Abstract
Background
Rapamycin is an inhibitor of the mechanistic target of rapamycin (mTOR) protein kinase, and preclinical data demonstrate that it is a promising candidate for a general gero- and neuroprotective treatment in humans. Results from mouse models of Alzheimer’s disease have shown beneficial effects of rapamycin, including preventing or reversing cognitive deficits, reducing amyloid oligomers and tauopathies and normalizing synaptic plasticity and cerebral glucose uptake. The “Evaluating Rapamycin Treatment in Alzheimer’s Disease using Positron Emission Tomography” (ERAP) trial aims to test if these results translate to humans through evaluating the change in cerebral glucose uptake following six months of rapamycin treatment in participants with early-stage Alzheimer’s disease.
Methods
ERAP is a six-month-long, single-arm, open-label, phase IIa biomarker-driven study evaluating if the drug rapamycin can be repurposed to treat Alzheimer’s disease. Fifteen patients will be included and treated with a weekly dose of 7 mg rapamycin for six months. The primary endpoint will be change in cerebral glucose uptake, measured using [18F]FDG positron emission tomography. Secondary endpoints include changes in cognitive measures, markers in cerebrospinal fluid as well as cerebral blood flow measured using magnetic resonance imaging. As exploratory outcomes, the study will assess change in multiple age-related pathological processes, such as periodontal inflammation, retinal degeneration, bone mineral density loss, atherosclerosis and decreased cardiac function.
Discussion
The ERAP study is a clinical trial using in vivo imaging biomarkers to assess the repurposing of rapamycin for the treatment of Alzheimer’s disease. If successful, the study would provide a strong rationale for large-scale evaluation of mTOR-inhibitors as a potential disease-modifying treatment in Alzheimer’s disease.
Trial registration
ClinicalTrials.gov ID NCT06022068, date of registration 2023–08-30.
Similar content being viewed by others
Background
For many decades, the “amyloid hypothesis” has been the dominant scientific lead in understanding and treating Alzheimer's disease (AD). Clinical trials that directly target amyloid plaques (such as amyloid antibodies) have however resulted in mixed success [1]. Only recently have two amyloid antibodies been given accelerated approval by the FDA. The drugs are prohibitively priced and questions about their efficacy and safety profile remain [2]. It is therefore crucial to explore new scientific approaches to find an efficient disease-modifying intervention. One such approach is to focus on the single largest risk factor for AD: advancing age.
It is estimated that the risk of developing AD doubles every five years over the age of 65 [3], and the risk of death from AD increases by about 700 times between the ages of 55 and 85 [4]. Within the field of geroscience, which focuses on the biology of aging, an increasing number of interventions have been shown to enhance the lifespan of model organisms and slow down or prevent age-related pathology [5]. One promising approach to understand and treat age-related diseases like AD is to study the effects of such interventions; defined as “geroprotective compounds”, in humans [6]. Pre-clinical data suggest that the drug rapamycin is a promising candidate for this purpose [6, 7].
Rapamycin, also known as sirolimus, is an immunosuppressive drug which has been in clinical use for more than two decades. In mice, treatment with rapamycin increases average lifespan by 10 to 15% [8]. The drug has also been shown to increase healthspan in model organisms by delaying the onset of age-related diseases [9]. For example, preclinical data support a beneficial effect of rapamycin (or its analogues) on periodontitis [10], retinal pathologies [11, 12], atherosclerosis [13, 14]; cardiac dysfunction [15, 16], and bone mass loss [17, 18]. Such diseases are commonly manifested with increasing age and are considered frequent comorbidities to AD [19,20,21,22,23,24,25,26,27,28,29,30,31].
There is a large body of preclinical data suggesting that repurposing rapamycin to treat AD could be effective [6, 7]. In several independent mice models of AD, rapamycin has been shown to prevent and reverse cognitive deficits [32, 33], reduce amyloid oligomers and tauopathies [34, 35], normalize synaptic plasticity [36], cerebral glucose uptake and [33] vascular cognitive impairment [37]. Additionally, in transgenic rodent models of AD, rapamycin has demonstrated neuroprotective effects by restoring blood–brain barrier function [32] and improving neurovascular coupling [38].
Despite promising preclinical data supporting rapamycin as an effective agent in alleviating or reversing AD pathology, no large-scale human clinical studies have been initiated. Currently, only one phase II trial is ongoing (ClinicalTrials.gov ID: NCT04629495).
Conducting randomized controlled trials (RCTs) with symptom ratings (such as cognitive ability) as endpoints is challenging due to the need for large sample sizes and high costs. An alternative approach is to assess the impact of candidate interventions on AD biomarkers before initiating such large-scale RCTs. By focusing on well-established and precise biomarkers of the disease rather than symptom ratings, evidence of slowing or even reversal of pathology can be obtained with much smaller sample sizes [39, 40].
The purpose of the study “Evaluating rapamycin treatment in Alzheimer’s disease using positron emission tomography” (ERAP) is to assess the effect of rapamycin in treating early-stage AD. This will be done by measuring changes in biomarkers using in vivo imaging modalities, such as positron emission tomography (PET) and magnetic resonance imaging (MRI), as well as biomarker changes in cerebrospinal fluid (CSF). We will test the hypothesis that rapamycin can reverse AD-associated brain pathologies, resulting primarily in an increase in neuronal glucose metabolism, and secondarily in an improved cerebral blood flow and a decrease in tau and amyloid protein aggregates in the CSF. We will also record the occurrence of adverse events and investigate pharmacokinetic properties of the drug. Further, we aim to explore the effect of rapamycin on other age-related pathologies in the body using different imaging techniques to assess changes in i) periodontal inflammation, ii) retinal structures, iii) bone mineral density, iv) atherosclerosis, as well as v) cardiac function. The results from this phase IIa trial will be used to inform on the feasibility of conducting a larger controlled trial in the future.
Methods
Study design
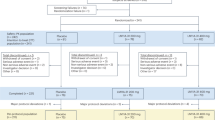
ERAP is a single-centre, open-label, one-arm, phase IIa intervention study. Fifteen patients diagnosed with early-stage AD will be recruited from the Karolinska University Hospital, Medical Unit Aging Memory clinic, located in Solna, Stockholm, Sweden. The unit is a specialized outpatient clinic that examines individuals referred by general practitioners in primary and occupational health care in the northern catchment of Stockholm, as well as individuals younger than 70 years in the entire Stockholm region [41].
Following baseline measurements, all participants will receive a weekly oral dose of 7 mg rapamycin (Tablet Rapamune®) for a duration of six months. Throughout the study, participants will be continuously monitored for safety and adverse events. By the end of the treatment period, follow-up measurements will be conducted. Figure 1 presents a schematic overview of the study timeline for each participant.
Participants
The study will enrol patients with early-stage AD, defined as fulfilling criteria for Alzheimer’s clinical syndrome, with either mild cognitive impairment (MCI) or mild dementia of the Alzheimer's type, according to the NIA-AA (National Institute of Aging-Alzheimer's Association) 2018 criteria [42] (see Table 1 for specific study eligibility criteria).
Study drug
Rapamycin was approved in 1999 in the USA and in 2001 in Europe as an immunosuppressive drug to prevent organ rejection in renal transplantation [43]. The drug and structurally analogous compounds (known as “rapalogs”), such as everolimus, have been approved for the treatment of several solid tumours [44, 45], and is currently the only pharmacological option when treating tuberous sclerosis complex (TSC) [46]. Rapamycin exerts its effect by inhibiting the intracellular protein kinase mTOR, which stands for “mechanistic target of rapamycin”. mTOR has been shown to be central in the regulation of several important functions in mammalian cells, such as cell growth and proliferation, protein synthesis, and autophagy [47].
The bioavailability of orally administered rapamycin is low (approximately 15%) and highly variable (SD = 9%). The drug is metabolized in the liver, primarily by CYP3A4, with a terminal half-life of 62 h, though also here with large interindividual variability (SD = 16 h) [48].
Adverse events, mitigation strategies and dosing
The side effect profile of rapamycin is well known from a large number of clinical trials and from long clinical use. The treatment is generally well tolerated, but common side effects, as described in the product information [43] are; stomatitis, diarrhea, and nausea. Changes in clinical laboratory values observed during rapamycin treatment include increased blood levels of cholesterol and triglycerides, and bone marrow depression manifesting as thrombocytopenia and anemia. The incidence of bacterial infections has been reported as increased in cancer patients treated with rapamycin, along with reports of cases of non-infectious pneumonitis [44].
Notably, the data on side effects is based on the use of rapamycin following organ transplantation, where the drug is commonly used together with other immunosuppressants. In the ERAP trial, we plan to deviate from the standard dosing of rapamycin in two ways. Typically, when used as an immunosuppressant, rapamycin is administered orally at a daily dose of 2 mg or above [43]. We will instead administer an overall lower dose but in an intermittent fashion; a weekly oral dose of 7 mg. This change is aimed at reducing the risk of adverse events. The rationale behind this is that positive effects of rapamycin are hypothesized to be caused by inhibition of the mTOR1 complex, while many of the side effects are hypothesized to be due to inhibition of the mTOR2 complex. While mTOR1 is sensitive to acute dosing treatment, mTOR2 requires sustained exposure of the drug to be effectively inhibited [45].
Patients will be monitored for side effects during the study, including the collection of blood samples at follow-up visits (see Table 2). These samples will be analysed for standard clinical measures, including parameters known to be affected by rapamycin: complete blood count with differential and platelet count, sodium, potassium, chloride, albumin, creatinine, bilirubin, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, glucose, cholesterol, triglycerides, calcium, phosphorus, and creatine phosphokinase.
Blood–brain-barrier passage
The extent to which rapamycin crosses the blood-brain barrier (BBB) in humans has not been thoroughly investigated. Rapamycin is a large molecule (molecular weight 914.2) and a substrate, albeit with low affinity, for the efflux pump P-glycoprotein [46]. Compounds with these properties are often considered unlikely to pass from intestine to blood and bind to an intracellular target [47]. It is however known, from long clinical use, that oral treatment with rapamycin in humans leads to intracellular mTOR inhibition. The molecule's ability to pass through cell membranes , likely facilitated by its high lipophilicity (logP estimated to be 4.3), supports its passage across the BBB despite its size .
After oral administration, detectable levels of rapamycin have been found in the brains of rodents [49, 50], and a large number of studies show clear effects in the central nervous system of animals [7]. Support for cerebral target engagement (i.e. mTOR inhibition) in humans comes from the use of rapamycin as a first-line treatment for the cerebral manifestations of TSC [51]. TSC is a genetic disorder that activates the mTOR pathway, leading to the growth of benign tumors in various organs, including the brain. Inhibition of mTOR with rapamycin analogues is the only approved pharmacological treatment of the disease, and the only feasible mechanism of action is mTOR inhibition in cells behind the BBB.
Visits and data collection
Table 2 and Supplementary Information (Additional file 1) outline the study visits, follow-ups, and corresponding assessments. In brief, participants will be invited to a first screening visit accompanied by a study partner. During this visit, the study will be explained in detail and written informed consent will be obtained. Basic clinical and demographic information will be collected, and the study eligibility criteria will be assessed (see Table 1).
Before initiating the study treatment, the following baseline examinations will be performed: [18F]Fluorodeoxyglucose ([18F]FDG) PET/CT imaging, brain and head MR imaging, cardiological MR imaging, retinal optical coherence tomography, lumbar puncture for collection of a CSF sample, as well as neuropsychological testing and physical aptitude. At the end of the treatment period, the same set of follow-up examinations will be conducted.
Throughout the treatment period, participants will attend three clinical follow-up visits. At every visit, information on side effects will be collected. During the second clinical follow-up, blood samples will be collected at four time points over 48 h to assess the drug’s pharmacokinetic properties (just before and 1,3, and 48 h after intake of the weekly dose). At the third clinical follow-up visit, which will occur after the final dose of the study drug, neuropsychological cognitive tests will be performed and a CSF sample will be collected. Additionally, participants will have at least two scheduled phone calls during the study to assess adverse events or changes in concomitant medications/supplements.
Objectives and endpoints
Table 3 presents the study objectives along with their respective outcomes and endpoints.
Primary objective
The primary objective of ERAP is to evaluate the effect of rapamycin on the progression of early-stage AD. The primary endpoint will be the change in [18F]FDG PET uptake in the cerebral grey matter between baseline and the end of the study. Multiple studies have demonstrated that cerebral glucose metabolism, assessed using [18F]FDG PET, declines progressively with normal aging and is further impaired in AD [52, 53]. Consequently, brain [18F]FDG uptake is commonly utilized as a diagnostic tool for AD and has served as a biomarker for disease progression when assessing the effectiveness of potential AD treatments [54].
Secondary endpoints for assessing treatment efficacy will be change between baseline and end-of-study in cerebral grey matter perfusion (blood-flow) measured by MRI and a pseudo-continuous arterial spin-labeling sequence; CSF levels of amyloid beta 42, phosphorylated tau and total tau; and change in the neuropsychological test the Montreal Cognitive Assessment (MoCA) total score.
Secondary objectives
The safety and tolerability of intermittently dosed rapamycin in early-stage AD will be assessed. We will monitor and record the incidence of treatment-emergent adverse events (AE), severe adverse events (SAE) through clinical follow-up examinations, where vital signs and blood tests will be evaluated (see Supplementary Information 2 and 3 (Additional file 1)).
The pharmacokinetic profile of rapamycin has not been thoroughly studied in the setting of an intermittent dosing scheme. As a secondary objective, we will assess the differences in whole blood concentration of the study drug among individuals by comparing peak (Cmax), trough (Ctrough), and area-under-the-curve (AUC) concentrations. This will also allow us to assess if any potential differences in the treatment effect are associated with drug whole blood concentration among participants.
Exploratory objectives
An exploratory objective of this study is to quantify changes in multiple age-related tissue pathologies before and after rapamycin treatment, using various imaging techniques (see Table 3). If beneficial effects on multiple such pathologies can be shown, it will lend support to the hypothesis that the study drug has a general geroprotective effect in humans.
Exploratory outcomes will include assessments of changes between baseline and end-of-study imaging outcomes, such as retinal nerve fibre layer thickness, periodontal oedema, arterial stiffness and [18F]FDG uptake in arterial plaques, cardiac diastolic function, myocardial strain, cardiac microvascular function, and bone mineral density.
Adverse events
Safety and tolerability will be assessed through monitoring and recording of all adverse events and serious adverse events. Clinically significant deviations in vital signs, laboratory evaluations, and physical examinations will be considered as adverse events and will be followed up. To the extent possible, all adverse events will be described by their severity grade, duration, and relationship to the study drug.
Statistics
Based on the relatively low variability in long-term test–retest data of [18F]FDG in humans [55, 56], a sample size of N = 15 is estimated to be sufficient to detect a 5% change in cerebral grey matter metabolic rate at 80% power with a significance level of 0.05. Such a hypothesized effect size is considered feasible given previous trials of AD using [18F]FDG as an outcome measure [57, 58].
The change in estimated metabolic rate of grey matter [18F]FDG between baseline and follow-up imaging will be assessed using a paired t-test. Additionally, grey matter differences in standardized uptake value ratios, using the cerebellum as a pseudo-reference region (denominator), will be evaluated as a complementary outcome measure of [18F]FDG uptake. The level of significance will be set at 0.05.
Paired t-tests will also be used to assess differences between baseline and end-of-study secondary outcome measures. We will also explore if pharmacokinetic parameters are correlated with 1) each other, 2) side effect burden, 3) treatment effect using linear models.
Ethical and regulatory considerations
The study will be conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation for Good Clinical Practice (ICH GCP E6). The study protocol and relevant documents were approved by the Swedish Medical Products Agency (Läkemedelsverket, number: 5.1–2023-8283), and the Swedish Ethical Review Authority (Etikprövningsmyndigheten, number: 2023–03075-02 and 2023–00611-01), EudraCT number: 2023–000127-36. The trial has been registered at ClinicalTrials.gov (NCT06022068, first release August 30, 2023). Prior to study enrolment, informed consent will be obtained from each participant and their study partner.
Discussion
The ERAP trial is a phase IIa, one-arm, open-label, single-centre study designed to investigate the potential of the drug rapamycin to be repurposed as a treatment for early-stage AD. Repurposing an approved drug for a new indication has the potential to substantially reduce the cost and time of drug development [59]. In the field of AD treatment research, 37% of candidate agents in the pipeline are repurposed drugs [60].
Possible mechanisms of action
Preclinical data suggest that rapamycin may be an effective drug for treating neurodegenerative disorders [6, 7]. Several non-mutually exclusive mechanisms have been hypothesized to underlie this putative effect:
-
1)
Autophagy Regulation: Inhibition of mTOR is known to upregulate cellular macro-autophagy [9]. Deteriorating autophagy and increased mTOR activity have been observed in normal aging and in the progression of AD [61, 62]. Autophagy plays a central role in clearing intracellular toxic aggregate-prone proteins. Stimulation of autophagy by rapamycin could facilitate intercellular clearance of misfolded proteins central to the pathophysiology of AD.
-
2)
Vasculoprotection: Reduced cerebral perfusion and compromised integrity of the blood-brain barrier (BBB) have been suggested as drivers behind AD pathology [63, 64], supported by observations of cerebrovascular dysfunction as one of the earliest detectable changes in AD patients [65]. Rapamycin has been shown to improve cerebral perfusion and BBB integrity in rodent models of AD, supporting the notion of the mTOR pathway as a potential target for brain vasculoprotection in AD [66].
-
3)
Immunomodulation: A sustained activation of microglia and ensuing inflammation is a central feature of neurodegenerative disorders, including in AD [67]. Rapamycin's effect on immune function is complex; while its main clinical use has been as an immunosuppressant, it has also been shown to augment immunity to certain pathogens [68], and improve response to influenza vaccination in elderly individuals [69]. Beneficial immunomodulatory effects could be driven by an increase in T-regulatory (Treg) cell function. Tregs might play an important role in the treatment of AD by suppressing microglia-mediated inflammation [70]. In line with this, a reduction in inflammatory CNS markers has been shown following rapamycin treatment [71], suggesting that this could be a potential mechanism for a treatment effect on AD.
Assessment of general geroprotective properties
In addition to its potential as a treatment for AD, rapamycin has also been hypothesized to have a general geroprotective effect by slowing multiple age-related processes in the human body. In the ERAP trial, we aim to collect data on a wide range of age-related pathological processes using imaging techniques such as PET, MRI, CT, and retinal OCT. If positive changes are observed in multiple outcomes reflecting various age-related pathologies in different organs and tissues, it would support the hypothesis that rapamycin has a general geroprotective effect. The logistics of collecting and quantifying the listed exploratory imaging outcomes in Table 3 are facilitated by the fact that participants are already undergoing whole-body PET/CT examinations and MRI procedures for the trial's primary and secondary endpoints. Adding sequences to quantify potential changes in additional pathologies can therefore be done with acceptable levels of additional discomfort and/or radiation exposure to participants.
Limitations
The main limitations of this study are the absence of a control group, small sample size, and short trial duration. Without a control group, detecting any potential inhibition of AD progression is not possible, and the current design relies on an increase in cerebral glucose metabolism in a relatively short time to demonstrate a positive treatment effect. However, ERAP is a phase IIa trial aimed at generating exploratory data on the effect of rapamycin on AD and assessing the feasibility of conducting a larger, longer and controlled clinical trial using imaging outcomes as endpoints in the future.
Conclusions
The study will measure a set of AD biomarkers before and after a 6-month dosing scheme, with the primary endpoint being change in [18F]FDG PET uptake in the cerebral grey matter, a well-established diagnostic and prognostic biomarker of AD disease progression. The findings from this repurposing effort of rapamycin can provide evidence of a novel treatment alternative for Alzheimer’s disease and form the basis for larger controlled phase IIb or III trials. This study will also investigate the potential general geroprotective effects of rapamycin on various age-related pathologies in the human body.
Availability of data and materials
No datasets were generated or analysed as part of this article. .
References
Ackley SF, Zimmerman SC, Brenowitz WD, Tchetgen Tchetgen EJ, Gold AL, Manly JJ, et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: instrumental variable meta-analysis. BMJ. 2021;372:n156.
Thambisetty M, Howard R. Lecanemab trial in AD brings hope but requires greater clarity. Nat Rev Neurol. 2023;19(3):132–3.
Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu YT, Prina M. World Alzheimer report 2015 - The global impact of dementia. An analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2015.
Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–28.
Nadon NL, Strong R, Miller RA, Harrison DE. NIA interventions testing program: investigating putative aging intervention agents in a genetically heterogeneous mouse model. EBioMedicine. 2017;21:3–4.
Kaeberlein M, Galvan V. Rapamycin and Alzheimer’s disease: Time for a clinical trial? Sci Transl Med. 2019;11(476):eaar428.
Querfurth H, Lee HK. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener. 2021;16(1):44.
Mannick JB, Lamming DW. Targeting the biology of aging with mTOR inhibitors. Nat Aging. 2023;3:642–60.
Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS. Modulating mTOR in Aging and Health. In: Interdisciplinary Topics in Gerontology and Geriatrics. 2015. p. 107–27.
An JY, Quarles EK, Mekvanich S, Kang A, Liu A, Santos D, et al. Rapamycin treatment attenuates age-associated periodontitis in mice. Geroscience. 2017;39:457–63.
Harder JM, Guymer C, Wood JPM, Daskalaki E, Chidlow G, Zhang C, et al. Disturbed glucose and pyruvate metabolism in glaucoma with neuroprotection by pyruvate or rapamycin. Proc Natl Acad Sci. 2020;117(52):33619–27.
Nakahara T, Morita A, Yagasaki R, Mori A, Sakamoto K. Mammalian target of Rapamycin (mTOR) as a potential therapeutic target in pathological ocular angiogenesis. Biol Pharm Bull. 2017;40(12):2045–9.
de Munck DG, de Meyer GRY, Martinet W. Autophagy as an emerging therapeutic target for age-related vascular pathologies. Expert Opin Ther Targets. 2020;24(2):131–45.
Silva AL, Fusco DR, Nga HS, Takase HM, Bravin AM, Contti MM, et al. Effect of sirolimus on carotid atherosclerosis in kidney transplant recipients: data derived from a prospective randomized controlled trial. Clin Kidney J. 2018;11(6):846–52.
Quarles E, Basisty N, Chiao YA, Merrihew G, Gu H, Sweetwyne MT, et al. Rapamycin persistently improves cardiac function in aged, male and female mice, even following cessation of treatment. Aging Cell. 2020;19(2):e13086.
Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy KE, Promislow DEL, et al. A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience. 2017;39(2):117–27.
Luo D, Ren H, Li T, Lian K, Lin D. Rapamycin reduces severity of senile osteoporosis by activating osteocyte autophagy. Osteoporosis Int. 2016;27(3):1093–101.
Wu J, Wang A, Wang X, Li G, Jia P, Shen G, et al. Rapamycin improves bone mass in high-turnover osteoporosis with iron accumulation through positive effects on osteogenesis and angiogenesis. Bone. 2019;121:16–28.
Abbayya K, Chidambar YS, Naduwinmani S, Puthanakar N. Association between periodontitis and alzheimer′s disease. N Am J Med Sci. 2015;7(6):241–6.
Wu H, Qiu W, Zhu X, Li X, Xie Z, Carreras I, et al. The periodontal pathogen fusobacterium nucleatum exacerbates Alzheimer’s pathogenesis via specific pathways. Front Aging Neurosci. 2022;14:14.
Czakó C, Kovács T, Ungvari Z, Csiszar A, Yabluchanskiy A, Conley S, et al. Retinal biomarkers for Alzheimer’s disease and vascular cognitive impairment and dementia (VCID): implication for early diagnosis and prognosis. Geroscience. 2020;42:1499–525.
Mutlu U, Colijn JM, Ikram MA, Bonnemaijer PWM, Licher S, Wolters FJ, et al. Association of retinal neurodegeneration on optical coherence tomography with dementia: a population-based study. JAMA Neurol. 2018;75(10):1256–63.
Tini G, Scagliola R, Monacelli F, La Malfa G, Porto I, Brunelli C, et al. Alzheimer’s disease and cardiovascular disease: a particular association. Cardiol Res Pract. 2020;2020:1–10.
Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and alzheimer disease. Arch Intern Med. 2006;166(9):1003–8.
Hughes TM, Craft S, Lopez OL. Review of ‘the potential role of arterial stiffness in the pathogenesis of Alzheimer’s disease.’ Neurodegener Dis Manag. 2015;5(2):121–35.
Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes? J Neurol Sci. 2012;322(1–2):141–7.
Peng TC, Chen WL, Wu LW, Chang YW, Kao TW. Sarcopenia and cognitive impairment: a systematic review and meta-analysis. Clin Nutr. 2020;39(9):2695–701.
Waite SJ, Maitland S, Thomas A, Yarnall AJ. Sarcopenia and frailty in individuals with dementia: a systematic review. Arch Gerontol Geriatr. 2021;92:104268.
Lary CW, Ghatan S, Gerety M, Hinton A, Nagarajan A, Rosen C, et al. Bone mineral density and the risk of incident dementia: a meta-analysis. J Am Geriatr Soc. 2023;72(1):194–200.
Bliuc D, Tran T, Adachi JD, Atkins GJ, Berger C, van den Bergh J, et al. Cognitive decline is associated with an accelerated rate of bone loss and increased fracture risk in women: a prospective study from the Canadian Multicentre osteoporosis study. J Bone Miner Res. 2021;36(11):2106–15.
Sui SX, Balanta-Melo J, Pasco JA, Plotkin LI. Musculoskeletal deficits and cognitive impairment: epidemiological evidence and biological mechanisms. Curr Osteoporos Rep. 2022;20(5):260–72.
Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, et al. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am J Physiol Heart Circ Physiol. 2018;314(4):693–703.
Lin AL, Jahrling JB, Zhang W, DeRosa N, Bakshi V, Romero P, et al. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J Cereb Blood Flow Metab. 2017;37(1):217–26.
Jiang T, Yu JT, Zhu XC, Zhang QQ, Cao L, Wang HF, et al. Temsirolimus attenuates tauopathy in vitro and in vivo by targeting tau hyperphosphorylation and autophagic clearance. Neuropharmacology. 2014;85:121–30.
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of Rapamycin (mTOR), amyloid-beta and tau: effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–20.
Wang H, Fu J, Xu X, Yang Z, Zhang T. Rapamycin activates Mitophagy and alleviates cognitive and synaptic plasticity deficits in a mouse model of Alzheimer’s Disease. J Gerontol. 2021;76(10):1707–13.
Jahrling JB, Lin AL, DeRosa N, Hussong SA, Van Skike CE, Girotti M, et al. mTOR drives cerebral blood flow and memory deficits in LDLR–/– mice modeling atherosclerosis and vascular cognitive impairment. J Cereb Blood Flow Metab. 2018;38(1):58–74.
Van Skike CE, Hussong SA, Hernandez SF, Banh AQ, DeRosa N, Galvan V. mTOR attenuation with rapamycin reverses neurovascular uncoupling and memory deficits in mice modeling Alzheimer’s disease. J Neurosci. 2021;41(19):4305–20.
Hampel H, Wilcock G, Andrieu S, Aisen P, Blennow K, Broich K, et al. Biomarkers for Alzheimer’s disease therapeutic trials. Prog Neurobiol. 2011;95(4):579–93.
Herholz K. Use of FDG PET as an imaging biomarker in clinical trials of Alzheimer’s disease. Biomark Med. 2012;6(4):431–9.
Rosenberg A, Öhlund-Wistbacka U, Hall A, Bonnard A, Hagman G, Rydén M, et al. β-Amyloid, Tau, neurodegeneration classification and eligibility for anti-amyloid treatment in a memory clinic population. Neurology. 2022;99(19):2102–13.
Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018;14(4):535–62.
Pfizer. Sirolimus (RAPAMUNE)[package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021083s059,021110s076lbl.pdf. Accessed 29 Nov 2023.
Sankhala K, Mita A, Kelly K, Mahalingam D, Giles F, Mita M. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Target Oncol. 2009;4(2):135–42.
Lamming DW. Rapamycin and rapalogs. In: Anti-aging pharmacology. Academic Press; Cambridge, Massachusetts: 2023. p. 89–118.
Yacyshyn BR, Bowen-Yacyshyn MB, Pilarski LM. Inhibition by rapamycin of p-glycoprotein 170-mediated export from normal lymphocytes. Scand J Immunol. 1996;43(4):449–55.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26.
Moes DJAR, Guchelaar HJ, de Fijter JW. Sirolimus and everolimus in kidney transplantation. Drug Discov Today. 2015;20(10):1243–9.
Serkova N, Jacobsen W, Niemann CU, Litt L, Benet LZ, Leibfritz D, et al. Sirolimus, but not the structurally related RAD (everolimus), enhances the negative effects of cyclosporine on mitochondrial metabolism in the rat brain. Br J Pharmacol. 2001;133(6):875–85.
Gottschalk S, Cummins CL, Leibfritz D, Christians U, Benet LZ, Serkova NJ. Age and sex differences in the effects of the immunosuppressants cyclosporine, sirolimus and everolimus on rat brain metabolism. Neurotoxicology. 2011;32(1):50–7.
Northrup H, Aronow ME, Bebin EM, Bissler J, Darling TN, de Vries PJ et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr Neurol. 2021;123:50–66.
Jiang J, Sun Y, Zhou H, Li S, Huang Z, Wu P, et al. Study of the influence of age in 18F-FDG PET images using a data-driven approach and its evaluation in Alzheimer’s disease. Contrast Media Mol Imaging. 2018;2018:3786083.
Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–18.
Chételat G, Arbizu J, Barthel H, Garibotto V, Law I, Morbelli S, et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020;19(11):951–62.
Fällmar D, Lilja J, Kilander L, Danfors T, Lubberink M, Larsson EM, et al. Validation of true low-dose 18F-FDG PET of the brain. Am J Nucl Med Mol Imaging. 2016;6(5):269–76.
Schaefer SM, Abercrombie HC, Lindgren KA, Larson CL, Ward RT, Oakes TR, et al. Six-month test-retest reliability of MRI-defined PET measures of regional cerebral glucose metabolic rate in selected subcortical structures. Hum Brain Mapp. 2000;10(1):1–9.
Gejl M, Gjedde A, Egefjord L, Møller A, Hansen SB, Vang K, et al. In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front Aging Neurosci. 2016;8:108.
Kadir A, Andreasen N, Almkvist O, Wall A, Forsberg A, Engler H, et al. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer’s disease. Ann Neurol. 2008;63(5):621–31.
Cummings J, Lee G, Nahed P, Kambar MEZN, Zhong K, Fonseca J, et al. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement. 2022;8(1):e12295.
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58.
Oddo S. The role of mTOR signaling in Alzheimer disease. Front Biosci. 2012;4:941.
Karabiyik C, Frake RA, Park SJ, Pavel M, Rubinsztein DC. Autophagy in ageing and ageing-related neurodegenerative diseases. Ageing Neurodegener Dis. 2021;1(2).
Bv Z. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–38.
Galvan V, Hart MJ. Vascular mTOR-dependent mechanisms linking the control of aging to Alzheimer’s disease. Biochim et Biophys Acta (BBA) - Mol Basis Disease. 2016;1862(5):992–1007.
Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC, Weiner MW, et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun. 2016;7(1):11934.
van Skike CE, Galvan VA. A rerfect sTORm: The role of the mammalian target of rapamycin (mTOR) in cerebrovascular dysfunction of Alzheimer’s disease: a mini-review. Gerontology. 2018;64(3):205–11.
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y). 2018;4(1):575–90.
Wolf S, Hoffmann VS, Sommer F, Schrempf M, Li M, Ryll M, et al. Effect of Sirolimus vs. Everolimus on CMV-Infections after kidney transplantation—a network meta-analysis. J Clin Med. 2022;11(14): 4216.
Mannick J, Giuseppe DG, Maria L, VN M, Jens P, Baisong H et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6(268).
Faridar A, Thome AD, Zhao W, Thonhoff JR, Beers DR, Pascual B, et al. Restoring regulatory T-cell dysfunction in Alzheimer’s disease through ex vivo expansion. Brain Commun. 2020;2(2):fcaa112.
Richardson A, Galvan V, Lin AL, Oddo S. How longevity research can lead to therapies for Alzheimer’s disease: The rapamycin story. Exp Gerontol. 2015;68:51–8.
Acknowledgements
We would like to thank Edvin Johansson, Martin Schain and Lars Johansson from Antaros Medical for their assistance with the design of MRI and PET imaging protocols. We would like to thank Lars Farde for his mentorship and valuable feedback on the study design.
Funding
Open access funding provided by Karolinska Institute. The study is supported by a Longevity Impetus grant from the Norn Group, Åhlén Stiftelsen, Demensfonden, The Swedish Society of Medicine (SLS), Loo and Hans Osterman Stiftelse, Stiftelsen för Ålderssjukdomar Karolinska Institutet, Stiftelsen för Gamla Tjänarinnor, Tore Nilssons Stiftelse för Medicinsk Forskning, Stiftelsen Stockholms Sjukhem, Region Stockholm (ALF grant), The Swedish Brain Foundation (PS2021-0012), and KI CIMED. None of the funding bodies had any role in the design of the study or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the development and/or the preparation of the study protocol; PPS and JS are responsible for conception and overall design of the study and obtained funding. PPS and JS prepared the first draft of the manuscript; all authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol and relevant documents were approved following review by the Swedish Medical Products Agency (Läkemedelsverket, number: 5.1-2023-8283), and the Swedish Ethical Review Authority (Etikprövningsmyndigheten, number: 2023-03075-02 and 2023-00611-01), EudraCT number: 2023-000127-36. The trial has been registered at ClinicalTrials.gov (NCT06022068, first released on August 30, 2023). Prior to study enrolment, informed consent will be obtained from each participant and their study partner.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary information 1. Expanding on information, data and outcomes collected at visits and follow-ups. Supplementary information 2. Expanding on procedures for measurements of endpoints. Supplementary information 3. Expanding on monitoring and handling of adverse events.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Svensson, J.E., Bolin, M., Thor, D. et al. Evaluating the effect of rapamycin treatment in Alzheimer’s disease and aging using in vivo imaging: the ERAP phase IIa clinical study protocol. BMC Neurol 24, 111 (2024). https://doi.org/10.1186/s12883-024-03596-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-024-03596-1