Abstract
Background
As a meaningful subtype of ischemic stroke in Asians, Branch atheromatous disease (BAD)-related stroke is associated with high early neurological deterioration (END) and disability, but is understudied and without recommended therapy. The mechanism of END still remains unclear. Branch atheromatous disease-related stroke study (BAD-study) therefore aims to investigate demographic, clinical and radiological features, and prognosis of BAD-related stroke in Chinese patients.
Methods/design
BAD-study is a nationwide, multicenter, consecutive, prospective, observational cohort study enrolling patients aged 18–80 years with BAD-related stroke within 72 h after symptom onset. Initial clinical data, laboratory tests, and imaging data are collected via structured case report form, and follow-ups will be performed at 7 days, 30 days, 90 days, 6 months and 12 months after enrollment. The primary outcome is the score on modified Rankin Scale at 90-day follow-up with single-blinded assessment. Secondary outcomes include END within 7 days, and National institute of health stroke scale score, Barthel index, cerebrovascular events, major bleeding complications, and all-cause mortality during 90-day follow-up. Characteristics of penetrating and parent artery will be assessed by high-resolution magnetic resonance imaging combined with other imaging techniques.
Discussion
BAD-study can provide demographic, clinical, radiological, and prognostic characteristics of BAD-related stroke, and thereby potentially figure out the vascular mechanism of early neurological deterioration and optimize therapy strategy with the aid of advanced imaging technique. Baseline data and evidence will also be generated for randomized controlled trials on BAD-related stroke in the future.
Similar content being viewed by others
Background
Stroke leads to millions of death and disability in China and other countries annually [1, 2]. To optimize stroke management and improve functional outcome, causative mechanism of stroke is of great importance. Branch Atheromatous Disease (BAD), first described by Caplan in 1989 [3], is increasingly becoming a clinical entity with the aid of neuroimage, characterized by stenosis or occlusion at the origin of a penetrating artery resulting from atherosclerosis and leading to ischemic lesion [4]. In our study, the term “Branch Atheromatous Disease (BAD)-related stroke” is used for ischemic stroke due to branch atheromatous disease.
Although proposed as a meaningful concept, BAD remained neglected and underused in clinical practice, researches and guidelines for the past three decades [4]. Epidemiological data on BAD is spare, and most studies were reported in Asian population [4]. Small vessel occlusion or intracranial atherosclerosis are more common in Asians, which is different from Western populations. BAD-related stroke accounted for 9.1% in Japan and 20.4% in Hongkong among ischemic stroke patients [5, 6]. Perforating arteries involve lenticulostriate artery (LSA), paramedian pontine artery (PPA), thalamoperforating artery, anterior choroidal artery and Heubner's artery [4, 6], while what have been widely studied Is LSA and PPA, indirectly via features of ischemic lesions [4, 7]. Based on clinical and neuroimage evidence, neurologists and researchers have proposed the diagnostic criteria for BAD-related stroke [7,8,9].
Of patients with BAD-related stroke, the mean age ranged from 54 to 75 years, slightly younger than that in large artery disease (LAD) or lacunar infarction (LACI), and male gender was more prevalent [4, 6, 10]. The median National Institutes of Health Stroke Scale (NIHSS) score of BAD-related stroke was 4, lower than 6 in LAD, but similar with that in LACI. The short-term disability (modified ranking scale [mRS] ≥ 3) rates of LAD, BAD-related stroke, and LACI were 74%, 61%, and 47%, respectively [6]. However, long-term outcomes were comparable between BAD-related stroke and LACI [11]. Finally, the clinical, neuroradiologic, and predictive data are insufficient for BAD-related stroke.
One challenge of BAD-related stroke was its high incidence of early neurological deterioration (END) [5, 12], which was strongly associated with poor outcome [13]. Compared with distal single subcortical infarction, BAD-related stroke showed higher-volume lesion (median, 1.79 vs 0.44 ml) and more frequent END [10, 14,15,16]. The rate of END following intravenous thrombolysis was estimated as 13.8%, measured by the deterioration of NIHSS score at 24 h (≥ 4) [12]. Intravenous thrombolysis seemed unable to prevent END and controversial about its effect on improving functional outcome among patients with BAD-related stroke [12, 17,18,19]. In addition, Seners P, et al. found that strongest predictors for END were consistent across thrombolysed and non-thrombolysed acute ischemic stroke patients including hyperglycemia, proximal arterial occlusion, large infarcts, no recanalization or re-occlusion, and etc. [12]. Thus, preventing the progression of stenosis and re-occlusion of proximal penetrating artery has become the essential node of the early management of BAD-related stroke. Multiple studies focused on the mechanism of END due to unique brain ischemia with poor collateral circulation [20]. However, no evidence-based therapy was recommended for secondary preventive treatment in acute phase of BAD-related stroke.
Previous study found that dual-antiplatelet or anticoagulation therapy might reduce END, but their effect on BAD-related stroke remained unclear and might increase the risk of bleeding [21, 22]. Tirofiban also showed efficacy on reducing END and improving outcomes in patients with endovascular therapy, intravenous thrombolysis or BAD-related stroke, but warranting further confirmation in large-sample patients with BAD-related stroke [23,24,25]. The optimal regimen for reducing END and improving outcome remains inconclusive [19].
In addition, many researchers focused on the role of inflammatory indicators in stroke pathogenesis and prognosis, including the effect of white blood cell, platelet, lipid level and etc. [12, 26,27,28]. For instance, a randomized controlled study reported that high-dose statins could reduce the level of inflammatory indicators and improve the outcome of ischemic stroke [29]. Elevated high-sensitivity C-reactive protein and inflammatory indicators also predicted poor outcome of stroke [30, 31]. However, the effect of inflammatory indicators on BAD-related stroke was unknown.
Another advance in BAD-related stroke is the application of high-resolution magnetic resonance imaging (HR-MRI) and images of vessel wall, which facilitates visualized analysis of LSA, PPA and their parent arteries [32, 33], and the further research of vascular pathophysiology of BAD-related stroke and END.
Although neurologists increasingly consider BAD-related stroke as a clinical entity and a subtype of ischemic stroke with relatively poor outcome, its demographic, clinical and radiological characteristics are still lacking with little evidence of therapy. Therefore, we describe here the protocol of BAD-study, a multicenter prospective cohort study in China to investigate demographic, clinical, radiological features, risk factors, and prognosis of BAD-related stroke and explore the optimal regiment for good outcome.
Methods/design
Study design
BAD-study is a nationwide, multicenter, consecutive, prospective, observational, cohort study recruiting patients with BAD-related stroke within 72 h after symptom onset. Due to February 28, 2022, this ongoing study has recruited 22 hospitals with the equipment of 3 Tesla (T) magnetic resonance imaging, with the leader of Peking Union Medical College Hospital in Beijing, China. BAD-study was approved by the Ethics Committee of Peking Union Medical College Hospital on May 25, 2021 (No. ZS-2982B), and written informed consents (model consent form) are required for all patients. In addition, protocol modifications, if any, would be approved by the ethics committee. The anticipated duration of the study will be about 2 years from June 1, 2021 until May 31, 2023. BAD-study has been registered on July 28, 2021 at clinicaltrials.gov, with identifier NCT04973774.
Study objectives
The primary objective of this study is to figure out the demographic, clinical, radiological characteristics, risk factors, and prognosis of BAD-related stroke in this clinical-radiological cohort with large sample, and evaluate the effects of different real-word therapies on outcome. Secondary objectives are to: 1) investigate the vascular mechanism of END; 2) establish the association between clinical, clinical, radiological characteristics and clinical outcome and model for predicting outcome; 3) find out the standardized process and criteria for analyzing penetrating arteries using vessel wall imaging (VWI) based on 3 Tesla-MRI; 4) explore the efficacy of safety of specific drugs, such as aspirin and tirofiban, providing baseline data for further clinical trials.
Study population
All consecutive ischemic stroke patients aged 18–80 years visiting any center of our study considering BAD-related stroke will be screened for eligibility according to the following inclusion and exclusion criteria. All eligible patients will be Invited to participate in this observational study.
-
Inclusion criteria:
-
1. Age:18–80 years.
-
2. Acute cerebral infarction, if the clinical manifestations are transient, new infarct lesion should be found on Diffusion Weighted Imaging (DWI) at the same time.
-
3. The time from symptom onset to enrollment is less than 72 h. If the onset time was unknown, the time of last known free of new ischemic symptoms to enrollment is less than 72 h.
-
4 Meet all the following radiological criterial:
-
1) DWI lesion: single (isolated) deep (subcortical) infarct;
-
2) The culprit vessels are the LSA or PPA, and the infarct lesion on DWI conforms to one of the following characteristics (A/B):
-
A. LSA: (1) “Comma-like” infarct lesions with “Fan-shaped” extension from bottom to top in the coronary position; OR (2) ≥ 3 layers (layer thickness 5–7 mm) on axial DWI images of the head;
-
B. PPA: the infarct lesion extends from the deep pons to the ventral pons on the axial DWI of the head.
-
-
3) No ≥ 50% stenosis on the parent artery of the criminal vessel (i.e. corresponding basilar or middle cerebral artery) (confirmed by magnetic resonance angiography [MRA] or computed tomography angiography [CTA] or digital substraction angiography [DSA]).
-
5 Signed informed consent by the patient or legally authorized representatives.
-
Exclusion criteria:
-
1. Intracranial hemorrhagic diseases, vascular malformations, aneurysms, brain abscesses, malignant space occupying lesions or other non-ischemic intracranial lesions observed by baseline head CT and MRI, MRA/CTA/DSA.
-
2. There was ≥ 50% stenosis of extracranial vessels with ipsilateral serial relationship.
-
3. Cardiogenic embolism: atrial fibrillation, myocardial infarction, valvular heart disease, dilated cardiomyopathy, infective endocarditis, atrioventricular block disease, heart rate less than 50 beats /min.
-
4. Have received or plan to receive acute endovascular treatment after onset of the disease.
-
5. Stroke caused by other clear causes, such as moyamoya disease, arterial dissection, vasculitis, etc.
-
6. mRS score prior to the onset of the disease was ≥ 2 points.
-
7. Known malignant tumor.
-
8. Life expectancy ≤ 6 months.
-
9. Contraindications of 3 T MRI examination.
-
10. Pregnant or lactating women.
-
11. Participation in another clinical within 3 months before enrollment, or taking part in another ongoing study.
Procedures
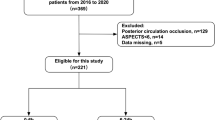
The board of primary investigator, neurologists, radiologists, epidemiologists, pharmacologist and statisticians was established on June 1, 2021. All hospital personnel have been trained on the reading of neuroimages in screening stage, the collection of data at baseline and each follow ups, and etc. The enrollment of first case in each center will be confirmed by the board. Eligible patients will be recruited at the time of signing the informed content. Baseline data included demographic and clinical assessments, neuroimages and laboratory data (first ones after stroke onset). Follow-ups will be conducted at 7 days, 30 days, 90 days, 6 months, and 12 months after enrollment, via face-to-face interviews or telephone, when the mRS, cerebrovascular events, adverse effects of medications, and etc. will be evaluated. At the follow-up of 7 days, the event of END will be assessed since stroke onset. The 90-day follow-up is required to be assessed by the neurologists, who are blinded to the clinical, radiological characteristics and treatment of selected patients. The flowchart of BAD-study is shown in Fig. 1.
The flowchart of BAD-study. BAD: Branch atheromatous disease; CTA: Computed tomography angiography; DSA: Digital subtraction angiography; e-CRF: Electronic case report form; END: Early neurological deterioration; HR-MRI: high-resolution magnetic resonance imaging; MRA: Magnetic resonance angiography; MRI: Magnetic resonance imaging; mRS: Modified Ranking scale; NIHSS: National institute of health stroke scale; pCASL: pseudo-continuous arterial spin labelling; PLATO: Platelet Inhibition and Patient Outcomes; SWI: Susceptibility weighted imaging; VWI: Vessel wall imaging. * CCA: Common carotid artery; ECG: Electrocardiograph; ICA: Internal carotid artery; VA: Vertebral artery; SubA: Subclavian artery; † TCD: Transcranial doppler; ‡ Drugs include Antiplatelet drugs, lipid-lowering drugs including statins, anticoagulant drugs, antihypertensive drugs, antidiabetics, and other neuroprotective drugs
Study measurements
Clinical data
Data of patients will be collected and reported using the electronic Case Report Form (e-CRF) at baseline and follow-up periods. The quality of data will be monitored dynamically by the central center. Clinical items include demographics, medical history, history of medication, clinical presentations, and treatments in hospital. The blood pressure and intake and output volume within 72 h after enrollment are also recorded. The details is shown in Table 1.
Laboratory tests
According to Chinese guidelines and clinical practice [34], initial clinical biochemistry routine tests including total blood cell count, liver and renal functions, lipid levels, and blood coagulation test will be recorded under standard lab procedures. If applicable, autoimmune biomarkers, homocysteine concentrations, glycosylated hemoglobin, fasting glucose, thyroid gland function, high sensitivity C reactive protein, Protein C, Protein S, Antithrombin III, and activated protein C will be measured at baseline. If applicable, lipid levels will be measured at 90-day follow up.
Imaging protocols
MRI
The MRI studies are conducted on 3 T MR scanners (GE Discovery 750 or SIEMENS Vida). Three dimensional (3D) T1, MRA, high-resolution vessel wall imaging and pseudo-continuous arterial spin labelling (ASL) are conducted, and the parameters are shown in Table 2. Based on Table 2, the imaging parameters are modified and verified at each center, if applicable. In addition, routine T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging, apparent diffusion coefficient (ADC) and susceptibility weighted imaging (SWI)/T2*WI will also be performed, using the standardized protocol in each center.
Other image techniques
Other assessment techniques for cerebral artery, carotid artery, vertebral artery, and subclavian artery, including MRA, CTA, DSA or vascular ultrasound are conducted according the standardized protocol in each center. In addition, the results of Holter, transcranial doppler sonography, and ultrasonic cardiogram will be recorded based on the criteria at each center, if applicable.
Image analysis and classification
The BAD-related stroke will be divided as two groups: 1) Lesion in area of LSA; and 2) Lesion in area of PPA. The plaque, stenosis, vessel wall, and cerebral perfusion of PPA and LSA will be assessed. The vascular lesion sites of penetrating artery and its parent artery are grouped into three types: 1) Plaque within parent artery blocking the branch orifice; 2) Plaque extending into the branch from parent artery; 3) Plaque originating in orifice of branch [3]. The length, thickness, square, volume, and signal of plaque will be measured. In LSA analysis based on 3 T-VWI, the number, length, and shape of LAS will also be evaluated [35].
The process of image collection, storage, synthesis and analysis are guided by a committee including senior radiologists and neurologists. All images will be evaluated by two experienced neurologists and will be reviewed by senior neurologist J Ni in case of disagreement.
Outcome measures
The primary outcome in our study is the score on the modified Rankin scale at 90 days, which is a 7-point scale ranging from 0 (no symptoms) to 6 (death) [36]. Secondary outcomes include END within 7 days, and NIHSS score, Barthel index, cerebrovascular events (myocardial infarction, new-onset ischemic stroke, new-onset intracranial hemorrhage), and major bleeding complications measured by PLATO (Platelet Inhibition and Patient Outcomes) definition during 90-day follow-up [37]. All-cause mortality within 90 days is also recorded. The follow up times are extended to 1 year, as the long-term outcome of BAD-related stroke remains inconclusive [4, 6].
The END is defined as: 1) From symptom onset to 7 days after enrollment; and 2) Occurrence of deterioration of neurologic deficits after initial assessment: A. An increase of the NIHSS score ≥ 4 points or the NIHSS motor score ≥ 1 point for ischemic patients; or B. The stereotyped attacks still repeat ≥ 3 times after arrival at hospital or progress to persistence status for patients with internal capsule warning syndrome or pontine warning syndrome [12, 38].
Sample size
There is no specific hypothesis for the primary outcome in our cohort study, thus, the sample size is estimated based on the number of variables, using the method recommended for registry study (10 times number of variable) [39]. The sample size is estimated as at least 450, and increases to 495 for 10% loss during follow-up.
Statistical analysis
Categorical variables will be presented as frequencies and percentages, such as gender, vascular risk factors, lesions of penetrating artery, and therapies. Continuous variables with non-normal distributions will be shown as median and interquartile range (IQR), while continuous variables with normal distributions as mean and 95% confidence interval (CI), such as age, NIHSS score, blood pressure, and hemoglobin concentration. For the statistical significance analysis of the outcomes, Pearson χ 2 or Fisher’s exact test, Wilcoxon tests, and t-tests will be used, where appropriate, with statistical uncertainty expressed by means of 95% CI. Cox proportional hazards model, Logistic regression model, and Poisson regression model will be used to assess the association between outcomes and clinical or radiological predictors, where appropriate. The good prognosis is defined as 0–2 mRS score, and poor prognosis as 3–6 mRS score at 90 days. Predictors including clinical and radiological features and different treatments will be evaluated. All analysis will be performed using SAS 9.4 and two-sided P < 0.05 is considered significant.
Data management
As mentioned above, the metadata of BAD-study are stored in the structured eCRF via internet, with central monitoring dynamically. Only anonymous data will be provided to researchers. In addition, data monitoring committee is not needed in this observational study.
Current status
The first patient of BAD-study was enrolled on August 16, 2021, after thorough assessment by the board. Until March 13, 2022, our ongoing study has recruited 143 patients. The brief demographic and clinical data of eligible patients are shown in Table3.
Discussion
BAD-study will be the first prospective clinical-radiological cohort with long-term follow-up and large sample, focusing on the BAD-related stroke, a neglected but clinically meaning subtype of stroke in Asians with poor collateral circulation and high disability [4, 10, 40]. The demographic clinical, radiological, and prognostic characteristics of BAD-related stroke in China will be reported.
In addition, we noticed that the BAD-related stroke was categorized as “stroke of undetermined etiology” in TOAST classification system, and thus failed to reflect its underlying stroke mechanism [4, 6, 40]. Our study will update the original TOAST classification, and prove the concept of BAD-related stroke. In addition, we noticed the higher frequency of cardiogenic embolism in elderly patients with lacunar infarcts, and excluded patients with cardiogenic stroke in our study [41].
With the documented data of laboratory tests, the role of inflammatory indicators on the prognosis of stroke will be assessed. The management of inflammatory indicators might be a novel therapy on reducing END and improving the outcome of BAD-related stroke [12, 28, 29, 31].
With the aid of MRI, MRA, HR-MRI, and ASL, BAD-study will analyze the cerebral artery, vessel wall of parent artery and penetrating artery, artery plaques, brain tissue, cerebral perfusion, and volume of lesion in BAD-related stroke. In addition, visualized analysis of LSA, and its association with outcomes will help neurologists understand the vascular mechanism of END [35, 42]. Radiological data of artery and lesion will provide clues for possible effective drugs. Moreover, quantitative analysis will be conducted to analyze the effect of lesion on the volume of gray matter and white matter, as global brain inflammation could be elicited by localized lesion [43, 44]
Besides, the comparison of different therapy strategies on primary outcome and END will provide direct clinical evidence to optimize the acute therapy in BAD-related stroke [13] and find the probably effective strategy of preventing END [12]. Based on the results of BAD-study, a risk prediction model will be generated for the prognosis, which may help neurologists individualize treatment strategy.
BAD-study will also assess the efficacy and safety of tirofiban, providing baseline data for further randomized controlled trials in patients with BAD-related stroke. And the diagnostic criteria of BAD-related stroke will be verified in clinical practice [7,8,9].
Availability of data and materials
The data of BAD-study will be available from the corresponding author on reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- ASL:
-
Arterial spin labeling
- BAD:
-
Branch atheromatous disease
- CCA:
-
Common carotid artery
- CI:
-
Confidence interval
- CTA:
-
Computed tomography angiography
- DSA:
-
Digital subtraction angiography
- DWI:
-
Diffusion weighted imaging
- e-CRF:
-
Electronic case report form
- ECG:
-
Electrocardiograph
- END:
-
Early neurological deterioration
- FA:
-
Flip angle
- FLAIR:
-
Fluid attenuated inversion recovery
- FOV:
-
Field of view
- HR-MRI:
-
High-resolution magnetic resonance imaging
- ICA:
-
Internal carotid artery
- IQR:
-
Interquartile range
- LACI:
-
Lacunar infarction
- LAD:
-
Large artery disease
- LD:
-
Labeling duration
- LSA:
-
Lenticulostriate artery
- MRA:
-
Magnetic resonance angiography
- MRI:
-
Magnetic resonance imaging
- mRS:
-
Modified Ranking scale
- NIHSS:
-
National institute of health stroke scale
- pCASL:
-
Pseudo-continuous arterial spin labelling
- PLATO:
-
Platelet Inhibition and Patient Outcomes
- PLD:
-
Post-labeling delay
- PPA:
-
Paramedian pontine artery
- SWI:
-
Susceptibility weighted imaging
- SubA:
-
Subclavian artery
- T:
-
Tesla
- TCD:
-
Transcranial Doppler
- TE:
-
Echo time
- TI:
-
Inversion time
- TIA:
-
Transient ischemic attack
- TOF-MRA:
-
Time-of-flight MR angiography
- VA:
-
Vertebral artery
- TR:
-
Repetition time
- VWI:
-
Vessel wall imaging
References
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392 10159:1736–88. https://doi.org/10.1016/S0140-6736(18)32203-7.
Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. https://doi.org/10.1016/S1474-4422(18)30500-3.
Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied, and underused concept. Neurology. 1989;39(9):1246–50. https://doi.org/10.1212/wnl.39.9.1246.
Petrone L, Nannoni S, Del Bene A, Palumbo V, Inzitari D. Branch Atheromatous Disease: A Clinically Meaningful. Yet Unproven Concept Cerebrovasc Dis. 2016;41(1–2):87–95. https://doi.org/10.1159/000442577.
Deguchi I, Hayashi T, Kato Y, Nagoya H, Ohe Y, Fukuoka T, et al. Treatment outcomes of tissue plasminogen activator infusion for branch atheromatous disease. J Stroke Cerebrovasc Dis. 2013;22(7):e168–72. https://doi.org/10.1016/j.jstrokecerebrovasdis.2012.10.012.
Kwan MW, Mak W, Cheung RT, Ho SL. Ischemic stroke related to intracranial branch atheromatous disease and comparison with large and small artery diseases. J Neurol Sci. 2011;303(1–2):80–4. https://doi.org/10.1016/j.jns.2011.01.008.
Men X, Chen W, Xu Y, Zhu Y, Hu W, Chen X, et al. Consensus of Chinese experts on perforator atherosclerosis. Chin J Stroke. 2021;16(5):508–14. https://doi.org/10.3969/j.issn.1673-5765.2021.05.016 (in Chinese).
Zhou L, Ni J. Progress in diagnosis and treatment of branch atheromatous disease. Chin J Stroke. 2020;15(12):1342–51. https://doi.org/10.3969/j.issn.1673-5765.2020.12.015 (in Chinese).
Adachi T, Takagi M. The clinical differences between lacunar infarction and branch atheromatous disease. Nihon Rinsho. 2006;64(Suppl 8):155–9.
Zhang C, Wang Y, Zhao X, Wang D, Liu L, Wang C, et al. Distal single subcortical infarction had a better clinical outcome compared with proximal single subcortical infarction. Stroke. 2014;45(9):2613–9. https://doi.org/10.1161/strokeaha.114.005634.
Suto Y, Nakayasu H, Maeda M, Kusumi M, Kowa H, Awaki E, et al. Long-term prognosis of patients with large subcortical infarctions. Eur Neurol. 2009;62(5):304–10. https://doi.org/10.1159/000235943.
Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015;86(1):87–94. https://doi.org/10.1136/jnnp-2014-308327.
Heitsch L, Ibanez L, Carrera C, Binkley MM, Strbian D, Tatlisumak T, et al. Early Neurological Change After Ischemic Stroke Is Associated With 90-Day Outcome. Stroke. 2021;52(1):132–41. https://doi.org/10.1161/STROKEAHA.119.028687.
Duan Z, Fu C, Chen B, Xu G, Tao L, Tang T, et al. Lesion patterns of single small subcortical infarct and its association with early neurological deterioration. Neurol Sci. 2015;36(10):1851–7. https://doi.org/10.1007/s10072-015-2267-1.
Seners P, Ben Hassen W, Lapergue B, Arquizan C, Heldner MR, Henon H, et al. Prediction of Early Neurological Deterioration in Individuals With Minor Stroke and Large Vessel Occlusion Intended for Intravenous Thrombolysis Alone. JAMA Neurol. 2021. https://doi.org/10.1001/jamaneurol.2020.4557.
Jeong HG, Kim BJ, Yang MH, Han MK, Bae HJ. Neuroimaging markers for early neurologic deterioration in single small subcortical infarction. Stroke. 2015;46(3):687–91. https://doi.org/10.1161/strokeaha.114.007466.
Park MG, Oh EH, Kim BK, Park KP. Intravenous tissue plasminogen activator in acute branch atheromatous disease: Does it prevent early neurological deterioration? J Clin Neurosci. 2016;33:194–7. https://doi.org/10.1016/j.jocn.2016.04.011.
Wu X, Liu Y, Nie C, Kang Z, Wang Q, Sun D, et al. Efficacy and Safety of Intravenous Thrombolysis on Acute Branch Atheromatous Disease: A Retrospective Case-Control Study. Front Neurol. 2020;11:581. https://doi.org/10.3389/fneur.2020.00581.
Ospel JM, Menon BK, Demchuk AM, Almekhlafi MA, Kashani N, Mayank A, et al. Clinical Course of Acute Ischemic Stroke Due to Medium Vessel Occlusion With and Without Intravenous Alteplase Treatment. Stroke. 2020;51(11):3232–40. https://doi.org/10.1161/STROKEAHA.120.030227.
Del Bene A, Palumbo V, Lamassa M, Saia V, Piccardi B, Inzitari D. Progressive lacunar stroke: review of mechanisms, prognostic features, and putative treatments. Int J Stroke. 2012;7(4):321–9. https://doi.org/10.1111/j.1747-4949.2012.00789.x.
Yi X, Zhou Q, Wang C, Lin J, Chai Z. Aspirin plus clopidogrel may reduce the risk of early neurologic deterioration in ischemic stroke patients carrying CYP2C19*2 reduced-function alleles. J Neurol. 2018;265(10):2396–403. https://doi.org/10.1007/s00415-018-8998-1.
Wang Q, Chen C, Chen XY, Han JH, Soo Y, Leung TW, et al. Low-molecular-weight heparin and early neurologic deterioration in acute stroke caused by large artery occlusive disease. Arch Neurol. 2012;69(11):1454–60. https://doi.org/10.1001/archneurol.2012.1633.
Yang J, Wu Y, Gao X, Bivard A, Levi CR, Parsons MW, et al. Intraarterial Versus Intravenous Tirofiban as an Adjunct to Endovascular Thrombectomy for Acute Ischemic Stroke. Stroke. 2020;51(10):2925–33. https://doi.org/10.1161/strokeaha.120.029994.
Wu C, Sun C, Wang L, Lian Y, Xie N, Huang S, et al. Low-Dose Tirofiban Treatment Improves Neurological Deterioration Outcome After Intravenous Thrombolysis. Stroke. 2019;50(12):3481–7. https://doi.org/10.1161/STROKEAHA.119.026240.
Liu B, Zhang H, Wang R, Qu H, Sun Y, Zhang W, et al. Early administration of tirofiban after urokinase-mediated intravenous thrombolysis reduces early neurological deterioration in patients with branch atheromatous disease. J Int Med Res. 2020;48(5):300060520926298. https://doi.org/10.1177/0300060520926298.
Zanoli L, Boutouyrie P, Fatuzzo P, Granata A, Lentini P, Ozturk K, et al. Inflammation and Aortic Stiffness: An Individual Participant Data Meta-Analysis in Patients With Inflammatory Bowel Disease. J Am Heart Assoc. 2017;6 10; doi: https://doi.org/10.1161/JAHA.117.007003.
Basili S, Raparelli V, Napoleone L, Talerico G, Corazza GR, Perticone F, et al. Platelet Count Does Not Predict Bleeding in Cirrhotic Patients: Results from the PRO-LIVER Study. Am J Gastroenterol. 2018;113(3):368–75. https://doi.org/10.1038/ajg.2017.457.
Tuttolomondo A, Pedone C, Pinto A, Di Raimondo D, Fernandez P, Di Sciacca R, et al. Predictors of outcome in acute ischemic cerebrovascular syndromes: The GIFA study. Int J Cardiol. 2008;125(13):391–6. https://doi.org/10.1016/j.ijcard.2007.03.109.
Tuttolomondo A, Di Raimondo D, Pecoraro R, Maida C, Arnao V, Della Corte V, et al. Early High-dosage Atorvastatin Treatment Improved Serum Immune-inflammatory Markers and Functional Outcome in Acute Ischemic Strokes Classified as Large Artery Atherosclerotic Stroke: A Randomized Trial. Medicine (Baltimore). 2016;95(13):e3186.
Wang S, Song X, Wang Y, Gao Y, Wu J. Elevated high-sensitivity C-reactive protein levels predict poor outcomes among patients with acute cardioembolic stroke. Ann Palliat Med. 2021;10(3):2907–16. https://doi.org/10.21037/apm-20-1927.
Hotter B, Hoffmann S, Ulm L, Montaner J, Bustamante A, Meisel C, et al. Inflammatory and stress markers predicting pneumonia, outcome, and etiology in patients with stroke: Biomarkers for predicting pneumonia, functional outcome, and death after stroke. Neurol Neuroimmunol Neuroinflamm. 2020;7 3; doi: https://doi.org/10.1212/NXI.0000000000000692.
Liang J, Liu Y, Xu X, Shi C, Luo L. Cerebral Perforating Artery Disease : Characteristics on High-Resolution Magnetic Resonance Imaging. Clin Neuroradiol. 2019;29(3):533–41. https://doi.org/10.1007/s00062-018-0682-4.
Liao S, Deng Z, Wang Y, Jiang T, Kang Z, Tan S, et al. Different Mechanisms of Two Subtypes of Perforating Artery Infarct in the Middle Cerebral Artery Territory: A High-Resolution Magnetic Resonance Imaging Study. Front Neurol. 2018;9:657. https://doi.org/10.3389/fneur.2018.00657.
Chinese Society of Neurology, Cerebrovascular disease group of Chinese Society of Neurology. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. 2018;51 9:666–82 (in Chinese); doi: https://doi.org/10.3760/cma.j.issn.1006-7876.2018.09.004.
Zhang Z, Fan Z, Kong Q, Xiao J, Wu F, An J, et al. Visualization of the lenticulostriate arteries at 3T using black-blood T1-weighted intracranial vessel wall imaging: comparison with 7T TOF-MRA. Eur Radiol. 2019;29(3):1452–9. https://doi.org/10.1007/s00330-018-5701-y.
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–7. https://doi.org/10.1161/01.str.19.5.604.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57. https://doi.org/10.1056/NEJMoa0904327.
Siegler JE, Martin-Schild S. Early Neurological Deterioration (END) after stroke: the END depends on the definition. Int J Stroke. 2011;6(3):211–2. https://doi.org/10.1111/j.1747-4949.2011.00596.x.
Noordzij M, Dekker FW, Zoccali C, Jager KJ. Sample size calculations. Nephron Clin Pract. 2011;118(4):c319–23. https://doi.org/10.1159/000322830.
Kim BJ, Kim JS. Ischemic stroke subtype classification: an asian viewpoint. J Stroke. 2014;16(1):8–17. https://doi.org/10.5853/jos.2014.16.1.8.
Arboix A, Garcia-Eroles L, Massons J, Oliveres M, Targa C. Lacunar infarcts in patients aged 85 years and older. Acta Neurol Scand. 2000;101(1):25–9. https://doi.org/10.1034/j.1600-0404.2000.00005.x.
Jiang S, Yan Y, Yang T, Zhu Q, Wang C, Bai X, et al. Plaque Distribution Correlates With Morphology of Lenticulostriate Arteries in Single Subcortical Infarctions. Stroke. 2020;51(9):2801–9. https://doi.org/10.1161/STROKEAHA.120.030215.
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18(11):1058–66. https://doi.org/10.1016/S1474-4422(19)30078-X.
Grau-Olivares M, Arboix A, Junque C, Arenaza-Urquijo EM, Rovira M, Bartres-Faz D. Progressive gray matter atrophy in lacunar patients with vascular mild cognitive impairment. Cerebrovasc Dis. 2010;30(2):157–66. https://doi.org/10.1159/000316059.
Acknowledgements
We would like to thank all researchers and patients for their collaboration and contribution to this study.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-D-007).
Author information
Authors and Affiliations
Contributions
SL and JN contributed to the conception, design of the work, the acquisition, analysis, interpretation of data for the work, and drafting of the work. DL, XF and FF conducted the interpretation of data, and analysis of neuroimage. JQ conducted the collection of imaging data. BP contributed to the study design and critical review of manuscript. MY, LZ, and YZ collected the data, critically read the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Peking Union Medical College Hospital on May 25, 2021 (No. ZS-2982B). In addition, ethical approval of BAD-study protocol at each center has been obtained, and all participants provide written consent (model consent form) at the time of enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, S., Ni, J., Fan, X. et al. Study protocol of Branch Atheromatous Disease-related stroke (BAD-study): a multicenter prospective cohort study. BMC Neurol 22, 458 (2022). https://doi.org/10.1186/s12883-022-02976-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02976-9