Abstract
Background
Myasthenia gravis (MG) is an autoimmune disorder affecting the neuromuscular junction. Despite the potential benefits of higher physical activity and lower sedentary behavior in MG patients, evidence from observational studies for the effect of physical activity on the risk of MG is limited and inconclusive.
Methods
We employed linkage disequilibrium score (LDSC) regression, two-sample Mendelian randomization (MR), and its multivariable extension analyses (MVMR) to assess the relationship between leisure screen time (LST), moderate-to-vigorous intensity physical activity during leisure time (MVPA) and the risk of MG using genome-wide association studies (GWAS) summary datasets. MR analyses were performed using the inverse-variance-weighted (IVW), weighted-median, and MR-Egger regression. Sensitivity analyses were further performed using alternative instruments to test the robustness of our findings.
Results
We found evidence of genetic overlap between LST (rg = 0.113, P = 0.023) and MG, as well as between MVPA (rg=-0.220, P = 0.0001) and MG, using LDSC method. The results of the MR suggested an association between genetic liability to LST and increased risk of MG (IVW OR = 1.609, 95% CI = 1.153 to 2.244; P = 0.005). This association was particularly notable for late-onset MG (IVW OR = 1.698, 95% CI = 1.145 to 2.518; P = 0.008), but not for early-onset MG. Consistent findings were obtained in the MVMR analysis using BMI as covariate (IVW OR = 1.593, 95% CI 1.167 to 2.173, P = 0.003). However, the MR analysis does not support a substantial causal effect of MVPA on the risk of MG.
Conclusion
Our findings support a causal effect of sedentary behavior as measured by LST on MG, indicating that lack of exercise may play a role in the development of MG. Longitudinal and interventional studies of this association are warranted.
Similar content being viewed by others
Background
Myasthenia gravis (MG) is a complex neurological disorder influenced by both genetic and environmental factors [1] and characterized by weakness of the skeletal muscles [2]. It is caused by autoantibodies that bind to functionally important molecules at the postsynaptic membrane at the neuromuscular junction [3]. Eighty per cent of patients with MG have detectable antibodies against the acetylcholine receptor (AChR), whereas a small minority instead have antibodies against muscle-specific kinase (MuSK) or lipoprotein-receptor-related protein 4 (LRP4) [3]. Nevertheless, the underlying factors contributing to the development of MG are not well understood [1]. The estimated heritability of MG is approximately 25.6%, which suggests there are genetic factors contributing to the disease that have yet to be identified [4]. In addition, the concordance of monozygotic MG twins is estimated to be about 35%, suggesting the important role of environmental variables in MG pathogenesis [5]. However, few lifestyle risk factors have been identified that are associated with the onset or progression of MG [6].
Physical activity (PA) is broadly defined as musculoskeletal movement that leads to energy expenditure [7]. There is growing interest in the potential of physical activity to preserve immunity and protect against multi-morbidity [8]. Conversely, excessive sedentary behavior has negative impacts on health regardless of other factors, suggesting that physical activity and sedentary behavior and may act as two independent risk factors predisposing individuals to poor health outcomes [9]. Despite several studies have evaluated the potential benefits of higher physical activity and lower sedentary behavior in MG patients [10,11,12,13], the relationship between PA and the risk of MG remains inconclusive due to the limited availability of longitudinal data.
Mendelian randomization (MR) overcomes these difficulties by using genetic variants associated with an exposure as instrumental variables (IVs) to inter the causality of the risk factor with respect to disease [14]. Leveraging the most recent GWAS datasets [15, 16], we employed linkage disequilibrium score (LDSC) regression, bidirectional MR, and its multivariable extension analyses (MVMR) to assess the relationship between leisure screen time (LST), moderate-to-vigorous intensity physical activity during leisure time (MVPA) and the risk of MG. Additionally, we explored the potential pleiotropic function of body mass index (BMI) [17], a notable confounder in research involving PA and sedentary behavior, in this interplay.
Methods
Study design
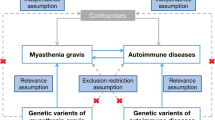
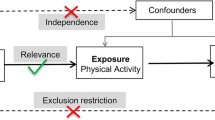
We performed this study under a two-sample, bidirectional, MR analysis framework. The MR approach we used was based on the following three assumptions: (1) genetic variants (single nucleotide polymorphisms (SNPs)) used as IVs are associated with exposures; (2) genetic variants are not associated with confounders; and (3) genetic variants influence the risk of outcomes only through interested exposures, not through other pathways. To begin, we performed a genetic correlation analysis using LDSC method to assess the shared genetic risks between LST and MVPA with MG. Next, we examined whether there was a bidirectional association between LST, MVPA, and the risk of MG. Finally, we conducted multivariable MR analysis to explore the potential mediation effect of body mass index (BMI) on the association between LST, MVPA, and the risk of MG.
Linkage disequilibrium score (LDSC) regression
Linkage Disequilibrium Score (LDSC) Regression (https://github.com/bulik/ldsc) was employed to evaluate the genetic correlation across features to investigate the possibility of a shared genetic architecture [18]. Precomputed LD scores of European individuals in the 1000 Genomes project for high-quality HapMap3 SNPs were used (‘eur_w_ld_chr’). Using LDSC regression, we estimated genetic correlation (rg) between two traits by incorporating LD scores and GWAS summary statistics in a regression model.
Mendelian randomization (MR) analyses
For selecting the most unbiased and representing instrumental genetic variables, we included SNPs associated with LST, MVPA and BMI at genome-wide significance (p < 5 × 10− 8). Then the index SNPs were obtained by clumping all significant SNPs within each linkage disequilibrium (LD) block from all GWAS using an R2 < 0.001 threshold (clumping window 10,000 kB). To prevent the effect estimates from aligning with different alleles, harmonization was performed to remove ambiguous SNPs showing non-concordant alleles. A more conservative strategy was performed to correct the strand for non-palindromic SNPs and drop all palindromic SNPs from the MR analysis. Overall, 91 instruments for LST and 12 instruments for MVPA were used in the two-sample MR analysis. The resulting lists of instrument SNPs for all phenotypes are provided in Supplemental Tables 2 and Supplemental Table 4.
The primary analyses were performed by the IVW method (hereafter referred to as standard MR analysis), which provides reliable estimates assuming all SNPs meet the IV assumptions and biases if average pleiotropic effect differs from zero. Then, the analyses were conducted utilizing the weighted median approach [19] and the MR-Egger regression method [20]. The weighted median regression calculates the effects that are robust if half or more of the SNPs are valid instruments, while the MR-Egger regression only relies on the instrument strength independent of direct effect assumption and not additionally on non-zero mean pleiotropy. Additionally, Cochran’s Q test and MR-Egger intercept test were utilized to assess the heterogeneity between causal estimates from different genetic variants, which can detect the presence of pleiotropy. The average pleiotropic effect of the genetic variants could be estimated by the intercept term (tested here using a p-value threshold of < 0.05). Besides, the leave-one-out analysis was conducted to assess the influence of potentially pleiotropic SNPs on the causal estimates. The multivariable MR analyses, in which BMI may potentially mediate the link across LST and MVPA with MG, were conducted similarly. Statistical analyses were carried out using the TwoSampleMR R package.
Results
Data sources
The data sources were selected from studies with publicly available GWAS summary data, and detailed information regarding the various GWAS datasets is displayed in Table 1. We extracted summary data from the most recent and largest meta-GWAS of physical activity which initially included four self-reported PA or sedentary behavior related traits: MVPA, leisure screen time (LST), sedentary commuting and sedentary behavior at work, and yield 88 loci associated with LST, 11 loci for MVPA, 4 loci for sedentary behavior at work, and no loci were identified for sedentary commuting [15]. To ensure adequate statistical power in instrumental variable and enrichment analyses, sedentary behavior at work and sedentary commuting were not included in the further post-GWAS analysis in the original dataset. Therefore, we also utilized LST (n = 526,725) and MVPA (n = 608,595) in our study. MG GWAS Summary statistics involving 1,873 patients versus 36,370 age/gender-matched controls collected from US and Italian [16] were used. To specify the age-dependent genetic heterogeneity of MG, we drew on summary statistics of early-onset (EOMG) (595 patients versus 2,718 controls, aged 40 years or younger) and late-onset (LOMG) (1,278 patients versus 33,652 controls) separately [16]. We also investigated the potential pleiotropic role of BMI in this interplay and GWAS summary of BMI which included 806,834 European ancestry individuals was retrieved from the GIANT consortium [17].
LDSC regression demonstrates a correlation between PA and MG
Using LDSC regression, we found a significant positive correlation between LST and MG (rg = 0.1134, P = 0.0230), as well as a significant negative correlation between MVPA and MG (rg = -0.2202, P = 0.0001) with both MG subtypes (Table 2). Furthermore, BMI was also significantly positively correlated with MG (rg = 0.1010, P = 0.0096) and LOMG (rg = 0.1129, P = 0.0046) (Supplemental Table 1). These novel findings support the genetic link hypothesis between PA and sedentary-related characteristics and MG.
MR analysis show LST was associated with increased risk of MG
The results of the MR suggested an association between LST and increased risk of MG (IVW OR = 1.609, 95% CI = 1.153 to 2.244; P = 0.005), and late-onset MG (IVW OR = 1.698, 95% CI = 1.145 to 2.518; P = 0.008). However, both weighted median and MR Egger analysis yielded a pattern of no effects. In addition, the MR estimate was not statistically significant when compared to LST and EOMG (IVW OR = 1.478, 95% CI = 0.747 to 2.927; P = 0.262). The causal relationships between MVPA and MG were not statistically significant (IVW OR = 0.570, 95% CI = 0.240 to 1.354; P = 0.203) (Fig. 1). The results of sensitivity analyses using Cochran’s Q test and MR-Egger intercept test suggested that there was no heterogeneity or horizontal pleiotropy among the SNP effect estimates (Supplemental Tables 3 and Supplemental Fig. 1). In addition, a leave-one-out analysis was conducted to examine the influence of pleiotropic genetic variants, however we did not identify a single genetic variant that affected the association between LST with MG and LOMG (Supplemental Fig. 2).
Univariant MR analysis for the effect of physical activity and sedentary-related traits on MG. (A) Forest plot of univariable MR-estimated effects sizes by the inverse-variance-weighted method. Data are expressed as OR values with 95% CI. LST, Leisure screen time; MVPA, Moderate-to-vigorous intensity physical activity during leisure time; MG, myasthenia gravis; EOMG, early-onset myasthenia gravis; LOMG, late-onset myasthenia gravis. P values in bold indicates P < 0.05/2. (B) Scatterplot of SNP potential effects on LST vs. MG, with the slope of each line corresponding to estimated MR effect per method
Reverse MR also did not demonstrate any causal effect of MG on LST (IVW OR = 0.991, 95% CI = 0.973 to 1.008; P = 0.301) and MVPA (IVW OR = 0.997, 95% CI = 0.986 to 1.009; P = 0.643). However, Cochran’s Q test of MG on LST (Q = 15.739; P = 0.015) suggested the possibility of heterogeneity SNP(s) in our dataset (Supplemental Table 3).
Since there is some evidence that levels of physical activity and BMI have a genetic association [15], we employed a multivariable MR approach for LST and MVPA with BMI as a confounding variable (Supplemental Table 4). Consistent with the results of univariate MR analyses that did not include BMI as a covariate, we found evidence confirming a causal association between LST with BMI against MG (OR = 1.593, 95% CI 1.167 to 2.173, P = 0.003), and LOMG (OR = 1.771, 95% CI 1.216 to 2.580, P = 0.003), but not EOMG. In contrast, the association between MVPA with BMI versus MG and MG subtypes was not significant after Bonferroni correction (Table 3).
Discussion
This is the first study to examine genetic correlations and causal associations across GWAS datasets between physical activity, sedentary behavior-related characteristics, and the risk of MG. Our analysis using the LDSC method revealed a significant relationship between LST and MVPA in relation to MG. Then, we applied MR analysis based on genetic instruments selected from the most recent large-scale GWASs, which suggested a causal relationship between LST and MG, independent of whether BMI was a confounder. Consistent with the prior research [1, 2], the generic difference between EOMG and LOMG was observed that only the LOMG had a significant association with LST in MR analysis.
The benefits of physical exercise for healthy individuals are well-established, particularly in terms of mitigating the risks of chronic lifestyle-related diseases [21]. PA can generate an anti-inflammatory response by significantly boosting T-regulatory cells, reducing immunoglobulin secretion and promoting the release of IL-6 from muscles [7] A recent study also utilized MR analysis and identified T-cell traits as causally protective factors for MG [22]. Therefore, PA is considered a protective factor for certain autoimmune disorders, including rheumatoid arthritis [23] and multiple sclerosis [24], which are also genetically associated with MG [15]. In the case of MG, previous studies indicate that lower levels of PA have a detrimental effect on muscle mass and strength [25, 26] and that PA restriction is a predictor of fatigue severity [12, 27]. Thus, the genetic liability of PA and MG is uncertain to distinguish if PA causally influences risk for MG, or whether there is reverse causality due to fatigue and reduction of PA in the prodromal phase. In our results, despite the association between MVPA and MG observed in LDSC, the univariable and multivariable MR analyses do not suggest a substantial causal effect of MVPA on the risk of MG. However, as the number of genetic variants for the MVPA trait is modest, resulting in low statistical effectiveness, our MR analyses may not be currently powered to detect weak effects.
Our results suggest a moderate causal relationship between genetic susceptibility to LST and MG. The LST was defined as leisure time in front of screens, mostly about TV, computer, and video games, which is generally considered a physical inactivity lifestyle. Physical inactivity can activate inflammatory pathways and lead to elevated serum/plasma concentrations of inflammatory markers, such as IL-6, IL-1β and TNF-α [28]. Recent research identified LST-associated genes that are enriched in skeletal muscle and altered by resistance training, some of which pointed to myopathy and T cell-related pathways [15]. This provides genetic insights into how complex traits such as sedentary behaviors are associated with MG. However, the complex and multifactorial association between MG and physical activity cannot be fully explored solely through MR analysis. Therefore, additional epidemiological evidence is necessary to establish causality. We approach the determination of LST as a potential causal risk factor for MG with caution.
Limitations
Several limitations merit consideration in our study. First, although we included data from the largest GWAS dataset on PA and sedentary behaviors, we are unable to carry out a more extensive analysis due to the lack of genetic variables for heterogenous PA or sedentary behavior-related factors, especially the MVPA traits which are just dichotomized in GWAS. Second, despite the selection of SNPs with strong associations, common SNPs cannot be considered exact proxies for exposure since they do not adequately explain the overall variance in complex traits. Thirdly, all the GWAS data were extracted primarily from European ancestry; hence, additional research from other ethnicities is required to bolster the conclusions.
Conclusions
Our findings support a causal effect of sedentary behavior as measured by LST on MG, indicating that lack of exercise may play a role in the development of MG; however, longitudinal and intervention studies are needed to determine the relationship.
Data Availability
The original contributions generated for this study are included in the article, and further inquiries can be directed to the corresponding author.
References
Punga AR, Maddison P, Heckmann JM, Guptill JT, Evoli A. Epidemiology, diagnostics, and biomarkers of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022;21:176–88.
Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14:1023–36.
Gilhus NE. Myasthenia Gravis. N Engl J Med. 2016;375:2570–81.
Renton AE, Pliner HA, Provenzano C, Evoli A, Ricciardi R, Nalls MA, et al. A genome-wide association study of myasthenia gravis. JAMA Neurol. 2015;72:396–404.
Avidan N, Le Panse R, Berrih-Aknin S, Miller A. Genetic basis of myasthenia gravis - a comprehensive review. J Autoimmun. 2014;52:146–53.
Maniaol AH, Boldingh M, Brunborg C, Harbo HF, Tallaksen CME. Smoking and socio-economic status may affect myasthenia gravis. Eur J Neurol. 2013;20:453–60.
Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y. Physical activity and autoimmune diseases: get moving and manage the disease. Autoimmun Rev. 2018;17:53–72.
Duggal NA, Niemiro G, Harridge SDR, Simpson RJ, Lord JM. Can physical activity ameliorate immunosenescence and thereby reduce age-related multi-morbidity? Nat Rev Immunol. 2019;19:563–72.
Dogra S, Stathokostas L. Sedentary behavior and physical activity are independent predictors of successful aging in middle-aged and older adults. J Aging Res. 2012;2012:190654.
Andersen LK, Aadahl M, Vissing J. Fatigue, physical activity and associated factors in 779 patients with myasthenia gravis. Neuromuscul Disord. 2021;31:716–25.
Birnbaum S, Bachasson D, Sharshar T, Porcher R, Hogrel J-Y, Portero P. Free-living physical activity and sedentary Behaviour in Autoimmune Myasthenia Gravis: a cross-sectional study. J Neuromuscul Dis. 2021;8:689–97.
Gilhus NE. Physical training and exercise in myasthenia gravis. Neuromuscul Disord. 2021;31:169–73.
Alsop T, Williams K, Gomersall S. Physical activity and sedentary behaviour in people with Myasthenia Gravis: a cross-sectional study. J Neuromuscul Dis. 2022;9:137–46.
Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization JAMA. 2017;318:1925–6.
Wang Z, Emmerich A, Pillon NJ, Moore T, Hemerich D, Cornelis MC, et al. Genome-wide association analyses of physical activity and sedentary behavior provide insights into underlying mechanisms and roles in disease prevention. Nat Genet. 2022;54:1332–44.
S S-A, N RC, C MAC, Rh B. R, Identification of genetic risk loci and prioritization of genes and pathways for myasthenia gravis: a genome-wide association study. Proc Natl Acad Sci USA. 2022;119.
Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of european ancestry. Hum Mol Genet. 2019;28:166–74.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–41.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some Invalid Instruments using a weighted median estimator. Genet Epidemiol. 2016;40:304–14.
Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45:1961–74.
Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29.
Zhong H, Jiao K, Huan X, Zhao R, Su M, Goh L-Y, et al. Herpesvirus entry mediator on T cells as a protective factor for myasthenia gravis: a mendelian randomization study. Front Immunol. 2022;13:931821.
Sandberg MEC, Wedrén S, Klareskog L, Lundberg IE, Opava CH, Alfredsson L, et al. Patients with regular physical activity before onset of rheumatoid arthritis present with milder disease. Ann Rheum Dis. 2014;73:1541–4.
Wesnes K, Myhr K-M, Riise T, Cortese M, Pugliatti M, Boström I, et al. Physical activity is associated with a decreased multiple sclerosis risk: the EnvIMS study. Mult Scler. 2018;24:150–7.
Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–4.
Cruz-Jentoft AJ, Sayer AA, Sarcopenia. Lancet. 2019;393:2636–46.
Sabre L, Westerberg E, Liik M, Punga AR. Diversity in mental fatigue and social profile of patients with myasthenia gravis in two different northern european countries. Brain Behav. 2017;7:e00653.
Rosa-Neto JC, Lira FS, Little JP, Landells G, Islam H, Chazaud B, et al. Immunometabolism-fit: how exercise and training can modify T cell and macrophage metabolism in health and disease. Exerc Immunol Rev. 2022;28:29–46.
Acknowledgements
The authors gratefully thank Dr. Zhe Wang, the Giant Consortium, the International Myasthenia Gravis Genomics Consortium, and all investigators and related consortia for sharing GWAS summary statistics on physical activity and sedentary-related traits, body mass index and myasthenia gravis.
Funding
This work was supported the Beijing Postdoctoral Research Foundation (2023-ZZ-004) and the Natural Science Foundation of Jiangsu Province (BK20200192).
Author information
Authors and Affiliations
Contributions
FW and JL conceived and designed the study, performed the analyses and interpretation. FW and CZ prepared the first draft and figures. HL, JG, ZL and JL revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
As the current study was based on published studies and public databases, no additional ethics approval or consent to participate was required.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Wang, F., Zhang, C. et al. Genetically predicted effects of physical activity and sedentary behavior on myasthenia gravis: evidence from mendelian randomization study. BMC Neurol 23, 299 (2023). https://doi.org/10.1186/s12883-023-03343-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03343-y