discharge and the standardized treatment.
Biomarkers of inflammatory response
Whole blood samples were collected upon admission and subsequently on day 2–3, 5–7, and 10–14. Plasma samples were then extracted and stored at -80℃. The concentrations of various inflammatory factors (IL-2, IL-4, IL-5, IL-8, IL-6, IL-10, IL-12p70, IL-17, TNF-α, IFN-γ, IFN-α, and IL-1β) were systematically measured by the 12-Cytokine Detection Kit (Raisecare, Qingdao, China) and Navios flow cytometer (Beckman Coulter, California, USA) following the manufacturer’s protocol.
Clinical characteristics
Demographics, medical history, stroke severity evaluation by NIHSS score assessment criteria [15] as well as stroke etiology based on Trial of Org 10,172 in acute stroke treatment (TOAST) criteria [16] were prospectively collected. Functional outcomes were assessed by modified Rankin Scale [17]. Table 1 provides detailed baseline characteristics of patients with AIS.
Statistical analysis
Baseline characteristics between the groups were compared by using the SPSS 20.0 system. Continuous variables including inflammatory factors are expressed as the mean ± standard deviation (SD) or median (interquartile range [IQR]), and comparisons were made using t-test or one-way ANOVA if applicable; otherwise, Mann–Whitney U test was used. Categorical variables are presented as frequency (percentage) and compared using chi-square test. A P value less than 0.05 was considered statistically significant.
Results
Baseline characteristics
Among the 978 AIS patients screened from the AISRNA study between January 2022 and December 2022, a total of 893 patients were excluded. The primary exclusions included: intravenous thrombolysis (n = 216), endovascular therapy (n = 136), NIHSS scores < 4 or > 24 (n = 205), posterior circulation infarction (n = 68), and invalid blood samples (n = 126). Therefore, 85 patients (44 patients received the standardized treatment [standardized group] and 41 patients received edaravone dexborneol plus standardized treatment [treatment group]) were included in the final analysis (Fig. 1). No significant differences in baseline characteristics were observed between these two groups (Table 1).
Association of edaravone dexborneol with clinical outcomes
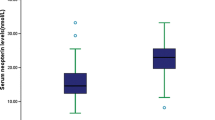
There was no significant difference in NIHSS scores on admission between the treatment group and standardized group (P = 0.826). However, the treatment group exhibited lower NIHSS scores compared to the standardized group on day 10–14 (P = 0.019). There were no significant differences in NIHSS scores on day 2–3 and day 5–7 between these two groups (P > 0.05, Table 2). Additionally, there were 29 (70.7%) patients in the treatment group and 21 (47.8%) patients in the standardized group with mRS score < 2 on day 90 (P = 0.031, Table 2; Fig. 2). However, we did not observe a significant difference between the treatment (n = 33, 80.5%) and standardized groups (n = 35, 79.5%) with mRS score ≤ 2 on day 90 (P = 0.914, Fig. 2).
Dynamic change of inflammatory factors after treatment
Given the role of inflammatory response in acute ischemic stroke [18, 19], we explored the influence of edaravone dexborneol on inflammatory response during the acute phase of ischemic stroke. We collected four samples at different time points: admission, day 2–3, day 5–7, and day 10–14. The results showed no significant differences in 11 inflammatory factors between the two groups upon admission except for IL-4 (P > 0.05, Table 3). However, compared to the standard group, the treatment group had lower levels of IL-1β and IL-17 on day 2–3, and higher levels of IL-4 and IL-10 on day 2–3 (P < 0.05, Fig. 3A, C, G, and I). On day 5–7, high levels of IL-4 and IL-10 were observed in the treatment group (Fig. 3C and G), but IL-6 and IL-17 exhibited an opposite effect in the treatment group (Fig. 3E and I). On day 10–14, we only found decreased levels of IL-6 in the treatment group (P < 0.001, Fig. 3E).
Dynamic change of inflammatory factors after admission in patients with ischemic stroke
Compared to the standard group, the treatment group had lower levels of IL-1β on day 2–3, IL-6 on day 5–7 and day 10–14, and IL-17 on both day 2–3 and day 5–7 (A, E, and I), as well as higher levels of IL-4 on day 2–3 and day 5–7 and higher levels of IL-10 on day 2–3 and day 5–7 (C and G). Other inflammatory factors were no statistical differences between the two groups (B, D, F, H, J, K, and L). IL, interleukin; TNF, tumor necrosis factor; IFN, interferon-γ; D, day. *P < 0.05, *P < 0.01, *P < 0.001
Discussion
The present study of AIS patients from the AISRNA study demonstrated an improvement role of edaravone dexborneol on functional outcome in acute ischemic stroke. Furthermore, edaravone dexborneol inhibited proinflammatory factors expression and increased anti-inflammatory factors levels during the acute phase of ischemic stroke.
Over the past ten years, a series of clinical studies focusing on neuroprotection have failed to show significant benefits for ischemic stroke [4, 7, 20,21,22]. However, a phase III clinical trial has shown that administering edaravone dexborneol within 48 h after stroke onset leads to improved functional outcomes at day 90 compared to edaravone alone [9]. Ischemic stroke results in damage through various pathways in brain ischemia. Previous drugs targeting neuroprotection only interfere with a single mechanism of brain damage. In contrast, edaravone dexborneol protected against ischemic injury by multifunctional cytoprotective pathways including inflammatory, excitotoxic, oxidative and apoptotic insults [8, 9, 23]. This study also demonstrates that edaravone dexborneol improves functional outcomes at day 90 in patients with acute ischemic stroke.
Poststroke inflammatory response is involved in brain injury [24]. Our previous study has shown that inflammatory factors have predictive value for stroke progression in patients undergoing endovascular therapy [25]. Edaravone has been reported to protect endothelial and neuronal cells during brain ischemia through suppressing inflammation and neurotoxicity [26, 27]. Bornel shows potential as a neuroprotective agent by suppressing reactive oxygen species generation and inhibiting adverse inflammatory responses while reducing NO and NO synthase levels [28]. A series of clinical and animal studies have shown that edaravone dexborneol alleviated ischemic brain damage by multiple molecular mechanisms including its effect on inflammatory response [8, 12, 29,30,31,32]. Additionally, a previous study showed that edaravone dexborneol reduced the levels of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in the APP/PS1 mice [33]. Importantly, edaravone dexborneol also suppressed the production of IL-1β, IL-6 and TNF-α and inhibited macrophages polarization to alleviate ischemic injury [34]. Another study demonstrated that edaravone dexborneol facilitated M2 polarization of microglia through inhibiting TL4/NF-κB pathway [35]. Furthermore, edaravone dexborneol exerted neuroprotective effect by inhibiting NF-κB/NLRP3/GSDMD signaling pathway and inflammatory factors (IL-1β and IL-18) in experimental ischemic stroke [32]. These studies suggested that edaravone dexborneol may suppress inflammatory response through regulating macrophages polarization and NLRP3 inflammasome. Our study demonstrated that edaravone dexborneol effectively inhibited proinflammatory factors and increased anti-inflammatory factors during the acute phase of ischemic stroke.
The strengths of our study include the exclusion of interference from intravenous thrombolysis and endovascular therapy on inflammatory response, as well as an opposite effect of edaravone dexborneol on proinflammatory and anti-inflammatory factors during acute phase of ischemic stroke. There are several limitations to acknowledge. Firstly, this study was an observational and single center. Secondly, whole blood samples were collected upon admission, on day 2–3, 5–7, and 10–14. Thus, blood sampling schedule was irregular. Thirdly, we only observed dynamic changes in inflammatory factors during the acute phase without exploring further potential mechanisms underlying the suppression of inflammatory response by edaravone dexborneol. Lastly, some patients received treatment approximately 48 h after stroke onset which may have influenced the levels of inflammatory factors on day 2–3 or day 5–7.
Conclusions
This study demonstrated that the 90-day good functional outcome favored patients treated with edaravone dexborneol who had lower levels of proinflammatory factors and higher levels of anti-inflammatory factors compared to patients without this treatment.
Data availability
All data supporting our findings are available from the corresponding authors upon reasonable.
Abbreviations
- AIS:
-
acute ischemic stroke
- modified Rankin Scale:
-
mRS
- NCCT:
-
non-contrast computer tomography
- MRI:
-
magnetic resonance imaging
- IL:
-
interleukin-2
- TNF:
-
tumor necrosis factor-α
- IFN:
-
interferon-γ
References
Collaborators GBDCoD. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet. 2017;390(10100):1151–210.
Nie X, Leng X, Miao Z, Fisher M, Liu L. Clinically ineffective reperfusion after endovascular therapy in Acute ischemic stroke. Stroke. 2023;54(3):873–81.
Chamorro A, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–81.
Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357(6):562–71.
Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020;395(10227):878–87.
Martin RH, Yeatts SD, Hill MD, Moy CS, Ginsberg MD, Palesch YY, et al. ALIAS (albumin in Acute ischemic stroke) trials: analysis of the Combined Data from Parts 1 and 2. Stroke. 2016;47(9):2355–9.
Saver JL, Starkman S, Eckstein M, Stratton SJ, Pratt FD, Hamilton S, et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med. 2015;372(6):528–36.
Wu HY, Tang Y, Gao LY, Sun WX, Hua Y, Yang SB, et al. The synergetic effect of edaravone and borneol in the rat model of ischemic stroke. Eur J Pharmacol. 2014;740:522–31.
Xu J, Wang A, Meng X, Yalkun G, Xu A, Gao Z, et al. Edaravone Dexborneol Versus Edaravone alone for the treatment of Acute ischemic stroke: a phase III, Randomized, Double-Blind, comparative trial. Stroke. 2021;52(3):772–80.
Fu Y, Wang A, Tang R, Li S, Tian X, Xia X, et al. Sublingual Edaravone Dexborneol for the treatment of Acute ischemic stroke: the TASTE-SL Randomized Clinical Trial. JAMA Neurol. 2024;81(4):319–26.
Xu J, Wang Y, Wang A, Gao Z, Gao X, Chen H, et al. Safety and efficacy of Edaravone Dexborneol versus edaravone for patients with acute ischaemic stroke: a phase II, multicentre, randomised, double-blind, multiple-dose, active-controlled clinical trial. Stroke Vasc Neurol. 2019;4(3):109–14.
Huang Y, Zhang X, Zhang C, Xu W, Li W, Feng Z, et al. Edaravone Dexborneol Downregulates Neutrophil Extracellular Trap expression and ameliorates blood-brain barrier permeability in Acute ischemic stroke. Mediators Inflamm. 2022;2022:3855698.
Li L, He G, Shi M, Zhu J, Cheng Y, Chen Y, et al. Edaravone dexborneol ameliorates cognitive impairment by regulating the NF-kappaB pathway through AHR and promoting microglial polarization towards the M2 phenotype in mice with bilateral carotid artery stenosis (BCAS). Eur J Pharmacol. 2023;957:176036.
Chinese Society of Neurology, Chinese Stroke Society. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. 2018;51(9):666–82.
Brott T, Adams HP Jr., Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–70.
Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–7.
Sun H, Li S, Xu Z, Liu C, Gong P, Deng Q, et al. SNHG15 is a negative regulator of inflammation by mediating TRAF2 ubiquitination in stroke-induced immunosuppression. J Neuroinflammation. 2022;19(1):1.
Li S, Lu G, Wang D, He JL, Zuo L, Wang H, et al. MicroRNA-4443 regulates monocyte activation by targeting tumor necrosis factor receptor associated factor 4 in stroke-induced immunosuppression. Eur J Neurol. 2020;27(8):1625–37.
Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354(6):588–600.
Ginsberg MD, Palesch YY, Hill MD, Martin RH, Moy CS, Barsan WG, et al. High-dose albumin treatment for acute ischaemic stroke (ALIAS) part 2: a randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013;12(11):1049–58.
Elkins J, Veltkamp R, Montaner J, Johnston SC, Singhal AB, Becker K, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16(3):217–26.
Hu X, Qian Z, Chen J, Chen M, Zhong W, Shen C, et al. Effects of edaravone dexborneol on neurological function and serum inflammatory factor levels in patients with acute anterior circulation large vessel occlusion stroke. Transl Neurosci. 2023;14(1):20220312.
Chamorro A, Hallenbeck J. The harms and benefits of inflammatory and immune responses in vascular disease. Stroke. 2006;37(2):291–3.
Deng QW, Huang S, Li S, Zhai Q, Zhang Q, Wang ZJ, et al. Inflammatory factors as potential markers of early neurological deterioration in Acute ischemic stroke patients receiving endovascular therapy - the AISRNA Study. J Inflamm Res. 2021;14:4399–407.
Mizuno A, Umemura K, Nakashima M. Inhibitory effect of MCI-186, a free radical scavenger, on cerebral ischemia following rat middle cerebral artery occlusion. Gen Pharmacol. 1998;30(4):575–8.
Lapchak PA. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin Pharmacother. 2010;11(10):1753–63.
Almeida JR, Souza GR, Silva JC, Saraiva SR, Junior RG, Quintans Jde S, et al. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. ScientificWorldJournal. 2013;2013:808460.
Xi Y, Ma J, Lu S. Favorable neuroprotective effect of intra-arterial application of edaravone dexborneol in ischemic stroke rats. J Stroke Cerebrovasc Dis. 2023;32(11):107356.
Shen G, Lou C, Li Q, Zhao B, Luo Y, Wu F et al. Edaravone dexborneol alleviates cerebral ischemia-reperfusion injury through NF-kappaB/NLRP3 signal pathway. Anat Rec (Hoboken). 2023.
Zhang H, Wang L, Zhu B, Yang Y, Cai C, Wang X, et al. A comparative study of the neuroprotective effects of dl-3-n-butylphthalide and edaravone dexborneol on cerebral ischemic stroke rats. Eur J Pharmacol. 2023;951:175801.
Hu R, Liang J, Ding L, Zhang W, Liu X, Song B et al. Edaravone dexborneol provides neuroprotective benefits by suppressing NLRP3 inflammasome-induced microglial pyroptosis in experimental ischemic stroke. Int Immunopharmacol. 2022;113(Pt A):109315.
Wang J, Du L, Zhang T, Chu Y, Wang Y, Wang Y, et al. Edaravone Dexborneol ameliorates the cognitive deficits of APP/PS1 mice by inhibiting TLR4/MAPK signaling pathway via upregulating TREM2. Neuropharmacology. 2024;255:110006.
Wang D, Wang Y, Shi J, Jiang W, Huang W, Chen K, et al. Edaravone dexborneol alleviates ischemic injury and neuroinflammation by modulating microglial and astrocyte polarization while inhibiting leukocyte infiltration. Int Immunopharmacol. 2024;130:111700.
Huang J, Hu X, Li J, Gong D. Edaravone dexborneol promotes M2 microglia polarization against lipopolysaccharide-induced inflammation via suppressing TLR4/MyD88/NF-kappaB pathway. Naunyn Schmiedebergs Arch Pharmacol. 2024.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Data curation, WXC and HQZ; formal analysis, QWD, ZZL, and WXC; investigation, HQZ and MW; project administration, YZ and YBC; writing—original draft, WXC; writing—review and editing, MW, YBC and YZ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol adhered to the Declaration of Helsinki and received approval from the Ethics Committee of Nanjing First Hospital, Nanjing Medical University (ID: KY20220518-03-KS-01). The informed consent was obtained from all individuals.
Consent to participate
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, W., Zhang, H., Li, Z. et al. Effects of edaravone dexborneol on functional outcome and inflammatory response in patients with acute ischemic stroke. BMC Neurol 24, 209 (2024). https://doi.org/10.1186/s12883-024-03712-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-024-03712-1