Abstract
Background
The goal of this research was to explore the role of Neutrophil to lymphocyte ratio (NLR) in Parkinson’s disease (PD).
Methods
From inception to 4 June 2023, PubMed, Web of Science, and ProQuest were searched for papers comparing NLR in PD to healthy individuals. Standardized mean difference (SMD) with a confidence interval (CI) of 95% were calculated.
Results
A random-effect model revealed that PD patients had elevated NLR values compared to healthy individuals (SMD = 0.81, 95% CI = 0.47 to 1.14, P < 0.001). The results of subgroup analysis were as follows: (1) study design: We observed that patients with PD had higher levels of NLR than healthy controls in either retrospective (SMD = 1.12, 95% CI = 0.58 to 1.66, P < 0.001) or prospective (SMD = 0.43, 95% CI = 0.18 to 0.68, P = 0.001) studies. (2) Ethnicity: We noticed that individuals with PD had higher levels of NLR than healthy controls, whether they were East Asian (SMD = 0.93, 95% CI = 0.22 to 1.63, P = 0.010) or Caucasian (SMD = 0.75, 95% CI = 0.40 to 1.10, P < 0.001).The pooled sensitivity of NLR in the prediction of PD was 0.67 (95% CI = 0.61–0.73), and the pooled specificity was 0.66 (95% CI, 0.61–0.70).
Conclusions
Increased levels of NLR is highly related with the presence of PD. Further research is needed to determine the potential clinical benefits of this simple and low-cost biomarker in the PD diagnosis.
Similar content being viewed by others
Background
Parkinson’s disease (PD) is the second most common neurological illness after Alzheimer’s disease, affecting approximately 7 to 10 million individuals globally [1, 2]. The disease’s most apparent symptoms are related to movement, such as rigidity, movement slowness, tremor, and postural instability [3, 4]. Despite the fact that PD is identified by the appearance of intraneuronal proteinacious cytoplasmic inclusions defined as Lewy bodies and a considerable decrease of dopaminergic neurons in the substantia nigra pars compacta, the illness’s origin remains unknown [5,6,7]. The antiparkinson medicines, including dopamine agonists and levodopa are often used to treat PD, and they are clinically beneficial in the early stages of disease. However, as the condition worsens, these treatments become ineffective and have adverse side effects [8, 9]. As a result, there is an urgent need to comprehend the etiology of PD and create innovative medicines to prevent or halt disease development.
Inflammation appears to play a prominent role in the pathologic characteristics and symptoms of PD, according to growing research [10,11,12,13,14,15]. Interleukins and tumor necrosis factors (TNFs) are crucial immune activation signaling molecules that affect both the brain and the periphery [16]. In vivo findings of positron emission tomography and postmortem in PD patients revealed elevated inflammatory reactions, such as microglial activation [17, 18], and higher levels of immunological markers in the brain [18,19,20].
In addition, several studies have compared inflammatory biomarkers in individuals with PD to healthy controls in an effort to better understand the origin of the condition and identify potential biomarkers for it [21,22,23,24].
Neutrophil to lymphocyte ratio (NLR) is a biomarker based on Complete blood count (CBC), and it shows the balance between immunity and systemic inflammation. Although several new studies found links between PD and NLR, the findings were contradictory [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. A meta-analysis on this topic is needed to resolve clinical evidence discrepancies. As far as we know, this is the first systematic review and meta-analysis on this matter.
Methods
Study design
We followed the most recent methodological suggestions provided by the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) to conduct this meta-analysis [44]. We registered our study in PEROSPERO (CRD42023429384).
Eligibility criteria
The following PICO terms served as our basis for inclusion:
-
(a)
Population: Patients suffering from PD.
-
(b)
Intervention: NLR.
-
(c)
Control: Healthy controls.
-
(d)
Outcomes: The diagnostic value of NLR.
-
(e)
Study design: case-control, cross-sectional, and cohort articles.
Conference abstracts, basic science investigations, animal studies, case reports and case series were all excluded.
Search strategy
We searched major data bases like Web of Science, ProQuest, and PubMed from inception until June 4, 2023. We used an extensive list of keywords related to NLR and Parkinson’s disease in our search strategy. We did not impose any language or publishing time constraints.
Study selection
Two review writers independently screened the titles, abstracts, and full texts of publications that appeared in our search to determine which met the criteria for inclusion. If there were any issues, a writer would step in and settle it. Following that, the reference and citation lists of the included papers were examined for further possibly relevant publications.
Data extraction and management
The extracted information from the selected articles are as follows:
Name of the first author, location, publication year, design of the study, demographic characteristics of the population, mean and standard deviation (SD) of the NLR, cut off point, specificity and sensitivity as effect measures.
Assessment of methodological quality
Two review authors independently assessed the methodological quality of the included papers using the Newcastle-Ottawa scale (NOS) [45].
Statistical analysis and data synthesis
Stata 11.2 (Stata Corp, College Station, TX) was employed to conduct the meta-analysis. To compare the NLR values between PD patients and controls, we employed standardized mean difference (SMD) with a 95% confidence interval (CI). To assess the heterogeneity of the included articles, the I2 and Cochran’s Q tests were used. Significant heterogeneity across included articles was noted as I2 > 50% and Q test p-value < 0.05. We also utilized the random-effects model to assess pooled effects since we noticed a substantial level of heterogeneity. We used the “metandi” command to measure negative likelihood ratio, positive likelihood ratio, diagnostic odds ratio (DOR), and pooled sensitivity and specificity of NLR for PD. In sensitivity analysis, we used “metaninf” command to investigate the influence of every single study on the overall meta-analysis estimate. We also prepared a summary receiver operating characteristic (SROC) curve. Finally, we employed the Egger test and the funnel plot to assess publication bias. GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach was used to assess the certainty of evidence [46].
Results
Search results and included studies
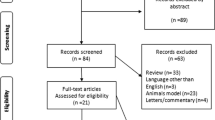
There were 1067 total results from the manual search of the article citation list and the database search. Eventually, after removing duplicates and irrelevant records, 20 studies were included to this review [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43, 47]. The PRISMA flow diagram, which illustrates the inclusion and exclusion procedure, appears in Fig. 1.
Characteristics of the population and quality assessment
Totally, 20 articles were included in the analysis, comprising 3584 patients with PD and 2487 healthy controls [25–43, 47]. The general features of the included articles are shown in Table 1. According to the NOS scale, the quality assessment revealed that all of the studies were of moderate to high quality (Table 1).
PD patients’ NLR levels
According to a random-effects model, NLR levels were higher in PD patients than in healthy controls (SMD = 0.81, 95% CI = 0.47 to 1.14, P < 0.001, Fig. 2). However, the certainty of evidence was very low (Table 2).
The results of subgroup analysis were shown in Table 3. In the subgroup analysis according to study design, we observed that PD patients exhibited increased levels of NLR in comparison to healthy controls in either retrospective (SMD = 0.93, 95% CI = 0.22 to 1.63, P < 0.010) or prospective (SMD = 0.43, 95% CI = 0.18 to 0.68, P = 0.001) studies (Fig. 3).
In the subgroup analysis according to ethnicity, we observed that PD patients exhibited increased levels of NLR in comparison to healthy controls in either East Asian (SMD = 0.93, 95% CI = 0.22 to 1.63, P = 0.010) or Caucasian (SMD = 0.75, 95% CI = 0.40 to 1.10, P < 0.001) patients (Fig. 4).
Sensitivity analysis
Our results were stable and not reversed after omitting each study in sensitivity analysis (Fig. 5, Supplemental Table I).
NLR’s diagnostic value in PD
The pooled sensitivity was 0.67 (95% CI = 0.61–0.73), and the pooled specificity was 0.66 (95% CI, 0.61–0.70). The pooled positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio (DOR) of NLR were 2.00(95%CI = 1.66–2.42), 0.48 (95%CI = 0.38–0.60), and 2.06 (95%CI = 1.65–2.58), respectively (Fig. 6).
Publication bias
There were some signs of publication bias among the included articles (Egger test 0.001, Supplemental Figure I).
Discussion
We undertook meta-analysis of 20 studies to evaluate the correlation of NLR with PD. It was discovered that PD patients had higher levels of NLR than healthy controls.
PD is a progressive neurodegenerative illness defined pathologically by emergence of lewy bodies and dopaminergic neuron loss in the substantia nigra. Neuroinflammation has a critical role in lewy body formation and substania nigra cell loss. Both the adaptive and innate immune systems are significantly altered in PD [48]. Inflammation is silent in the brain. The response relies on the production of inflammatory components by microglia in the brain. Microglia produce inflammatory cytokines that appear to play significant roles [49]. Interleukin-1 (IL-1), IL-6, and Tumor necrosis factor-alpha (TNF) are examples of inflammatory cytokines that amplify and sustain immune responses and inflammation. It is interesting that PD patients’ CSF basal ganglia contain elevated levels of TNFα, IL-6, and IL-1b [50]. The BBB’s integrity may be lost as a result of the release of pro-inflammatory cytokines by microglia and also upregulation of adhesive molecules (VCAM and ICAM), which cause infiltration of peripheral immune cell in brain [51]. The NLR is a low-cost, simple-to-use indicator of peripheral inflammation [52, 53]. Increased NLR has been shown in many CNS conditions [54]. Several studies evaluated association of increased NLR with PD and our meta-analysis confirmed this association. This may show important role of inflammation and it may exist several years before clinical manifestation of PD and the immune system involvement is promising for therapeutic targeting. In addition to diagnostic utilities, NLR can be used as a prognostic factor in PD. For example, in 2022, Muñoz-Delgado et al. conducted a retrospective cohort study on 211 PD patients as primary-cohort. For replication goals, they also included 344 separate patients with PD in Parkinson’s Progression Markers Initiative group as PPMI-cohort. They showed that NLR is associated with nigrostriatal dopaminergic system degeneration, assessed using striatal dopamine transporter (DAT) density. Patients with higher levels of NLR had significantly lower DAT levels in the putamen (primary-cohort: P = 0.02; PPMI-cohort: P = 0.02) and the caudate (primary-cohort: P < 0.001; PPMI-cohort: P = 0.05) [55]. In addition, Song et al. prospectively followed up 26,210 participants for 21 years. During the follow-up, 486 incidences of PD were reported. The results showed that NLR (HR = 1.09; 95% CI: 1.00- 1.19), was related to an increased risk of PD [56].
Although the role of chronic inflammation is confirmed in PD, it is unclear whether inflammation is the root cause of neurodegeneration or whether it develops as a consequence of cell degeneration and selective damage process [57]. Also the trigger factor for inflammation such as viral infection or autoimmunity remain to be elucidated. The role of inflammation may be different in various subtypes of PD. Use of anti-inflammatory, anti-viral drug and immune-modulating (immunotherapy)for PD treatment and even preventing of PD can be a new era for management of neurodegenerative disease [51, 58].
Due to the extensive evidence that inflammation and immune activation are characteristics of PD, it is not strange that immune-targeting interventions and anti-inflammatory drugs have progressed in the treatments for PD, as they have in other neurodegenerative illnesses such as amyotrophic lateral sclerosis and Alzheimer’s disease [59, 60]. Trials on anti-inflammatory medication in PD have been profoundly unsatisfactory, as they have been in other neurodegenerative illnesses, and such trials need adjusting the strategy [52]. The incorporation of immune-related end points might be a significant therapeutic factor that influences the design of future, possibly more effective trials that use combination medicines that target many processes, like immunological responses [52, 61].
As the neurodegenerative field grows, it is becoming more important to investigate the role of peripheral and central inflammation in the progression and pathogenesis of PD, one of the key obstacles is the creation of innovative methods, including advanced patient-derived models, machine learning, or single-cell multi-omics that will empower the field to thoroughly examine how immune cells predispose or protect neuron injury, whether or not inflammation and the immune system are significant elements of the disease, and at which stages immune processes play crucial roles and are prognostic of illness course [62, 63]. Therefore, further research in these areas is required. Better knowledge and identification of the immunological processes behind the initial indications of PD may result in innovative medicines, and physicians may one day be able to successfully intervene with repurposed or novel immunomodulatory and anti-inflammatory medications to postpone or slow progression of the illness from the periphery to the CNS [64].
There were several limitations to this study. First, only summary data, rather than individual patient data, can be utilized. Second, we only considered studies that reported mean and SD. Third, there was a significant publication bias in our results. Forth, we could not perform trial sequential analysis (TSA), because it can be applied to analyses on dichotomous data like odds ratio (OR) and hazard ratio (HR), but not on SMD, which was reported in our meta-analysis. We tried to extract dichotomous data like OR from included studies and conduct a new meta-analysis and then, perform TAS; however, except to one study [33], no study had reported OR, so further studies reporting TSA results are needed. Finally, neutrophil and lymphocyte counts are nonspecific measures that might be affected by other circumstances such as infections, inflammation, and medicines. These contemporaneous variables may affect how NLR is measured in some of the included studies since they were not specifically controlled.
Conclusion
In conclusion, high NLR can be regarded as an indicator of inflammation in PD. Still, it needs more investigations to study its diagnostic value, association with the stage and progression of the disease, and therapeutic value. Our results expanded immunological information from Parkinson’s disease patients to build better preclinical models, with the long-term objective of allowing early detection of at-risk people to postpone, and treat the illness more properly.
Data Availability
The full text of this article also contains the dataset used to support its findings.
Abbreviations
- PD:
-
Parkinson’s disease
- TNFs:
-
Tumor necrosis factors
- NLR:
-
Neutrophil to lymphocyte ratio
- CBC:
-
Complete blood count
- PRISMA:
-
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- SD:
-
Standard deviation
- NOS:
-
Newcastle-Ottawa scale
- SMD:
-
Standardized mean difference
- CI:
-
Confidence interval
- DOR:
-
Diagnostic odds ratio
- SROC:
-
Summary receiver operating characteristic
- IL:
-
Interleukin
8. References
Leigh RM. Parkinson’s disease. Rumi Michael Leigh; 2019.
Tan E-K, Chao Y-X, West A, Chan L-L, Poewe W, Jankovic J. Parkinson disease and the immune system—associations, mechanisms and therapeutics. Nat Reviews Neurol. 2020;16(6):303–18.
Zesiewicz TA. Parkinson disease. CONTINUUM: Lifelong Learning in Neurology. 2019;25(4):896–918.
Berg D, Borghammer P, Fereshtehnejad S-M, Heinzel S, Horsager J, Schaeffer E, et al. Prodromal Parkinson disease subtypes—key to understanding heterogeneity. Nat Reviews Neurol. 2021;17(6):349–61.
Balestrino R, Schapira A. Parkinson disease. Eur J Neurol. 2020;27(1):27–42.
Simon DK, Tanner CM, Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med. 2020;36(1):1–12.
Limousin P, Foltynie T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat Reviews Neurol. 2019;15(4):234–42.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. The Lancet. 2021;397(10291):2284–303.
Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, Ray Chaudhuri K, et al. Parkinson disease-associated cognitive impairment. Nat Reviews Disease Primers. 2021;7(1):1–21.
Pajares M, Rojo I, Manda A, Boscá G, Cuadrado L. Inflammation in Parkinson’s disease: mechanisms and therapeutic implications. Cells. 2020;9(7):1687.
Han X, Sun S, Sun Y, Song Q, Zhu J, Song N, et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: implications for Parkinson disease. Autophagy. 2019;15(11):1860–81.
Borsche M, König IR, Delcambre S, Petrucci S, Balck A, Brüggemann N, et al. Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain. 2020;143(10):3041–51.
Romano S, Savva GM, Bedarf JR, Charles IG, Hildebrand F, Narbad A. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. npj Parkinson’s Disease. 2021;7(1):1–13.
Marogianni C, Sokratous M, Dardiotis E, Hadjigeorgiou GM, Bogdanos D, Xiromerisiou G. Neurodegeneration and Inflammation—An interesting interplay in Parkinson’s disease. Int J Mol Sci. 2020;21(22):8421.
Armstrong MJ, Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323(6):548–60.
Rolli-Derkinderen M, Leclair-Visonneau L, Bourreille A, Coron E, Neunlist M, Derkinderen P. Is Parkinson’s disease a chronic low-grade inflammatory bowel disease? J Neurol. 2020;267(8):2207–13.
Joshi N, Singh S. Updates on immunity and inflammation in Parkinson disease pathology. J Neurosci Res. 2018;96(3):379–90.
McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:3–S7.
Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol. 2022:1–17.
Chen L, Mo M, Li G, Cen L, Wei L, Xiao Y, et al. The biomarkers of immune dysregulation and inflammation response in Parkinson disease. Translational Neurodegeneration. 2016;5(1):1–6.
Wu Y, Le W, Jankovic J. Preclinical biomarkers of Parkinson disease. Arch Neurol. 2011;68(1):22–30.
Schapira AH. Recent developments in biomarkers in Parkinson disease. Curr Opin Neurol. 2013;26(4):395.
Miller DB, O’Callaghan JP. Biomarkers of Parkinson’s disease: present and future. Metabolism. 2015;64(3):40–S6.
Delenclos M, Jones DR, McLean PJ, Uitti RJ. Biomarkers in Parkinson’s disease: advances and strategies. Parkinsonism Relat Disord. 2016;22:106–S10.
Akıl E, Bulut A, Kaplan İ, Özdemir HH, Arslan D, Aluçlu MU. The increase of carcinoembryonic antigen (CEA), high-sensitivity C-reactive protein, and neutrophil/lymphocyte ratio in Parkinson’s disease. Neurol Sciences: Official J Italian Neurol Soc Italian Soc Clin Neurophysiol. 2015;36(3):423–8.
Ataç Uçar C, Gökçe Çokal B, Ünal Artık HA, İnan LE, Yoldaş TK. Comparison of neutrophil-lymphocyte ratio (NLR) in Parkinson’s disease subtypes. Neurol Sciences: Official J Italian Neurol Soc Italian Soc Clin Neurophysiol. 2017;38(2):287–93.
Contaldi E, Magistrelli L, Cosentino M, Marino F, Comi C. Lymphocyte Count and Neutrophil-to-lymphocyte ratio are Associated with mild cognitive impairment in Parkinson’s Disease: a Single-Center Longitudinal Study. J Clin Med. 2022;11(19):5543.
Jiang L, Zhong Z, Huang J, Bian H, Huang W. Monocytohigh-density lipoprotein ratio has a high predictive value for the diagnosis of multiple system atrophy and the differentiation from Parkinson’s disease. Front Aging Neurosci. 2022;14.
Jiang S, Wang Y, Gao H, Luo Q, Wang D, Li Y et al. Cell ratio differences in peripheral blood between early-and late-onset Parkinson’s disease: a case-control study. BioMed Research International. 2019;2019.
Jin H, Gu H-y, Mao C-j, Chen J, Liu C. -f. Association of inflammatory factors and aging in Parkinson’s disease. Neurosci Lett. 2020;736:135259.
Kara SP, Altunan B, Unal A. Investigation of the peripheral inflammation (neutrophil–lymphocyte ratio) in two neurodegenerative diseases of the central nervous system. Neurol Sci. 2022;43(3):1799–807.
Kenangil G, Ari B, Kaya F, Demir M, Domac F. Red cell distribution width levels in Parkinson’s disease patients. Acta Neurol Belgica. 2020;120(5):1147–50.
Liu Z, Fan Q, Wu S, Wan Y, Lei Y. Compared with the monocyte to high-density lipoprotein ratio (MHR) and the neutrophil to lymphocyte ratio (NLR), the neutrophil to high-density lipoprotein ratio (NHR) is more valuable for assessing the inflammatory process in Parkinson’s disease. Lipids Health Dis. 2021;20(1):1–12.
Madetko N, Migda B, Alster P, Turski P, Koziorowski D, Friedman A. Platelet-to-lymphocyte ratio and neutrophil-tolymphocyte ratio may reflect differences in PD and MSA-P neuroinflammation patterns. Neurol Neurochir Pol. 2022;56(2):148–55.
Muñoz-Delgado L, Macías‐García D, Jesús S, Martín‐Rodríguez JF, Labrador‐Espinosa M, Jiménez‐Jaraba MV, et al. Peripheral Immune Profile and Neutrophil‐to‐lymphocyte ratio in Parkinson’s Disease. Mov Disord. 2021;36(10):2426–30.
Paul KC, Kusters C, Furlong M, Zhang K, Yu Y, Folle AD, et al. Immune system disruptions implicated in whole blood epigenome-wide association study of depression among Parkinson’s disease patients. Brain Behav Immunity-Health. 2022;26:100530.
Pekel, NB, Yildiz 1 D, Siğirli 2 D, Yabaci 2 A, Seferoğlu1 M, Güneş1 A. Parkinson’s Disease: Is it Actually an inflammatory disorder? Turkish J Geriatr. 2018;21(4):483–9.
Sanjari Moghaddam H, Ghazi Sherbaf F, Mojtahed Zadeh M, Ashraf-Ganjouei A, Aarabi MH. Association between Peripheral inflammation and DATSCAN Data of the Striatal nuclei in different motor subtypes of Parkinson Disease. Front Neurol. 2018;9:234.
Solmaz V, Genç EP, Aksoy D, Çevik B, Kurt SG, Benli İ. Parkinson hastalarında nötrofil/lenfosit oranları, C reaktif protein ve sedimantasyon hızlarının değerlendirilmesi. Cukurova Med J (Çukurova Üniversitesi Tıp Fakültesi Dergisi).43(2):1-.
Wang L-x, Liu C, Shao Y-q, Jin H, Mao C-j, Chen J. Peripheral blood inflammatory cytokines are Associated with Rapid Eye Movement Sleep Behavior Disorder in Parkinson’s Disease. Neurosci Lett. 2022:136692.
Wang Y, Gao H, Jiang S, Luo Q, Han X, Xiong Y, et al. Principal component analysis of routine blood test results with Parkinson’s disease: a case-control study. Exp Gerontol. 2021;144:111188.
Xing N, Dong Z, Wu Q, Kan P, Han Y, Cheng X et al. Identification and validation of key molecules associated with humoral immune modulation in Parkinson’s disease based on bioinformatics. Front Immunol. 2022;13.
Yazar HO, Yazar T. Serum inflammation biomarkers are associated with stages of Parkinson’s disease. 2019.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford; 2000.
Goldet G, Howick J. Understanding GRADE: an introduction. J Evidence-Based Med. 2013;6(1):50–4.
Muñoz-Delgado L, Macías-García D, Periñán MT, Jesús S, Adarmes-Gómez AD, Bonilla Toribio M, et al. Peripheral inflammatory immune response differs among sporadic and familial Parkinson’s disease. npj Parkinson’s Disease. 2023;9(1):12.
Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:210–S2.
Hirsch EC, Standaert DG. Ten unsolved questions about neuroinflammation in Parkinson’s disease. Mov Disord. 2021;36(1):16–24.
Tiwari PC, Pal R. The potential role of neuroinflammation and transcription factors in Parkinson disease. Dialogues in clinical neuroscience. 2022.
De Virgilio A, Greco A, Fabbrini G, Inghilleri M, Rizzo MI, Gallo A, et al. Parkinson’s disease: autoimmunity and neuroinflammation. Autoimmun rev. 2016;15(10):1005–11.
Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Translational Neurodegeneration. 2015;4(1):1–9.
Song S-Y, Zhao X-X, Rajah G, Hua C, Kang R-j, Han Y-p, et al. Clinical significance of baseline neutrophil-to-lymphocyte ratio in patients with ischemic stroke or hemorrhagic stroke: an updated meta-analysis. Front Neurol. 2019;10:1032.
Yu S, Arima H, Bertmar C, Clarke S, Herkes G, Krause M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurol Sci. 2018;387:115–8.
Muñoz-Delgado L, Labrador‐Espinosa M, Macías‐García D, Jesús S, Benitez Zamora B, Fernández‐Rodríguez P et al. Peripheral inflammation is Associated with Dopaminergic Degeneration in Parkinson’s Disease. Mov Disord. 2023.
Song L, Zhang S, Li H, Hansson O, Sonestedt E, Borné Y. Comparison of risk factors for Parkinson’s disease, coronary events and ischemic stroke. npj Parkinson’s Disease. 2022;8(1):107.
Gelders G, Baekelandt V, Van der Perren A. Linking neuroinflammation and neurodegeneration in Parkinson’s disease. Journal of immunology research. 2018;2018.
More SV, Kumar H, Kim IS, Song S-Y, Choi D-K. Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators of inflammation. 2013;2013.
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2018;4:575–90.
Lyon MS, Wosiski-Kuhn M, Gillespie R, Caress J, Milligan C, Inflammation. Immunity, and amyotrophic lateral sclerosis: I. etiology and pathology. Muscle Nerve. 2019;59(1):10–22.
Lu C-H, Allen K, Oei F, Leoni E, Kuhle J, Tree T et al. Systemic inflammatory response and neuromuscular involvement in amyotrophic lateral sclerosis. Neurology-Neuroimmunology Neuroinflammation. 2016;3(4).
Vivekanantham S, Shah S, Dewji R, Dewji A, Khatri C, Ologunde R. Neuroinflammation in Parkinson’s disease: role in neurodegeneration and tissue repair. Int J Neurosci. 2015;125(10):717–25.
Troncoso-Escudero P, Parra A, Nassif M, Vidal RL. Outside in: unraveling the role of Neuroinflammation in the progression of Parkinson’s Disease. Front Neurol. 2018;9:860.
Boyd RJ, Avramopoulos D, Jantzie LL, McCallion AS. Neuroinflammation represents a common theme amongst genetic and environmental risk factors for Alzheimer and Parkinson diseases. J Neuroinflamm. 2022;19(1):1–20.
Acknowledgements
Not applicable.
Funding
This systematic review and meta-analysis was not funded in any way.
Author information
Authors and Affiliations
Contributions
S.H designed the study, designed data collection, and revised the initial version of our manuscript. A.A and M.Kh supervised data collection and statistical analyses and critically reviewed the manuscript. A.Gh and A.B drafted the initial manuscript and revised the manuscript. J.P and H.B collected data and conducted the initial analyses. S.Kh conceptualized and designed the study, designed data collection and reviewed the manuscript. Finally, N.Sh. and R.Gh helped in the revision of manuscript, conducting further analyses, and submitting the protocol of the manuscript in the PROSPERO. All authors read and approved the final manuscript and are responsible for data review.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hosseini, S., Shafiabadi, N., Khanzadeh, M. et al. Neutrophil to lymphocyte ratio in parkinson’s disease: a systematic review and meta-analysis. BMC Neurol 23, 333 (2023). https://doi.org/10.1186/s12883-023-03380-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03380-7