Abstract
Background
Patients with severe thalassemia may experience adverse effects from transfusion such as fever, rash, and iron overload after long-term transfusion therapy. Severe headaches as a side effect of blood transfusion in patients with thalassemia are not commonly observed, especially when combined with superficial siderosis of the central nervous system, which is easily misdiagnosed and requires excessive examination and treatment.
Case Presentation
A 31-year-old woman was admitted with severe headache and vomiting over 3 days following blood transfusion. She was diagnosed with intermediate α-thalassemia at 2 years of age and had a history of irregular blood transfusions. Physical examination revealed horizontal nystagmus with no other abnormal neurological signs. Magnetic resonance (MR) imaging, MR venography, MR arteriography, and cerebrospinal fluid analysis were normal. However, susceptibility-weighted imaging showed abnormal signals in the bilateral and fourth ventricles. Initial antibiotics, antivirals, decompression of intracranial pressure, iron chelation, and symptomatic treatments were administered; subsequently, small intermittent blood transfusions were cautiously administered for severe anemia. The patient’s headache was gradually relieved, and she was discharged on day 9. At the 5-month follow-up, the patient’s headache recurred following another transfusion.
Conclusions
Severe post-transfusion headache in patients with thalassemia has not been fully recognized and is easily misdiagnosed, leading to excessive examination and treatment. Understanding the clinical features of transfusion-related headaches can help identify this complication, but the exact pathophysiological mechanism requires further research.
Similar content being viewed by others
Background
Thalassemia is a hemolytic anemia in which normal adult-type hemoglobin synthesis is reduced owing to a defect in one or more peptide-chain genes, leading to impaired biosynthesis of the peptide chain [1]. Thalassemia is a hereditary hematological disorder caused by gene mutations or deletions, resulting in disorders of globin synthesis. Thalassemia can be divided into α and β types according to different peptide-chain synthesis disorders and further divided into mild, intermediate, and severe [1, 2]. The primary characteristic of thalassemia is the impaired production of hemoglobin, leading to a decrease in the number of red blood cells (RBCs), abnormally shaped RBCs, and shortened RBC lifespan, resulting in anemia. Due to the extensive destruction of RBCs, patients with Mediterranean anemia often suffer from severe anemia. RBC transfusion therapy is often required to correct anemia. After receiving RBC transfusion, certain patients may experience headaches, possibly associated with physiological changes during the transfusion process. However, there is insufficient comprehensive data and understanding of the related mechanisms. Severe headache as a post-transfusion complication in patients with thalassemia is not well known to clinicians, especially when combined with cerebral superficial siderosis (CSS), which is easily misdiagnosed in patients with thalassemia.
Hemoglobin disorders present a significant health problem in 71% of 229 countries, with this 71% including 89% of all births worldwide. Over 330,000 infants born annually are affected, with 17% affected by thalassemia [3]. Thalassemia is mainly concentrated in tropical and subtropical regions and is prevalent on the Mediterranean coast, North Africa, Southeast Asia, and the Indian subcontinent. The incidence of thalassemia is also high in southwestern and southern China [4]. Blood transfusion is an important treatment for severe thalassemia. Blood transfusion-related central nervous system (CNS) complications (e.g., headache, cognitive impairment, and cerebral hemorrhage [5,6,7]) are rare in patients with thalassemia and must be distinguished from migraine, intracranial infections, and intracranial venous sinus thrombosis. To the best of our knowledge, there have been no reports of patients with thalassemia with CSS developing severe headaches after transfusion.
Here, we present a case report demonstrating this scenario. We outline the implications of the case study, summarize the clinical characteristics, and analyze the possible mechanisms of transfusion-related CNS complications to improve our understanding and management of rare transfusion-related neurological complications.
Case presentation
A 31-year-old woman was admitted owing to severe headache and vomiting lasting 1 day in July 2021. The patient had received a blood transfusion 5 days before headache onset. On the morning of admission, she developed an explosive headache, which worsened when sitting or standing, accompanied by dizziness, vomiting, blurred vision, and waist and leg pain. On the day of headache onset, emergency brain computed tomography (CT) showed no obvious abnormalities (Fig. 1A). The patient was previously diagnosed with intermediate α-thalassemia by genetic testing at 2 years of age, after which she received irregular blood transfusions. Transfusion of 2 units of packed RBCs every half a year in 2016, transfusion of 2 units of packed RBCs every 3 months in 2017, and transfusion of 4 units of packed RBCs every 3 months in 2018 were administered owing to anemia. Changes in hemoglobin levels are shown in Fig. 2. A year before admission, liver and cardiac magnetic resonance imaging (MRI) indicated normal cardiac iron levels but revealed severe iron overload in the liver. The patient had been consistently taking deferasirox (1000 mg/day) since 2018, up to the current hospital admission. Changes in ferritin levels are shown in Fig. 3. The patient underwent a splenectomy for thalassemia. She had a history of cholecystectomy, hepatitis, and induced abortion.
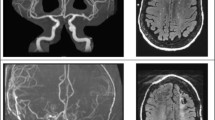
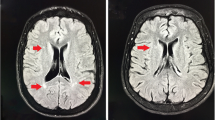
The patient’s CT and MR imaging findings. The initial brain CT (A), brain MR imaging (B and C, T1-WI; D, T2-WI; E and F, fluid-attenuated inversion recovery sequences; G, diffusion-WI; H, apparent diffusion coefficient), enhanced MR imaging (I), MR venography (J), and MR arteriography (K) show no obvious abnormalities. Susceptibility-weighted imaging (L-O) shows abnormal signals in bilateral ventricles, the fourth ventricle, and the brainstem surface. CT, computed tomography; MR, magnetic resonance; WI, weighted imaging
Physical examination upon admission showed horizontal nystagmus while looking left, right, and up; negative meningeal signs; and no other abnormal neurological signs. Glycosylated hemoglobin levels were within normal limits, and the results of thyroid and kidney function tests, nucleic acid test for COVID-19, and Coombs test were normal. Abnormal hematological findings are shown in Table 1. Cerebrospinal fluid analysis on day 3 revealed normal pressure, white blood cell count, and glucose and protein levels. Echocardiography showed mild tricuspid regurgitation, chest CT showed atherosclerotic changes in the aorta, and brain MRI (Fig. 1B-H), gadolinium-enhanced MRI (Fig. 1I), MR venography (Fig. 1J), and MR arteriography (Fig. 1K) on day 4 showed no obvious abnormalities. Susceptibility-weighted imaging (Fig. 1L-O) showed abnormal signals in the bilateral and fourth ventricles. Heart and liver iron levels measured by T2-weighted MRI showed severe hepatic iron overload.
The initial diagnosis, headache, may be secondary to intracranial infection, cerebral venous sinus thrombosis, or reversible cerebral vasoconstriction syndrome (RCVS); therefore, antibiotic (ceftriaxone 2 g/day for 6 days) and antiviral (acyclovir 0.75 g/day for 8 days) treatment, decompression of intracranial pressure (mannitol 375 mL/day), deferasirox 1 g/day, and symptomatic treatment were administered. However, these considerations were not supported by the results of the auxiliary examination. Therefore, transfusion-related headache was considered. The hemoglobin level was 67 g/L on day 3, and the desired transfusion target was a pre-transfusion hemoglobin level of 95–105 g/L (typically to achieve post-transfusion hemoglobin of 130–150 g/L) as per the guidelines [8, 9]. Small intermittent blood transfusions were administered with caution. A RBC suspension (2 units) was transfused on days 4, 5, and 6, and the hemoglobin level was 113 g/L following transfusion. The patient’s headache was gradually relieved, and she was discharged on day 9. Headache recurred after another blood transfusion at the 5-month follow-up; however, it was not as severe as that observed at admission. In the year after discharge, liver and cardiac MRI indicated normal cardiac iron levels but revealed severe iron overload in the liver. The timeline of the patient’s condition is shown in Fig. 4. The patient was satisfied with the treatment and recovered well.
This study was approved by the Ethics Review Board of the First Affiliated Hospital of Shenzhen University (No. 2023-169-01PJ). Informed consent was obtained from the patient.
Discussion and conclusions
We report a rare case of thalassemia in a patient with CSS who developed severe headache after blood transfusions. After excluding intracranial infection, cerebral venous sinus thrombosis, and RCVS, transfusion-related neurological complications were considered. There is a lack of reports on severe headaches caused by transfusions, particularly when combined with CSS, which can be easily misdiagnosed and lead to over-treatment.
Pathogenesis of transfusion-related neurological complications
The plasma renin concentration increases after transfusion in patients with thalassemia and returns to normal after a few days [10]. Studies have shown that, in some cases, plasma renin activity and plasma angiotensin II levels started to rise after 6–8 h of transfusion, reached their highest point after 24 h, and then gradually decreased [11]. In conclusion, the evidence suggests the potential for an increase in renin levels following transfusion, although this may be influenced by various factors. Patients with chronic anemia show increased production of endocenesterin-1 (a powerful vasoconstrictor) [12, 13], which may cause arterial spasms and vasoconstriction, leading to headaches. Multiple transfusions of RBCs over a short time can cause changes in blood flow velocity [14], which may lead to headaches. Intermediate and severe thalassemia require long-term blood transfusion and can lead to iron overload, resulting in a hypercoagulable state [2, 15]. The histopathological results in patients with thalassemia who died of cerebrovascular accidents after blood transfusion showed that the findings were similar to those of hypertensive cerebral hemorrhage and hypertensive encephalopathy [6]. In the present case, the patient’s blood pressure was within normal ranges during hospitalization, and hypertensive encephalopathy was not supported.
Blood transfusion may lead to neurological complications, possibly involving immune reactions, electrolyte imbalance, infectious factors, and the characteristics of the blood components themselves [16]. First, during transfusion, the patient may develop antibodies against foreign blood components [17, 18], which may cross-react with structures of the nervous system, leading to inflammation and nerve damage [19]. Second, transfusion may cause electrolyte imbalance, especially changes in potassium and calcium ion concentrations, which may affect the excitability and conduction function of nerve cells. Third, transfusion may spread viruses, bacteria, and other pathogens, which may directly invade the nervous system or indirectly affect neurological function by causing systemic infections. Finally, the large amount of coagulation factors in fresh-frozen plasma may lead to blood clot formation, thereby triggering stroke [20].
Ferritin and iron deposition
Ferritin levels are closely linked to iron deposition in the brain. Elevated ferritin levels are often associated with increased iron deposition on the brain surface. Conversely, high ferritin levels may indicate or manifest iron deposition on the brain surface. Elevated ferritin levels can lead to iron deposition and damage the brain, liver, and other organs. The spleen is responsible for clearing aging and abnormal RBCs, which release iron as they break down, leading to iron deposition in the spleen. Iron deposits usually appear first in the spleen. Prolonged iron deposition may cause spleen dysfunction, resulting in splenomegaly; this patient underwent splenectomy to treat thalassemia. Pancreatic iron deposition typically occurs in cases of severe imbalance in iron metabolism. The liver is the main organ for iron metabolism and storage. Iron deposition in the liver usually progresses more slowly than in the spleen and pancreas, but once it begins, the accumulation rate may accelerate. Cardiac iron deposition may occur in the middle to late stages of the disease, leading to myocardial damage and heart failure. The timing of iron deposition in the brain is not fully understood, but it may damage nerve cells and lead to decreased cognitive function.
Clinical characteristics of transfusion-related neurological complications
Thalassemia patients experienced a higher prevalence of headaches compared to the general population [21]. Headaches may result from insufficient oxygen supply to the brain [22]. The body responds to the lack of oxygen due to anemia by raising heart rate and blood flow speed. This compensatory mechanism can cause the dilation of cerebral blood vessels, leading to headaches. Severe headache after blood transfusion is rarely reported, and this aspect is not fully understood. In 1979, Wasi et al. first reported that eight patients with thalassemia developed hypertension, convulsions, severe headache, and cerebral hemorrhage after transfusion of 3–7 units of blood; the authors emphasized that this was a unique clinical syndrome caused by blood transfusion [5]. CNS complications (e.g., convulsions [5] and disturbance of consciousness [23]) may occur in patients with thalassemia after transfusions [6, 23]. Some transfusion-related headaches are secondary to cerebral hemorrhage, but severe headaches alone are also one of the complications of transfusion.
The characteristics of transfusion-related headache alone in patients with thalassemia are as follows: (1) severe headaches are the most common, while hypertension, loss of consciousness, convulsions, cerebral hemorrhage, etc., may not appear; (2) headaches usually occur 2–15 days after the last transfusion and often occur after multiple transfusions within a short time, as shown in this case report. The onset of headache may not be accompanied by an obvious neurological disease. To the best of our knowledge, there are few reports of headaches caused by transfusion in other diseases, and transfusion-related headaches may be relatively specific to patients with thalassemia.
Differential diagnosis of transfusion-related headaches
The patient in this study exhibited unique signs of CSS, and it was crucial to determine whether the patient’s headache was related to CSS. CSS is caused by the deposition of hemosiderin and iron ions on the surface of the CNS, and its clinical features include binaural sensorineural hearing loss, progressive cerebellar ataxia, and pyramidal tract sign [24]. CSS deposits are mainly located under the pia mater and on the surface of the brain tissue, cranial nerves, and spinal cord [25, 26]. CSS is characterized by the deposition of hemosiderin in the pial and subpial regions of the brain, resulting from chronic bleeding in the superficial layers of the cortex and spinal cord [27]. The etiologies of subarachnoid bleeding leading to CSS are broad and heterogeneous, including tumors, vascular malformations [28], nerve root avulsions, and iatrogenic [29], traumatic, and pathological dural causes, as well as cerebral amyloid angiopathy [30]. In this case, CSS may have been caused by long-term chronic hemolysis, and repeated blood transfusions may have led to iron overload [31, 32]. CSS can lead to a spectrum of neurological deficits and symptoms, including transient focal neurological episodes, mild cognitive impairment, dementia, and stroke [33, 34]. The condition can result in sensorineural hearing loss, ataxia, pyramidal signs, and vestibular deficits, which are characteristic of CSS [35]. There was a strong temporal correlation between the headache and blood transfusion; therefore, we considered headache as a possible CNS complication associated with blood transfusion.
RCVS must also be ruled out, which mainly presents with reversible, sudden thunderclap pain with or without neurological deficits and diffuse cerebral vasoconstriction that can recover within 3 months [1, 36]. Exercise, swimming, sexual intercourse, dysthymia, stress, and cough are common predisposing factors of RCVS, and RCVS is common in people who use vasoactive drugs and during postpartum [37]. There were no obvious abnormalities on MR arteriography during headache in the present case; therefore, a diagnosis of RCVS was not supported. Intracranial infection was excluded based on clinical information including signs and results of lumbar puncture.
Treatment of transfusion-related headaches
Symptomatic treatment is the main treatment for the acute phase of headache in such patients, and there is currently a lack of specific treatment. Iron chelation therapy helps reduce the damage caused by the deposition of iron in organs such as the brain and liver. In severe cases, higher dosages of deferasirox, typically ranging from 20 to 40 mg/kg/day, can be considered, depending on the amount of transfusional iron loading, even in children [38]. Alternatively, critically ill patients can be administered intravenous deferoxamine at an initial dose of 1000 mg, followed by 500 mg every 4–12 h [39]. It is essential to gradually maximize the dosage of these iron chelators in severe cases to achieve optimal therapeutic outcomes while minimizing adverse effects. In certain clinical situations, the use of hydroxyurea can increase HbF levels and improve anemia [40, 41]. Supplementing with antioxidants and magnesium may help alleviate post-transfusion vasoconstriction and secondary headaches [42, 43]. Timely identification of transfusion-related headaches can help minimize unnecessary tests and treatments. Formulation of a more scientific and reasonable blood transfusion protocol may help reduce transfusion-related headaches in patients with thalassemia.
Limitations
The mechanisms of severe headache in patients with thalassemia after blood transfusion may be related to a variety of factors, and blood transfusion is an important trigger; however, the exact mechanism remains unclear. Further research will help prevent and treat these serious complications.
Conclusions
Post-transfusion headache in patients with thalassemia has not been fully recognized and is easily misdiagnosed, leading to excessive examination and treatment. Understanding the clinical features of transfusion-related headaches can help identify these complications. However, the exact mechanism requires further research to help prevent and treat this serious complication.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CNS:
-
Central nervous system
- CSS:
-
Cerebral superficial siderosis
- CT:
-
Computed tomography
- MR:
-
Magnetic resonance
- MRI:
-
Magnetic resonance imaging
- RBC:
-
Red blood cell
- RCVS:
-
Reversible cerebral vasoconstriction syndrome
References
Fukaguchi K, Goto T, Fukui H, Sekine I, Yamagami H. Reversible cerebral vasoconstriction syndrome: the importance of follow-up imaging within 2 weeks. Acute Med Surg. 2020;7(1):e559. https://doi.org/10.1002/ams2.559.
Arab-Zozani M, Kheyrandish S, Rastgar A, Miri-Moghaddam E. A systematic review and meta-analysis of stature growth Complications in β-thalassemia major patients. Ann Glob Health. 2021;87(1):48. https://doi.org/10.5334/aogh.3184.
Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):480–7. https://doi.org/10.2471/blt.06.036673.
Zhuang J, Jiang Y, Wang Y, Zheng Y, Zhuang Q, Wang J, et al. Molecular analysis of α-thalassemia and β-thalassemia in Quanzhou Region Southeast China. J Clin Pathol. 2020;73(5):278–82. https://doi.org/10.1136/jclinpath-2019-206179.
Wasi P, Na-Nakorn S, Pootrakul P, Sonakul D, Piankijagum A, Pacharee P. A syndrome of Hypertension, convulsion, and Cerebral Haemorrhage in thalassaemic patients after multiple blood-transfusions. Lancet. 1978;2(8090):602–4. https://doi.org/10.1016/s0140-6736(78)92824-6.
Wiwanitkit V. Brain pathology in a syndrome of Hypertension, convulsion, and Cerebral Haemorrhage in thalassaemic patients after multiple blood-transfusions: a summary in reported Thai autopsy cases. J Hypertens. 2006;24(3):601. https://doi.org/10.1097/01.hjh.0000203844.49561.fd.
Tartaglione I, Manara R, Caiazza M, Carafa PA, Caserta V, Ferrantino T, et al. Brain functional impairment in beta-thalassaemia: the cognitive profile in Italian neurologically asymptomatic adult patients in comparison to the reported literature. Br J Haematol. 2019;186(4):592–607. https://doi.org/10.1111/bjh.15959.
Red Blood Cell Disease (Anemia) Group, Chinese Society of Hematology, Chinese Medical Association. Chinese guideline for diagnosis and treatment of transfusion dependent β- thalassemia(2022). Chin J Hematol. 2022;43(11):889–96. https://doi.org/10.3760/cma.j.issn.0253-2727. 2022.11.002.
Farmakis D, Porter J, Taher A, Domenica Cappellini M, Angastiniotis M, Eleftheriou A. 2021 Thalassaemia International Federation guidelines for the management of transfusion-dependent thalassemia. Hemasphere. 2022;6(8):e732. https://doi.org/10.1097/HS9.0000000000000732.
Chuansumrit A, Isarangkura P, Hathirat P, Thirawarapan S. A syndrome of post-transfusion Hypertension, convulsion and Cerebral Hemorrhage in beta-thalassemia hb E Disease: a case report with high plasma renin activity. J Med Assoc Thai. 1986;69(Suppl 2):1–5.
Oelkers W, Köhler A, Belkien L, Fuchs-Hammoser R, Maiga M, Scherer B, et al. Studies on the mechanism by which ACTH stimulates renin activity and angiotensin II formation in man. Acta Endocrinol (Copenh). 1982;100(4):573–80. https://doi.org/10.1530/acta.0.1000573.
Viprakasit V, Kankirawatana S, Akarasereenont P, Durongpisitkul K, Chotewuttakorn S, Tanphaichitr VS. Baseline levels of plasma endothelin-1 (ET-1) and changes during transfusion in thalassemic patients. Am J Hematol. 2002;70(3):260–2. https://doi.org/10.1002/ajh.10129.
Li C, Gonsalves CS, Eiymo Mwa Mpollo MS, Malik P, Tahara SM, Kalra VK. MicroRNA 648 targets ET-1 mRNA and is cotranscriptionally regulated with MICAL3 by PAX5. Mol Cell Biol. 2015;35(3):514–28. https://doi.org/10.1128/MCB.01199-14.
Jordan LC, Rodeghier M, Donahue MJ, DeBaun MR. Reduction in transcranial doppler ultrasound (TCD) velocity after regular blood transfusion therapy is associated with a change in hemoglobin S fraction in sickle cell anemia. Am J Hematol. 2020;95(11):E308–10. https://doi.org/10.1002/ajh.25954.
Manakeng K, Prasertphol P, Phongpao K, Chuncharunee S, Tanyong D, Worawichawong S, et al. Elevated levels of platelet- and red cell-derived extracellular vesicles in transfusion-dependent β-thalassemia/HbE patients with pulmonary arterial Hypertension. Ann Hematol. 2019;98(2):281–8. https://doi.org/10.1007/s00277-018-3518-z.
Lee SJ, Wang H, Ahn SH, Son MK, Hyun GH, Yoon SJ, et al. Metabolomics approach based on multivariate techniques for blood transfusion reactions. Sci Rep. 2019;9(1):1740. https://doi.org/10.1038/s41598-018-37468-9.
Norris PJ, Schechtman K, Inglis HC, Adelman A, Heitman JW, Vilardi R, et al. Influence of blood storage age on immune and coagulation parameters in critically ill transfused patients. Transfusion. 2019;59(4):1223–32. https://doi.org/10.1111/trf.15250.
Gong Y, Tang Y, Xue Y, Chen L. Impact of intraoperative allogenic and autologous transfusion on immune function and prognosis in patients with hepatocellular carcinoma. Med (Baltim). 2020;99(41):e22568. https://doi.org/10.1097/MD.0000000000022568.
Zhu SH, Ji MH, Gao DP, Li WY, Yang JJ. Association between perioperative blood transfusion and early postoperative cognitive dysfunction in aged patients following total hip replacement Surgery. Ups J Med Sci. 2014;119(3):262–7. https://doi.org/10.3109/03009734.2013.873502.
Mariscalco G, Biancari F, Juvonen T, Zanobini M, Cottini M, Banach M, et al. Red blood cell transfusion is a determinant of neurological Complications after cardiac Surgery. Interact Cardiovasc Thorac Surg. 2015;20(2):166–71. https://doi.org/10.1093/icvts/ivu360.
Premawardhena A, Ranawaka U, Pilapitiya T, Weerasinghe G, Hapangama A, Hettiarachchi S, et al. Headache: an important symptom possibly linked to white matter lesions in Thalassaemia. Br J Haematol. 2019;185(3):541–8. https://doi.org/10.1111/bjh.15825.
Barakat H, Qureshi KA, Alsohim AS, Rehan M. The purified siderophore from streptomyces tricolor hm10 accelerates recovery from iron-deficiency-induced anemia in rats. Molecules. 2022;27(13):4010. https://doi.org/10.3390/molecules27134010.
Ngim CF, Ng CS, Lai NM. Post-transfusion Hypertension and seizure in congenital hemolytic anemia: a case report and literature review. J Trop Pediatr. 2014;60(3):253–6. https://doi.org/10.1093/tropej/fmu003.
Teranishi T, Ohba S, Kawazoe Y, Adachi K, Murayama K, Yamada S, et al. Superficial siderosis of the central nervous system caused by glioneuronal Tumor: a case report and literature review. Neurol India. 2020;68(4):894–6. https://doi.org/10.4103/0028-3886.293458.
LeVine SM, Chakrabarty A. The role of iron in the pathogenesis of experimental allergic encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2004;1012:252–66. https://doi.org/10.1196/annals.1306.021.
Chen X, Zeng C, Luo T, Ouyang Y, Lv F, Rumzan R, et al. Iron deposition of the deep grey matter in patients with multiple sclerosis and neuromyelitis optica: a control quantitative study by 3D-enhanced susceptibility-weighted angiography (ESWAN). Eur J Radiol. 2012;81(4):e633–9. https://doi.org/10.1016/j.ejrad.2012.01.003.
Feldman HH, Maia LF, Mackenzie IR, Forster BB, Martzke J, Woolfenden A. Superficial siderosis: a potential diagnostic marker of cerebral amyloid angiopathy in Alzheimer Disease. Stroke. 2008;39(10):2894–7. https://doi.org/10.1161/STROKEAHA.107.510826.
Leussink VI, Flachenecker P, Brechtelsbauer D, Bendszus M, Sliwka U, Gold R, et al. Superficial siderosis of the central nervous system: pathogenetic heterogeneity and therapeutic approaches. Acta Neurol Scand. 2003;107(1):54–61. https://doi.org/10.1034/j.1600-0404.2003.02001.x.
Kumar R, Jacob JT, Welker KM, Cutrer FM, Link MJ, Atkinson JL, et al. Superficial siderosis of the central nervous system associated with incomplete dural closure following posterior fossa Surgery: report of 3 cases. J Neurosurg. 2015;123(5):1326–30. https://doi.org/10.3171/2014.12.JNS141920.
Vernooij MW, Ikram MA, Hofman A, Krestin GP, Breteler MM, van der Lugt A. Superficial siderosis in the general population. Neurology. 2009;73(3):202–5. https://doi.org/10.1212/WNL.0b013e3181ae7c5e.
Koeppen AH, Michael SC, Li D, Chen Z, Cusack MJ, Gibson WM, et al. The pathology of superficial siderosis of the central nervous system. Acta Neuropathol. 2008;116(4):371–82. https://doi.org/10.1007/s00401-008-0421-z.
Bonomo G, Cusin A, Rubiu E, Iess G, Bonomo R, Boncoraglio GB, et al. Diagnostic approach, therapeutic strategies, and surgical indications in intradural thoracic disc herniation associated with CSF leak, intracranial hypotension, and CNS superficial siderosis. Neurol Sci. 2022;43(7):4167–73. https://doi.org/10.1007/s10072-022-06059-y.
Shoamanesh A, Akoudad S, Himali JJ, Beiser AS, DeCarli C, Seshadri S, et al. Cortical superficial siderosis in the general population: the framingham heart and rotterdam studies. Int J Stroke. 2021;16(7):798–808. https://doi.org/10.1177/1747493020984559.
Malhotra K, Theodorou A, Katsanos AH, Zompola C, Shoamanesh A, Boviatsis E, et al. Prevalence of clinical and neuroimaging markers in cerebral amyloid angiopathy: a systematic review and meta-analysis. Stroke. 2022;53(6):1944–53. https://doi.org/10.1161/STROKEAHA.121.035836.
Lee JH, Kim MS, Park BR. Vestibular end organ injury induced by middle ear treatment with ferric chloride in rats. Hum Exp Toxicol. 2017;36(2):146–59. https://doi.org/10.1177/0960327116639365.
Gupta S, Zivadinov R, Ramasamy D, Ambrus JL Jr. Reversible cerebral vasoconstriction syndrome (RCVS) in antiphospholipid antibody syndrome (APLA): the role of centrally acting vasodilators. Case series and review of literature. Clin Rheumatol. 2014;33(12):1829–33. https://doi.org/10.1007/s10067-013-2434-9.
Cossu G, Daniel RT, Hottinger AF, Maduri R, Messerer M. Malignant PRES and RCVS after brain Surgery in the early postpartum period. Clin Neurol Neurosurg. 2019;185:105489. https://doi.org/10.1016/j.clineuro.2019.105489.
Masera N, Cattoni A, Decimi V, D’Apolito V, Arosio C, Mariani R, et al. Efficacy of deferasirox for the treatment of iron overload in a child affected by juvenile hemochromatosis. Case Rep Clin Med. 2013;02(02):126–8. https://doi.org/10.4236/crcm.2013.22033.
Poonkuzhi Naseef P, Elayadeth-Meethal M, Mohammed Salim KT, Anjana A, Muhas C, Abdul Vajid K, et al. Therapeutic potential of induced iron depletion using iron chelators in Covid-19. Saudi J Biol Sci. 2022;29(4):1947-1956. https://doi.org/10.1016/j.sjbs.2021.11.061.
Steinberg MH, McCarthy WF, Castro O, Ballas SK, Armstrong FD, Smith W, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: A 17.5 year follow-up. Am J Hematol. 2010;85(6):403-8. https://doi.org/10.1002/ajh.21699.
Ware RE, Aygun B. Advances in the use of hydroxyurea. Hematol Am Soc Hematol Educ Program. 2009;62–9. https://doi.org/10.1182/asheducation-2009.1.62.
Mu YP, Huang QH, Zhu JL, Zheng SY, Yan FR, Zhuang XL, et al. Magnesium attenuates endothelin-1-induced vasoreactivity and enhances vasodilatation in mouse pulmonary arteries: modulation by chronic hypoxic pulmonary Hypertension. Exp Physiol. 2018;103(4):604–16. https://doi.org/10.1113/EP086655.
Eren F, Koca Yozgat A, Firat Oğuz E, Neşelioğlu S, Firat R, Gürlek Gökçebay D, et al. A new perspective for potential organ damage due to Iron-mediated oxidation in Thalassemia Major patients. J Clin Med. 2023;12(6):2422. https://doi.org/10.3390/jcm12062422.
Acknowledgements
We thank the “Double-First Class” Application Characteristic Discipline of Hunan Province (Pharmaceutical Science) for their support.
Funding
This study was supported by the Scientific Research Projects of the Health Commission of Hunan Province (No. 202203075030), Project of Teaching Reform in Shenzhen Second People’s Hospital (No. 202209), and Project of Teaching Reform in School of Medicine of Shenzhen University (No. XBJG202205). The funders provided the article processing charge for this study but did not contribute to the study in any other way.
Author information
Authors and Affiliations
Contributions
X.L and L.C conceived of and designed the study. X.L and H.J wrote the original draft. X.L was responsible for data collection and treatment. L.C and L.R edited and reviewed the manuscript. L.C and L.R acquired funding for this study. X.L and H.J contributed equally to this work and share first authorship. L.C and L.R contributed equally to this work and share corresponding authorship. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Review Board of the First Affiliated Hospital of Shenzhen University (No. 2023-169-01PJ). Informed consent was obtained from the patient.
Consent for publication
Written informed consent for publication was obtained from the participant.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, X., Jiang, H., Ren, L. et al. Post-transfusion severe headache in a patient with thalassemia with superficial siderosis of the central nervous system: a case report and literature review. BMC Neurol 24, 21 (2024). https://doi.org/10.1186/s12883-024-03526-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-024-03526-1