Abstract
Background
Pontocerebellar hypoplasia (PCH) is increasingly known as a degenerative disease rather than simple “hypoplasia”. At least 21 disease-causing genes have been identified for PCH so far. Because PCH is very heterogenous, prognostic prediction based solely on clinical or radiologic findings is not feasible.
Case presentation
Here, we report two siblings who had a fulminant neonatal course. The documentation of pontocerebellar hypoplasia by postmortem brain CT imaging in one of the siblings and a subsequent complex and comprehensive whole genome analysis established that both siblings had bi-allelic compound heterozygous variants (a splicing variant and a deletion) in the SLC25A46 gene which encodes a solute carrier protein essential for mitochondrial function. Long-read whole genome sequencing was required to confirm the presence of the deletion. The fulminant courses suggest that SLC25A46-related PCH is an acutely progressive degenerative condition starting in utero, rather than a simple static hypoplasia.
Conclusion
The genomic analysis was instrumental and essential to solving the enigma of the unexplained neonatal deaths of these two siblings and to provide accurate genetic counseling.
Similar content being viewed by others
Background
Pontocerebellar hypoplasia (PCH) is characterized by the degeneration of the cerebellum and pons and was first described in 1917. Other features include severe psychomotor retardation, optic nerve atrophy, progressive myoclonic ataxia, and axonal peripheral neuropathy [1]. Indeed, “Hypoplasia” is a misnomer because the loss of volume in the cerebellum and pons is caused by the degeneration of Purkinje cells, rather than by true developmental hypoplasia [2]. Although there are no indications for surgical interventions for PCH, recognition of this progressive entity is critical because the natural course is completely different from those of other non-progressive disorders.
PCH has been categorized into several subtypes. So far, at least 21 disease-causing genes are known [3, 4]. One such causative gene, SLC25A46 encodes solute carrier family 25 member 46, a modified carrier protein that is recruited to the mitochondrial outer membrane and is involved in the maintenance of mitochondrial cristae structure by interacting with mitofusin2 (MFN2), Optic Atrophy 1 (OPA1), and the mitochondrial contact site and cristae organizing system [5]. So far, SLC25A46 variants have been reported in 19 PCH patients from 14 unrelated families [6, 7].
Here, we report two siblings with SLC25A46 variants who had fulminant and lethal neonatal courses. Genomic diagnosis was achieved through the results of sophisticated whole genome sequencing methods, which correlated with post-mortem brain CT.
Case presentation
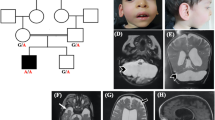
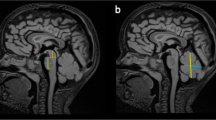
The proband was a 15-day-old male born at 37 weeks and 1/7 days of gestation to unrelated parents. His birthweight was 2578 g (− 0.16 SD), his body length was 47 cm (− 0.22 SD), and his head circumference was 35.3 cm (1.92 SD). The pregnancy was a spontaneous one, and amniotic fluid overload, fetal effusion, and fetal ascites were noted after 36 weeks of pregnancy. The patient’s Apgar Score was severely depressed (i.e., 1 at 1 min, 1 at 5 min, and 2 at 10 min, with all scores for pulse only), and all other parameters such as appearance, grimace, activity and respiration were zero [8]. At birth, the baby was not breathing spontaneously and did not respond to resuscitation, resulting in severe neonatal hypoxic-ischemic injury. Further treatment included cardiovascular support, inhaled nitric oxide therapy, and high frequency oscillatory ventilation, but the response to any treatment was poor, with worsening edema, increased pleural effusions, ascites, and anuria. The pleural fluid was not chyle, and lymphatic hypoplasia was ruled out. The lungs of the baby were hypoplastic. Because of the patient’s critical condition, imaging evaluation of the brain could not be performed, but trans fontanel ultrasonography showed an enlarged cisterna magna. The patient died at the age of 19 days. Postmortem CT imaging of the head showed cerebellar hypoplasia (Fig. 1). No autopsy was performed.
A significant family history included an elder brother who had died at the age of 12 h. The brother had been born at 36 weeks and 6 days of gestation and had been noted to have a breech presentation and polyhydramnios during the fetal period; he passed away because of severe neonatal asphyxia and cardiopulmonary failure with hypoplastic lungs. The parents had no diseases of note.
An exome analysis from the peripheral blood of the proband and his parents was performed, as previously reported [9]. The proband had an apparently homozygous variant of the SLC25A46 gene (Chr5(GRCh37):g.110081969G > A, NM_138773.2 c.385-1G > A) (Fig. 2a). This variant is located within the canonical consensus “AG” dinucleotide. The predicted effect of this variant on splicing was high (0.8 within the 0–1.0 scale) according to the deep learning-based tool SpliceAI [10]. As for the deceased brother, the dried umbilical cord had been preserved because for religious reasons. Targeted sequencing was performed on the cord-derived DNA, and an apparently homozygous variant identical to that of the patient was detected. The mother was heterozygous for the c.385-1G > A allele, but the father did not have this variant. We considered two possibilities: uniparental isodisomy, or a heterozygous deletion derived from the father. Uniparental isodisomy was less likely based on the recurrence of “apparent homozygosity” in the two affected siblings. Furthermore, homozygosity mapping of the exome data using the computer program H3M2 excluded uniparental disomy [11].
Molecular analysis of the patient and his family members. (a) A Sanger sequencing of the DNA derived from the peripheral blood of the proband and his parents and from dried umbilical cord of his deceased brother revealed that the patient and his deceased brother had a hemizygous variant of the SLC25A46 gene, Chr5(GRCh37):g.110081969G > A, NM_138773.2 c.385-1G > A, which was derived from his mother. (b) Paternally derived 80-kb deletion spanning the TMEM232 and SLC25A46 loci. Top: Coverage diagram of the short-read whole genome sequencing in the father. Bottom: Coverage diagram of the short-read whole genome sequencing and long-read sequencing of PCR products amplified across the deletion. The deletion spans highly homologous L1 elements (L1PA4 in the intron of theTMEM232 locus and L1PA2 at the intergenic region telomeric to the SLC25A46). (c) Detailed view of the extent of the deleted region based on GRCh37 (hg19) coordinates
Multiple programs for the detection of copy number variants from exome data, including XHMM, Excavator2, and FishingCNV were used to search for a potential deletion involving the SLC25A46 locus [12,13,14]. None of these programs detected a deletion spanning the SLC25A46 locus. Whole genome sequencing of the proband’s sample was performed using a short-read sequencer (NovaSeq6000; Illumina) according to the manufacturer’s instructions. Samples were processed through an alignment and structural variant detection pipeline using the DRAGEN 3.5 suite for the Illumina data. The presence of an 80-kb deletion (chr5:110,032,569-110,113,234) (GRCh37/hg19) spanning SLC25A46 and TMEM232 was detected in both patients and the father (Fig. 2b and c). TMEM232 is not known to be associated with human diseases.
To better characterize the breakpoint, we designed polymerase chain reaction (PCR) primers for the amplification of genomic regions spanning the breakpoints. The left primer was 5′-CCCGAGCTATTAGTCCTCAAAC-3′, and the right primer was 5′-AATGATTTTGCCCCATCATTAC-3. The amplicon was further analyzed using a long-read sequencer (PromethION system; Oxford Nanopore Technologies). Briefly, a sequencing library was constructed from the amplicon using the Ligation Sequencing Kit SQK-LSK109 and the Native barcoding Expansion Kit EXP-NBD104, following the manufacturer’s instructions; sequencing was then performed using the FLO-PRO002 R9.4.1 Flow Cell on the PromethION system. Basecalling and demultiplexing were performed using Guppy Basecaller v.4.3 on the same instrument. The obtained long read sequences were mapped against the GRCh37/hg19 human reference genome using the LRA program v1.1.2 [15], and the breakpoint of the deletion was determined by manual inspection using the Integrative Genomics Viewer [16]. Both breakpoints were within highly homologous LINE transposon sequences, L1PA4 and L1PA2. The deletion was likely to be mediated through non-allelic homologous recombination (Fig. 2c).
DISCUSSION and CONCLUSIONS
Here, we report two siblings with PCH caused by bi-allelic compound heterozygous variants in the SLC25A46 gene. A genomic analysis was instrumental and essential to solving the enigma of the unexplained neonatal deaths of the two siblings. The fulminant courses indicate that SLC25A46-related PCH is as an acutely progressive degenerative condition starting in utero, rather than a simple static hypoplasia.
Our observation gives further credence to the emerging notion that biallelic loss-of-function variants (e.g., truncating and splicing variants or gene deletion) of SLC25A46 lead to PCH. The fulminant neonatal course of PCH is in sharp contrast to other phenotypic expressions of biallelic variants of SLC25A46, including “Neuropathy, hereditary motor and sensory, type VIB (OMIM: 616505),” optic atrophy and parkinsonism.
The concept of PCH was first coined by Dr. Peter G. Barth [17] as a degenerative brain disorder with a fetal onset. Later it became apparent that some types of PCH are progressive whereas others are non-progressive. Furthermore, a large number of disorders have imaging patterns that are compatible with PCH (i.e., reduced volumes in the pons and cerebellum) [18, 19]. Hence, a genomic etiologic investigation is desirable, in addition to radiologic studies in the evaluation of reduced pons and cerebellum volumes because both the prognosis and recurrence risk depend on the etiologic diagnosis.
The identification of both mutant alleles was crucial in providing a precise genetic diagnosis and subsequent genetic counseling, including information on possible preimplantation diagnosis. A conventional exome analysis using short-read sequencing succeeded in the detection of one mutant allele, but not the other mutant allele. The utilization of long-read whole genome sequencing resulted in the detection of the deletion and its extent, ultimately leading to a confirmatory diagnosis. The diagnostic clue for finding the deletion in the presently reported family was “apparent homozygosity” for the splice-disrupting allele detected using short-read sequencing. The lack of the allele in one of the parents (i.e., the father) despite “apparent homozygosity” prompted a further analysis using short-read whole genome sequencing and subsequent long-read whole genome sequencing. The serial application of increasingly sophisticated analytic methods led to the eventual success in the detection of the deletion derived from the father.
In view of recent studies showing the central role of mitochondrial defects in degenerative forms of PCH, various drugs used for mitochondrial protection may be helpful for patients with SLC25A46 defects [20]. A prompt and precise definition of the causative gene in each patient with PCH during the neonatal period may open a door to potential pharmacologic interventions in the future.
Abbreviations
- PCH:
-
PontoCerebellar hypoplasia
- SLC25A46 :
-
Solute Carrier Family 25 Member 46
- MFN2:
-
Mitofusin2
- OPA1:
-
Optic Atrophy 1
References
van Dijk T, Baas F, Barth PG, Poll-The BT. What's new in pontocerebellar hypoplasia? An update on genes and subtypes. Orphanet J Rare Dis. 2018;13:92.
Namavar Y, Barth PG, Poll-The BT, Baas F. Classification, diagnosis and potential mechanisms in pontocerebellar hypoplasia. Orphanet Journal of Rare Diseases. 2011;6:50.
Appelhof BBP, Baas F. Classification of pontocerebellar hypoplasia: where does it end? Eur Med J Neurol. 2019;7:52–61.
Appelhof B, Wagner M, Hoefele J, Heinze A, Roser T, Koch-Hogrebe M, et al. Pontocerebellar hypoplasia due to bi-allelic variants in MINPP1. Eur J Hum Genet. 2021;29:411–21.
Lahiri S, Chao JT, Tavassoli S, Wong AK, Choudhary V, Young BP, et al. A conserved endoplasmic reticulum membrane protein complex (EMC) facilitates phospholipid transfer from the ER to mitochondria. PLoS Biol. 2014;12:e1001969.
Abrams AJ, Fontanesi F, Tan NBL, Buglo E, Campeanu IJ, Rebelo AP, et al. Insights into the genotype-phenotype correlation and molecular function of SLC25A46. Hum Mutat. 2018;39:1995–2007.
Braunisch MC, Gallwitz H, Abicht A, Diebold I, Holinski-Feder E, Van Maldergem L, et al. Extension of the phenotype of biallelic loss-of-function mutations in SLC25A46 to the severe form of pontocerebellar hypoplasia type I. Clin Genet. 2018;93:255–65.
American Academy Of Pediatrics Committee On F, Newborn, American College Of O, Gynecologists Committee On Obstetric P. The Apgar score. Pediatrics. 2015;136:819–22.
Yamada M, Suzuki H, Watanabe A, Uehara T, Takenouchi T, Mizuno S, et al. Role of chimeric transcript formation in the pathogenesis of birth defects. Congenit Anom (Kyoto). 2021;61:76–81.
Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, et al. Predicting splicing from primary sequence with deep learning. Cell. 2019;176(535–548):e524.
Magi A, Tattini L, Palombo F, Benelli M, Gialluisi A, Giusti B, et al. H3M2: detection of runs of homozygosity from whole-exome sequencing data. Bioinformatics. 2014;30:2852–9.
Fromer M, Moran JL, Chambert K, Banks E, Bergen SE, Ruderfer DM, et al. Discovery and statistical genotyping of copy-number variation from whole-exome sequencing depth. Am J Hum Genet. 2012;91:597–607.
Shi Y, Majewski J. FishingCNV: a graphical software package for detecting rare copy number variations in exome-sequencing data. Bioinformatics. 2013;29:1461–2.
D'Aurizio R, Pippucci T, Tattini L, Giusti B, Pellegrini M, Magi A. Enhanced copy number variants detection from whole-exome sequencing data using EXCAVATOR2. Nucleic Acids Res. 2016;44:e154.
Ren J, Chaisson MJP. Lra: a long read aligner for sequences and contigs. PLoS Comput Biol. 2021;17:e1009078.
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–6.
Barth PG, Vrensen GF, Uylings HB, Oorthuys JW, Stam FC. Inherited syndrome of microcephaly, dyskinesia and pontocerebellar hypoplasia: a systemic atrophy with early onset. J Neurol Sci. 1990;97:25–42.
Marin-Valencia I, Gerondopoulos A, Zaki MS, Ben-Omran T, Almureikhi M, Demir E, et al. Homozygous mutations in TBC1D23 Lead to a non-degenerative form of pontocerebellar hypoplasia. Am J Hum Genet. 2017;101:441–50.
Rusch CT, Bolsterli BK, Kottke R, Steinfeld R, Boltshauser E. Pontocerebellar hypoplasia: a pattern recognition approach. Cerebellum. 2020;19:569–82.
Singh A, Faccenda D, Campanella M. Pharmacological advances in mitochondrial therapy EBioMedicine. 2021;65:103244.
Acknowledgements
We thank Ms. Chika Kanoe, Ms. Yumi Obayashi and Ms. Keiko Tsukue for their technical assistance in the preparation of this article.
Data availability and materials
The data that support the findings of this study are openly available in Database of Pathogenic Variants at DPV: 12211 (https://dpv.cmg.med.keio.ac.jp/dpv-pub/variants/12211).
Funding
This work was supported by Initiative on Rare and Undiagnosed Diseases (grant number JP21ek0109549) and Practical Research Project for Rare and Intractable Diseases (grant number JP21ek0109485) from the Japan Agency for Medical Research and Development.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MY, HS, HA, AN, FM, TT, and KK. The first draft of the manuscript was written by MY and HS (equally contributed), and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Bioethics Committee of Keio University school of medicine (No. 20110262). Written informed consent for molecular studies and publication of identifiable images was obtained from the patient’s parents.
Competing interests
All authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yamada, M., Suzuki, H., Adachi, H. et al. Diagnosis of SLC25A46-related pontocerebellar hypoplasia in two siblings with fulminant neonatal course: role of postmortem CT and whole genomic analysis: a case report. BMC Neurol 22, 20 (2022). https://doi.org/10.1186/s12883-021-02540-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-021-02540-x