Abstract
Background
Cognitive dysfunction affects up to 70% of people with progressive MS (PMS). It can exert a deleterious effect on activities of daily living, employment and relationships. Preliminary evidence suggests that performance can improve with cognitive rehabilitation (CR) and aerobic exercise (EX), but existing data are predominantly from people with relapsing-remitting MS without cognitive impairment. There is therefore a need to investigate whether this is also the case in people with progressive forms of the disease who have objectively identified cognitive impairment. It is hypothesized that CR and EX are effective treatments for people with PMS who have cognitive impairment, in particular processing speed (PS) deficits, and that a combination of these two treatments is more effective than each individual treatment given alone. We further hypothesize that improvements in PS will be associated with modifications of functional and/or structural plasticity within specific brain networks/regions involved in PS measured with advanced MRI techniques.
Methods
This study is a multisite, randomized, double-blinded, sham controlled clinical trial of CR and aerobic exercise. Three hundred and sixty subjects from 11 sites will be randomly assigned into one of four groups: CR plus aerobic exercise; CR plus sham exercise; CR sham plus aerobic exercise and CR sham plus sham exercise. Subjects will participate in the assigned treatments for 12 weeks, twice a week.
All subjects will have a cognitive and physical assessment at baseline, 12 weeks and 24 weeks. In an embedded sub-study, approximately 30% of subjects will undergo structural and functional MRI to investigate the neural mechanisms underlying the behavioral response. The primary outcome is the Symbol Digit Modalities Test (SDMT) measuring PS. Secondary outcome measures include: indices of verbal and non-verbal memory, depression, walking speed and a dual cognitive-motor task and MRI.
Discussion
The study is being undertaken in 6 countries (11 centres) in multiple languages (English, Italian, Danish, Dutch); with testing material validated and standardized in these languages. The rationale for this approach is to obtain a robustly powered sample size and to demonstrate that these two interventions can be given effectively in multiple countries and in different languages.
Trial registration
The trial was registered on September 20th 2018 at www.clinicaltrials.gov having identifier NCT03679468. Registration was performed before recruitment was initiated.
Similar content being viewed by others
Background
A recent comprehensive review of rehabilitation studies in progressive MS (PMS) encompassing balance, weakness, cardiovascular fitness, ataxia, fatigue, bladder dysfunction, spasticity, pain, cognitive deficits, depression and pseudobulbar affect concluded that there was a striking dearth of studies devoted solely to people with secondary progressive MS (SPMS) or primary progressive MS (PPMS) [1]. Furthermore, when including progressive patients alongside patients with relapsing-remitting disease (RRMS), subject numbers has generally been very small while analysis of treatment effects have not accounted for the possible influence of disease course. Finally, in the few studies reporting benefits of treatment for patients with progressive disease, the ecological validity of the results remained uncertain.
The present study focuses on improving cognition in people with PMS for two reasons.
First, up to 70% of people with PMS are impaired in this domain [2] and second, people with MS have themselves identified cognitive dysfunction as a primary area of concern [3]. Furthermore, there are now a number of studies suggesting that cognitive rehabilitation (CR) can result in significant improvements in numerous cognitive domains. A consistent picture is emerging of CR bringing about improvements in learning/memory [4, 5] and processing speed [6, 7]. Of note is that home-based CR programs have also reported significant cognitive gains [8, 9] as have interventions administered in a group setting [10, 11]. Complementing these data are findings suggesting that exercise too can provide physical, cognitive and emotional benefits [12,13,14].
These results, while encouraging, are also limited by various factors including small sample size, single centre administration and sample composition predominately limited to people with RRMS. Whether people with progressive disease will derive the same benefits from these interventions is therefore not known, although a single study suggests that improvement in memory may be possible with CR [4]. The gaps in our knowledge therefore suggest a number of complementary ways forward. Multisite replication of these preliminary, promising findings is a good place to start. However, as people with progressive MS constantly remind us, time is short. This suggests that an additional effort is required, a bolder approach, one that combines more than one intervention with the aim of producing synergistic effects, an improvement in one area boosting the putative benefits of therapy in another, the overall outcome exceeding the sum of the individual treatments. Such an approach often reflects the clinical reality of PMS where multiple neurological difficulties rather than an isolated problem must be addressed simultaneously. While a powered joint CR and exercise clinical trial has yet to be done in people with MS of any disease course there is tentative evidence from three small studies in people with RRMS that this approach may be more beneficial than either intervention alone [15,16,17]. Thus, a methodologically rigorous, well-powered study is needed now in PMS specifically in an effort to inform clinical practice. It is only through such a study that we can ensure that final conclusions and recommendations are not limited by sample size, generalizability or methodological concerns.
Methods/design
The protocol adheres to the Spirit guidelines.
Aim, design and setting of the study
The study has the following primary aims:
-
1.
To assess whether CR and exercise (EX) in combination have beneficial synergistic effects in the treatment of impaired processing speed in people with PMS.
-
2.
To determine whether CR and EX are individually effective treatments for impaired processing speed in people with PMS.
Secondary aims:
-
1.
To assess the everyday life impact of cognitive and/or physical changes after the different rehabilitation interventions.
-
2.
To assess brain functional and structural substrates of cognitive changes after the different rehabilitation interventions.
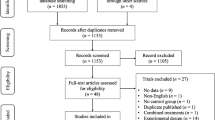
This study is a multicenter, multi-arm, randomized, double-blinded, sham-controlled trial that includes follow up periods of 12- and 24 weeks (+/− 2 weeks) post randomization. As depicted in Fig. 1, after the baseline assessment each participant will be randomized to one of four arms with different combinations of CR and EX and their respective shams (−S). That is: CR + EX; EX + CR-S; EX-S + CR; EX-S + CR-S.
Study flow-chart. RRMS, Relapsing-Remitting Multiple Sclerosis; EDSS, Expanded Disability Status Scale; CNS, Central nervous system; PMS, Progressive Multiple Sclerosis; GLTEQ, Godin Leisure-Time Exercise Questionnaire; BDI-II Beck Depression Inventory-II; SDMT, Symbol Digit Modalities Test; CR-S, cognitive rehabilitation - sham; EX-S, exercise-sham; EX, exercise; CR, cognitive rehabilitation
The study protocol will be carried out at 11 sites in six different countries (Canada (1 site), USA (2 sites), United Kingdom (2 sites), Denmark (1 site), Belgium (1 site) and Italy (4 sites)). Each site has at least one blinded and one unblinded research assistant. The blinded measurement assessor (research assistant) will screen potential participants and perform all baseline and 6-month testing, whereas the 3-month follow up interview will be made by the unblinded research assistant. If a participant meets the inclusion criteria (see later), the unblinded research assistant is responsible for randomizing participant using REDCap (a web-based system) and conducting the interventions for which the participant is assigned. As the study design aims to mask the intent of the intervention given to the participants, the unblinded research assistant will be strictly instructed not to discuss participant allocation and participants will likewise be instructed not to reveal details that can indicate their group allocation to the blinded assessor. The MRI analysis will be undertaken by experienced technicians blinded to group allocation.
Recruitment and screening of participants
Participants are being recruited via specialized in and out-patient MS clinics, as well as via media advertising. Prior to enrollment, all potential participants will undergo a two-step screening procedure. First, a pre-screening examination in person or via telephone will collect basic information. If the participant passes the initial pre-screen, a detailed face-to-face screening for neurological, psychiatric, cognitive, and medical variables will take place at the participating center. Inclusion and exclusion criteria for the two screening steps are summarized in Tables 1 and 2, respectively.
When participants complete the study they will revert to their neurologist and usual care programs.
Randomization
Participants will be randomized to a treatment arm using block randomization by site. Block sizes will be blinded to study personnel and principal investigators. Randomization assignment will be conducted in REDCap (a secure web application for building and managing databases) within 24 h after the baseline assessment.
Interventions
For all interventions, comprehensive manuals were developed and distributed to all participating sites to ensure optimal standardization. This was complemented by face-to-face and distance training at the beginning of the study, and supplemented as necessary during the course of the study. All interventions will be delivered within the hospital/clinic setting or research center under individual supervision, twice per week over a 12 week period. All intervention sessions will start with either CR or CR-S followed by EX or EX-S. In case of holiday, sickness or other unforeseen circumstances the intervention period may be extended by up to two additional weeks, allowing the intervention period to be a maximum of 14 weeks. During the intervention period, compliance to the exercise prescription (attended/planned sessions; actual intensity/target intensity; actual duration/target duration) and cognitive rehabilitation protocols will be monitored by an independent person who will provide regular feedback to sites to optimize the fidelity of the treatment regimens.
Any potential reason for discontinuing will be discussed on individual basis between the principal investigator of the site and the Data Safety Monitoring Board. If a situation occurs where precaution has to be taken in order for the participant to continue in the study, unblinding will be permissible if permitted by the study steering committee.
Cognitive rehabilitation (CR)
The CR component comprises the computerized RehaCom program (Pearson’s Clinical Assessment group, Bloomington, MA, USA) that will be performed on non-consecutive days. To address processing speed, the modules “divided attention 1 and 2”, “attention and control”, “sustained attention” and “vigilance 2” will be used. Participants will begin at level 1 and advance the program as dictated by their performance. Each session will be programmed to last 45 min. The RehaCom program has previously been shown to have positive effects on processing speed in persons with RRMS [6, 8, 18].
Sham cognitive rehabilitation (CR-S)
The CR-S consists of Internet training [19], beginning with more basic tasks such as learning to use a computer and the internet to search for information, including locating information regarding medications, gardening, getting directions, etc. Each session is programmed to last 45 min and will also take place on non-consecutive days. The control session is designed to match the CR on social and computer contact. Similar training procedures have previously been shown not to impact PS in a normal aging sample [19].
Exercise (EX)
The exercise intervention is aerobic and performed on a recumbent stepper (NuStep T5XR, Ann Arbor, MI, USA). All sessions are supervised and performed on an individual basis. Training is performed on non-consecutive days thereby permitting recovery between sessions. The EX intervention consists of twice weekly sessions, one of which is continuous exercise and the other of which is high intensity interval training. It complies with the basic principle of progressive overload. The continuous training ranges from 10 min of exercise at a work rate corresponding with 50–60% of VO2peak at week one, progressing to 30 min of exercise at a work rate corresponding with 70–80% of VO2peak in week 12. The interval training begins with 5 × 1 min of exercise at a work rate corresponding with 80–90% VO2peak followed by 1 min of active breaks with a work rate at 15 watts. At week 12 the protocol will have progressed to 10 × 2 min of exercise at a work rate corresponding with 90% VO2peak followed by 2 min of active breaks with a work rate of 15 watts. Protocols built on the same principles have previously improved VO2peak in persons with MS [20, 21]. The recumbent stepper represents an acceptable modality of aerobic exercise in people with PMS [22] as has interval training using this device. See Table 3 for further details.
Sham exercise (EX-S)
The EX-S does not put strain on the cardiovascular system, so as to avoid a potential aerobic effect, and avoids incorporating progressive resistance strengthening (the use of weights are not permitted) as improvement in aerobic capacity [23] and lower limb muscle strength [24] have been associated with faster cognitive processing speed. Moreover, no focused dual task activities are performed during the training sessions, so as to avoid any potential cognitive training. Hence the EX-S is focusing on balance, co-ordination and stretching, which is a credible sham exercise comparator.
The EX-S sessions have been designed to reflect the EX intervention for time and attention, hence the frequency and duration of sessions, and the manner in which the training times progress throughout the 12 week program mimic that described above. During all EX-S sessions at least one exercise from each of six different categories is performed to allow variation; these are selected on the basis of individual need (exercises summarized in Table 4). To ensure that exercises are at a light intensity, heart rate and rate of perceived exertion (RPE) are monitored throughout the training sessions, after completion of each exercise (i.e. a minimum of six times). Should either the HR or RPE increase above pre-set criteria, an enforced rest is required by the participant to prevent any potential aerobic effect of the exercise. The EX-S protocol builds on a sham intervention that was applied in a previous study in people with MS [25].
Follow up
To encourage people to continue exercising post supervision the goals of the person with MS will be discussed and taken into account at the beginning of the program, and then reviewed on a further three occasions (every 4 weeks) during the 12 week intervention. This is a practical, and commonly used strategy for positively affecting behavioral change and engagement with rehabilitation programs [26].
Study status
By March 2020, a total of 135 participants have been randomized into the study. Of these, 40 are now in the intervention phase, 90 have completed the immediate follow up, 63 have completed the 3-month follow up and 37 have completed the 6-month follow up.
Outcomes and assessments
Primary outcome
The primary outcome of this study is the change in processing speed (PS) over the 12 weeks of training, assessed with the Symbol Digit Modalities Test (SDMT). The SDMT is available in several versions, and in the present study, 3 versions will be used in a randomized order to minimize practice effects when repeated [27]. There are several reasons why we chose PS as the primary outcome measure. First, it is well known that PS is the primary cognitive impairment in persons with MS [28] and as a primary cognitive construct, impaired PS itself can lead to problems in higher cognitive functioning such as executive abilities [29]. As such, improving PS may also improve other cognitive areas. Second, there is good preliminary data from smaller studies from multiple laboratories that both CR and exercise improve PS [24, 30,31,32]. Third, the existing RehaCom literature shows the most consistent and significant effect on PS [18, 33,34,35,36]. Fourth, a recent topical review of the SDMT found strong evidence supporting the reliability and validity of the test and recommended a responder definition of SDMT change approximating 4 points or 10% in magnitude [37]. Lastly, after an extensive review of potential cognitive outcome measures by the Multiple Sclerosis Outcome Assessment Consortium, the SDMT was recommended to the Federal regulators as the cognitive test of choice to be included in MS clinical trials [38].
Secondary outcome
All secondary outcomes will be assessed during the in-person interview or the baseline assessment, at the post 12-week assessment and at the 24-week follow-up assessment.
Study assessments
Study assessments are composed of the neuropsychological and exercise assessments, MRI, and the completion of the patient reported outcomes (PROs).
For a detailed overview of all outcomes and the timing of assessment see Table 5.
Neuropsychological evaluation
This is conducted in one session to document current levels of cognitive performance. The neuropsychological assessment includes a standard, widely accepted assessment battery for MS, the BICAMS [39]. The BICAMS consists of our primary outcome measure, the SDMT, as well as two other cognitive tests of verbal and visual learning and memory that will be used as secondary outcome measures, namely the California Verbal Learning Test (CVLT) and the Brief Visuospatial Memory Test (BVMT-R). The BICAMS is available in the languages represented within our study sample (English, Italian, French, Dutch and Danish). Language specific normative data are available in all cases [40,41,42,43] except in the case for Denmark, where the Dutch norm-data will be applied. Z-scores computed for inclusion criteria used regression-based norms adjusting for linear and non-linear age, sex and total years of education for either the raw or scaled scores from the respective normative data. To provide an assessment of cognitive reserve the Wechsler Test of Adult Reading (WTAR) [44] will be administered at baseline. The WTAR is validated in the participant’s primary language, and an estimated IQ will be computed based on performance. This estimated IQ score will serve as a common metric across all participants for inclusion in analyses. For those countries which do not have WTAR data, the comparable Adult National Reading Test (ANART) [45] will be used.
Physical performance
Height and weight will be used to calculate the Body Mass index (BMI). An incremental cardiopulmonary exercise test (CPET) will be conducted to assess peak aerobic capacity and power using the recumbent stepper that is also used for the exercise intervention. The incremental CPET will be undertaken in a standardized manner, and with scripted instructions to the participant. Expired gases will be collected using a 2-way non-rebreathable valve (e.g. Hans Rudolph, Kansas City, MO, USA or the like) and oxygen consumption will be continuously measured using an open circuit spirometry system (e.g. TrueOne, Parvo Medics, Sandy, UT, USA or similar). Participants will complete a 1-min warm-up at 15 W. The initial work rate will be set to 15 W and gradually increase until the participant reaches volitional fatigue. The work rate will be increased by 10 W per minute or 5 W per minute for participants with mild to moderate (i.e., EDSS of 4.0–5.5) or severe disability (i.e., EDSS of > 6.0), respectively. Participants will be encouraged to maintain a stepping rate of 60–100 steps per minute throughout the test depending on the work rate. Heart rate (Polar FT1 Heart Rate Monitor, Polar Electro Inc., Bethpage, NY, USA), and Ratings of Perceived Exertion (RPE) via the Borg Rating of Perceived Exertion Scale will be recorded every minute. The highest recorded 20-s rate of oxygen consumption value (VO2) will be recorded as peak oxygen consumption (VO2peak), expressed in mL/kg/min, optimally when two or more of the following criteria is satisfied: (1) respiratory exchange ratio (RER) of 1.10 or greater; (2) peak heart rate within 10 beats per minute of age-predicted maximum (i.e., 220-age); or (3) RPE of 17 or greater. The highest recorded power achieved during a 20-s period will be recorded as peak power output (Wpeak). This CPET protocol has previously been used in persons with MS [46], and will be used for measuring changes in aerobic capacity and for prescribing the recumbent stepper exercise training sessions [21].
Walking performance will be assessed by the 6 min walk test (6MWT). Subjects will be instructed to walk at their fastest speed, and to cover as much distance as possible, according to the script of Goldman et al. [47]. Subjects will be notified, without further encouragement, about each expired minute. Distances walked per minute and total distance will be recorded. Subjects walk back and forth along a 30-m hallway turning around cones at each end. In centers without this facility, a square trajectory is allowed given that this has been shown not to compromise results [48].
Cognitive-motor interference during walking will be quantified by a dual task cost (DTC) calculation in the motor and cognitive domains. The DTC calculation is based on a comparison of the performance on a single motor or cognitive task and the motor and cognitive performance during a concurrent motor plus cognitive dual task. The formula is DTC = ((DT-ST)/ST)*100. There are data suggesting that this dual modality testing can give additional insights into the putative benefits of the proposed interventions [49,50,51]. The single motor task requires the participant to walk at the fastest possible speed while maintaining safety for 60 s. The distance covered is measured. The setting for the 6MWT (30 m corridor) will be used for this test. The single cognitive task entails performing the alternating Latin alphabet for 60 s [52]. During the task, subjects list alternating letters of the alphabet as fast and accurately as possible (i.e., A, C, E, G, etc.). The number of correct letters provided by the subjects is recorded. The test requires working memory and inhibitory control. The cognitive-motor dual task involves walking at fast speed for 60 s with the alternating alphabet test as a concurrent task. Subjects will be instructed to divide attention equally on walking while correctly naming alternating letters of the alphabet.
Physical activity will be determined by accelerometry. Participants will wear the accelerometer (Actigraph; http://actigraphcorp.com/) on an elastic belt around the waist located above the non-dominant hip during the waking hours for 7 days before the first intervention week and during the week following completion of the intervention. This method has proven reliable in people with MS [53]. It will provide data on the degree of lifestyle physical activity (i.e. steps/day and minutes/day of moderate to vigorous physical activity) immediately before and after the intervention phase.
Patent reported outcomes (PRO’s)
PRO’s include the HADS, BDI-II, MFIS and PDQ. The MSWS-12, MSIS-29 version 2 and EQ-5D-5L are all standardized self-report outcome measures having strong reliability and validity in people with MS [54, 55] and with evidence supporting their responsiveness in rehabilitation trials [55,56,57]. Their wide use in MS interventional studies will enable comparisons between studies. The MSWS-12 provides information on the subjective impact of MS on walking and related activities and therefore adds important information regarding what can be obtained from objective measures of walking. Furthermore, it has strong psychometric properties [58]. The MSIS-29 is a disease specific measure of the impact of MS; it has a preference-based tariff [59] for use in sensitivity analyses for the Quality Adjusted Life Year (QALY) outcome, and has been endorsed for use in health economic analyses in MS studies [60]. This will complement the EQ-5D-5L, which is recommended for use in health policy decision making [60]. The FAMS was specifically developed for use with the MS population and has been shown to have adequate reliability and validity within this population. The measure contains 59 questions organized into 6 subscales: mobility, symptoms, emotional well-being, general contentment, thinking/fatigue, and family/social [61]. The thinking/fatigue subscale is composed of 9 items, including questions pertaining to task initiation, task completion, new learning, memory, concentration, and slowness of thought. Participants rate their symptoms on a 5-point Likert type scale. This assessment focuses on the person as a whole, investigating impairments, functional limitations, and disability in many areas of the person’s life. This overview of functional status at all levels is important in determining the impact of cognitive treatment on an individual’s everyday life. The FAMS has demonstrated good internal consistency, reliability and validity [61]. The scale has been used successfully to measure change from before to after cognitive rehabilitation [62]. To provide an assessment of anxiety and depression at each assessment, the Hospital Anxiety Depression Scale (HADS) [63] will be administered. The Modified Fatigue Impact Scale (MFIS) [64] will additionally be administered to assess fatigue at each assessment. Subjective cognitive deficits will be assessed with the Perceived Deficits Questionnaire (PDQ) [65].
Brain MRI protocol
Brain MRI scans will be obtained using 3.0 Tesla scanners. Budgetary constraints dictate that the MRI is obtained in one third of the sample (i.e. 120 subjects divided equally (n = 30) between the four treatment arms). The following sequences will be collected at baseline, termination of the interventions and after 24 weeks of follow up, following a standardized protocol of acquisition and careful guidelines for patients repositioning: axial T2 weighted Turbo Spin Echo (TSE); axial FLAIR; high resolution 3D sagittal T1-weighted sequence; axial DT sequence (55 contiguous, 2.5 mm thick, slices, #DW direction = 64) and T2*-weighted single-shot echo-planar imaging (EPI) during and active cognitive fMRI task and at rest.
For active fMRI, the Go/No-go task will be administered using a block-design, as previously described [66]. Reaction times, omission errors (no response although required), commission errors (false response without adequate cue), and the proportion of correct responses will be recorded using a response-box. Before imaging, participants will be familiarized with the paradigm. The fMRI Go/no-Go paradigm has been used both in cross-sectional [67] and longitudinal [68] studies of people with MS. Notably, a longitudinal (median follow up 20 months) neuropsychological and fMRI evaluation detected significant correlations between worsening of SDMT performances and modification of activation during the Go/no-Go task in several supra- and infratentorial brain regions [68]. Most importantly, the Go/no-Go task has already been validated for multicentric acquisition [66]. During resting state (RS) fMRI, subjects will be instructed to remain motionless, to close their eyes and not to think about anything in particular. Movements will be minimized using foam padding and ear blocks.
The total duration of MRI acquisition (structural plus functional MRI) will be approximately 50 min.
MRI analysis: MRI data acquired for the study will be analyzed centrally at one Neuroimaging Research Unit (Hospital San Raffaele, Milan, Italy).
Lesion and atrophy analysis: Brain T2-hyperintense and T1-hypointense lesion volumes (LV) will be measured on FLAIR and 3D T1-weighted scans, respectively, using a local thresholding segmentation technique (Jim 7.0, Xinapse Systems, West Bergholt, UK). New lesions at follow-up will be counted. Normalized brain (NBV), WM (WMV) and GM (GMV) volumes will be measured on 3D T1-weighted scans using the SIENAx software, after T1-hypointense lesion refilling.168 Hippocampal volume will be estimated using FIRST software.
Mapping changes in gray matter (GM) and white matter (WM) structures: Voxel-based Morphometry (VBM) with DARTEL method will be applied to determine between-group differences of GM volumes at baseline, using SPM12 and 3D T1-weighted images. Tensor-based Morphometry (TBM) [69] will be applied to map the longitudinal regional variations of GM volume at the different time points.
Diffusion-weighted images will be corrected for distortions induced by the eddy currents and for head movements, and transformed to MNI (Montreal Neurological Institute) space. Then, using the FMRIB’s Diffusion Toolbox (http://www.fmrib.ox.ac.uk), the DT will be estimated in each voxel by linear regression [70] and mean diffusivity (MD), radial diffusivity (RD), axial diffusivity (AD) and fractional anisotropy (FA) maps derived. Tract-based Spatial Statistics (TBSS) will be used to define the patterns of microstructural WM abnormalities on diffusion tensor images at baseline and their variations during the follow up.
Analysis of fMRI data: Active and RS fMRI data will be pre-processed using SPM12. Activations during the Go/no-Go task will be estimated using SPM12. An independent Component Analysis (ICA) will be used to decompose RS fMRI data into spatially independent maps and time courses, using the GIFT software [71]. Individual functional maps will be converted to Z-scores before entering group statistics, to obtain voxel values comparable across subjects. A systematic process will be applied to inspect and select the components of interest from the estimated ones. The association of each component spatial map with a priori probabilistic maps of GM, WM, and CSF within the MNI space will contributed to identifying the components with a signal change correlated to the GM. Components with a high correlation with cerebrospinal fluid or WM, or with a low correlation with the GM, will be excluded. In addition, to identify components with potentially functional relevance, a frequency analysis of IC time courses will be performed to detect those with a high (50% or greater) spectral power at a low frequency (between 0.01 and 0.05 Hz) [72]. The spatial patterns of the remaining ICs will be sorted out on the basis of their matching with relevant RSNs found in previous studies [73,74,75,76] A seed-base RS functional connectivity (RS FC) analysis, using the thalami as a seed, will also be performed to assess modifications of RS FC of the thalamic network in the main study groups and their correlations with clinical scales [77].
Standardisation and data quality
To promote data quality, all assessment activites are manualised. Further, before initiating recruitment all PI’s, blinded and unblinded assessors participated in a training session at which all of the tests were discussed and demonstrated. Following this, every site performed a rehearsal incremental CPET on at least one MS patient and sent the data to the responsible PI for review. To ensure that all sites initialise their accelerometers correctly, the assessors themselves wore a device for 7 days, and the data sent to the same PI for review. Similarly, a mock MRI scan will occur at the four centers participating in the MRI substudy. All data will be entered into REDCap [78]. Before entering any data in the actual study database, all assessors will undertake practice lessons and complete a practice certification consisting of a set of ficticious data. Data will be downloaded regularly for quality control purposes and basic reports will be generated via the REDCap system and SAS. Data forms will employ validation and skip pattern logic that provides constrained input whenever possible. Errors or questionable data will be turned into data queries and will be sent by the Data Coordinating Center to the sites for correction and/or clarification. Reports will be produced and include information on data quality, completeness and protocol adherence.
Confidentiality is guaranteed through anonymity. Each participant is given an unique case number, and the Data Coordinating Center at Washington University are not given any identifying data.
Power analysis
The primary outcome measure is the SDMT. A four-point improvement on the SDMT is considered clinically useful [37, 38]. Evidence for the reliability of the test was initially obtained in a study of 80 adults administered the test in 2 test sessions approximately 30 days [79]. Comparisons of test scores obtained at times 1 and 2 resulted in a test-retest correlation of 0.80. Individuals tested at baseline obtained a score of 56.79 ± 9.84. Scores increased an average of 3.67 points to 60.46 ± 11.16 at retesting. Such an increase in scores at the second test reflects a “practice effect,” which is commonly seen in test-retest situations [80]. This level of test-retest correlation has been reproduced multiple times and is often higher. We expect a learning curve to be present in all groups and this will in effect wash out in the analyses as our comparisons will utilize comparative changes in means.
Camp et.al. in a primary progressive population studied longitudinally revealed standard deviations of 13.96 at baseline, 15.24 at 1 year and 14.32 at 2 years [81]. Sonder et.al. showed a standard deviation of 14.3. Further, the change over time was generally impacted slightly by the learning curve returning to baseline as the time scale extended [82]. Thus, we can think of changes from baseline to 12 weeks or 6 months in the present study as potentially representing a zero change except for the intervention effect.
The reliability of the SDMT is high, 0.80 or higher as noted above, implying that the correlation between two measures that are not materially changing have high reliability and thus the standard deviation of the change should be relatively small. If we assume that the correlation between two measures is only 0.50, then our estimated standard deviation of the change would be the same as the cross-sectional value (i.e. 10 to 16). However, given the high test-retest correlation, we should assume the correlation between the measures is higher. Assuming it is 0.80, then the estimated standard deviation would be between 6.3 and 10.1 (assuming the cross-sectional standard deviation is 10 or 16 respectively). Thus assuming a standard deviation of the changes of 7 to 8, seems reasonable and 8 may be slightly high, since 16 is at the upper end of most reports. If the standard deviation is 12 cross-sectionally, then the estimated change standard deviation is 7.6. Nevertheless, for sample size estimation it is better to be a bit conservative.
We propose to treat four groups of participants as noted above. We estimated our sample size using a standard 1-factor analysis of variance approach with a Type I error set at 5%. We computed the sample size necessary to achieve 80% power for such a design assuming conservative changes. For simplicity we used 4 points for the combined treatments, assuming that we want to demonstrate a clinically meaningful difference on average and that the two interventions are additive; 4 more correct answers has been suggested by the FDA as a meaningful change [37]. We also assumed a change of 2 answers for each of the single interventions and 0 for the sham group [83]. (Note again that the learning curve and practice effects will contribute equally to each group and the results shown below are the same if we had chosen 6, 4, 4, 2 or other combinations with the same relative spread to accommodate the practice effects, etc.)
Table 6 shows that with 90 participants per treatment group at the time of analysis, there is over 80% power to detect differences as specified (4,2,2,0) across the four groups when the standard deviation of the change is 8 points and the overall Type I error to detect any mean differences is 0.05.
In order to assess the sensitivity of these calculations to assumptions, we conducted a few additional patterns of response and standard deviations. Clearly for any standard deviation smaller than 8, we have more power. For example, if the standard deviation of the change were 7 instead of 8, the power is 91% with 90 patients per group and 81% with 70 per treatment group. If the treatment difference pattern is 4,1,1,0; the power is 86% with four groups assuming a standard deviation of 8.
If the change is less in the combined treatment group, and the pattern is say 3,2,1,0 and a standard deviation of the change is 8, the power is 59%. If the pattern is 3,2,1,0 and the standard deviation of the change is 7, then the power is 71%. Thus, the power is reasonably high for a variety of the patterns. Obviously increasing the sample size in each treatment group increases the power, but we will increase the number recruited and randomized to account for potential dropouts, thus should have ample power under these assumptions.
Statistical analyses
The statistical analyses will begin with descriptive analyses of baseline characteristics (age, sex, disease duration, EDSS, other physiologic parameters at baseline and over time, medications, etc.), by treatment allocation ((1) EX + CR, (2) EX + CR-S (3) EX-S + CR or (4) EX-S + CR-S). Continuous variables will be summarized using the statistics mean, median, SD, minimum and maximum. Categorical variables will be summarized with frequency counts and percentages. During the trial, drop-outs and losses to follow-up will be compared between groups to ensure high follow-up rates and comparability and that no particular demographic or site is differentially dropping out of one treatment group. The currently supported version of SAS software will be used to perform all data analyses.
Summary tables will indicate the number of subjects with complete data for each measurement, event or outcome. All analyses will be based on available data, unless otherwise stated, and the intent-to-treat principle. Secondary analyses will examine the per protocol analysis population. All confidence intervals will be two-sided and will use 95% confidence levels. Any analyses requiring significance testing will use a two-sided test at the 5% significance level, unless otherwise specified.
Differences in baseline characteristics between groups will be examined in continuous variables, such as age and disease duration using an ANOVA and categorical variables using a Chi-square tests of association. Informative censoring will be examined with potentially biased imputation and non-informative censoring as well as missing-at random assumptions using multiple imputation to provide sensitivity analyses of the primary results.
The primary analysis will utilize an ANOVA and include an interaction term for the combined treatments. A priori contrasts (stated above in the hypothesis section) will be conducted if the overall test of differences amongst the treatment groups achieves statistical significance. For the pairwise comparisons Dunnett’s test will be used to preserve the Type I error rate. Additional analyses will be conducted as sensitivity analyses using Analysis of Covariance (ANCOVA). These will include site, gender, age and other covariates that may be seen to differ amongst the groups at baseline. Multiple imputation will be used to assess the sensitivity of the primary results to dropouts.
Secondary outcomes will be similarly assessed using ANOVA and ANCOVA procedures and, repeated measures mixed models will be used for measurements that are taken between baseline and 12 weeks.
Statistical analysis of MRI data
In each group, longitudinal hierarchical linear models will be used to assess changes over time of WM tract DTI measures and average Z-scores of RS functional connectivity (FC), accounting for the repeated measurement design. Statistical analyses of VBM, TBM, MRI active and RS FC maps derived from ICA will be performed using the SPM12 software (whole brain analysis, p < 0.05, family-wise error [FWE], corrected for multiple comparisons).
Voxelwise differences of MD, RD, AD and FA values between groups at baseline, and their within-group changes at follow up will be tested using a permutation method (“Randomize” program within FSL) and two-sample and paired t tests, as appropriate (p < 0.05 FWE).
Linear regression analysis (using SPM12) will be used to assess the correlations between fMRI activations, RS FC maps and clinical and neuropsychological data.
Discussion
The present study will be the first combined CR and exercise trial to date in persons with PMS with the potential to change clinical practice; this trial further is the largest combined CR and exercise study in any MS phenotype. As the study is a large international multicenter study, efforts have been made to ensure trial feasibility. Moreover, the collaborating sites have access to patient populations that should allow sufficient recruitment. Efforts have been made to ensure optimal standardization and subsequently best possible data quality. Such efforts relate to 1) comprehensive and detailed assessment and intervention manuals as well as a combination of face-to-face and distance training on how to deliver these, 2) weekly quality control and feedback of the delivered interventions, 3) hotlines in case of questions relating to delivery of the interventions and 4) weekly telephone conferences allowing the widely dispersed centers close communication.
There are well described challenges related to the development of appropriate sham interventions when it comes to exercise trials [84, 85]. Adding to this challenge is the fact that the underlying mechanisms mediating potential exercise induced effects on brain function are poorly understood [86]. A sham-concept that does not significantly improve cardio-respiratory function but still involves attentional and social contact components was therefore chosen as a counterweight to the aerobic exercise intervention [13]. Choosing the appropriate sham treatment is important as it limits the potential of this intervention to alter brain function that is known to accompany aerobic exercise.
The imaging component to our study broadens the scope of our inquiry. MRI techniques are currently being applied to investigate mechanisms related to structural and functional brain plasticity in healthy individuals following training and in neurologically impaired individuals following spontaneous recovery and after rehabilitation interventions. Several authors have used fMRI during active tasks or at rest to evaluate the effects of motor [87, 88] and cognitive [18, 36, 89,90,91,92] rehabilitation in MS patients. All of these studies have demonstrated that a modulation of function in brain regions have a crucial role in the trained function which occurs in MS after rehabilitation and is associated with clinical improvement. Whether such changes are possible in people with PMS, is not yet known. Our study therefore has the potential to address the dearth of data in this population and shed light on the degree to which neural plasticity is retained in the context of a progressive disease course.
The impact of cognitive dysfunction in the lives of people with MS is considerable. It is associated with difficulties finding and sustaining employment, maintaining intimate relationships and friendships, pursuing leisure activities and managing basic activities of daily living [93]. Given that we have chosen a primary outcome measure, namely the SDMT, in which a 10% (or 4-point) change over time is known to be clinically significant, should our interventions achieve this threshold, they will acquire the imprimatur of an elusive ecological validity. Moreover, the multinational composition of the research teams all pursuing a shared methodology has the potential to demonstrate that the chosen interventions can transcend language, cultural and indeed institutional barriers that make it difficult to extrapolate results from only a single centre.
The study is expected to conclude by the end of 2022. With a robust sample size that ensures adequate statistical power, the findings, if positive, have the potential to guide clinically meaningful interventions for people with PMS who struggle with all the functional limitations associated with slowed processing speed.
Availability of data and materials
At completion of the study, only the data coordinating center at Washington University will have access to the final dataset.
At conclusion of the study, results will be communicated through individual MS societies from each participating country.
Abbreviations
- ACSM:
-
American College of Sports Medicine
- AD:
-
Axial Diffusivity
- AHA:
-
American Heart Association
- ANART:
-
Adult National Reading Test
- ANCOVA:
-
Analysis of Covariance
- ANOVA:
-
Analysis of Variance
- BDN-II:
-
Beck Depression Inventory – II
- BICAMS:
-
Brief International Cognitive Assessment for MS
- BVMT-R:
-
Brief Visuospatial Memory Test-Revised
- CMI:
-
Cognitive-Motor Interference
- CNS:
-
Central Nervous System
- CPET:
-
Cardiopulmonary Exercise Test
- CR:
-
Cognitive Rehabilitation
- CSF:
-
Cerebrospinal Fluid
- CVLT:
-
California Verbal Learning Test
- CT:
-
Continuous Training
- DCT:
-
Dual Task Cost
- EDSS:
-
Expanded Disability Status Scale
- EPI:
-
Echo-planar Imaging
- EQ -5D-5L:
-
European Quality of Life – 5 Dimensions
- EX:
-
Exercise
- FA:
-
Fractional Anisotropy
- FAMS:
-
Functional Assessment of Multiple Sclerosis
- fMRI:
-
Functional Magnetic Resonance Imaging
- GLTEQ:
-
Godin Leisure-Time Exercise Questionnaire
- GM:
-
Grey Matter
- GMV:
-
Grey Matter Volume
- HADS:
-
Hospital Anxiety and Depression Scale
- HIIT:
-
High Intensity Interval Training
- HR:
-
Heart Rate
- ICA:
-
Independent Component Analysis
- IET:
-
Incremental Exercise Test
- IQ:
-
Intelligence Quotient
- LSD:
-
Lysergic Acid Diethylamide
- MD:
-
Mean Diffusivity
- MFIS:
-
Modified Fatigue Impact Scale
- MNI:
-
Montreal National Institute
- MRI:
-
Magnetic Resonance Imaging
- MS:
-
Multiple Sclerosis
- MSIS-29V2:
-
Multiple Sclerosis Impact Scale – 29 Items Version 2
- MSWS-12:
-
Multiple Sclerosis Walking Scale 12 Items
- NART:
-
National Adult Reading Test
- NBV:
-
Normalized Brain Volume
- PCP:
-
Phencyclidine
- PDQ-20:
-
Perceived Deficits Questionnaire 20 Items
- PI:
-
Principal Investigator
- PMS:
-
Progressive Multiple Sclerosis
- PPMS:
-
Primary Progressive Multiple Sclerosis
- PRO:
-
Patient Reported Outcomes
- PS:
-
Processing Speed
- QALY:
-
Quality Adjusted Life Year
- RD:
-
Radial Diffusivity
- RPE:
-
Rate of Perceived Exertion
- RRMS:
-
Relapsing Remitting Multiple Sclerosis
- RS:
-
Resting State
- RSFC:
-
Resting State Functional Connectivity
- -S:
-
Sham
- SD:
-
Standard Deviation
- SDMT:
-
Symbol Digit Modalities Test
- SPMS:
-
Secondary Progressive Multiple Sclerosis
- TBM:
-
Tensor-based Morphometry
- TBSS:
-
Tract-based Spatial Statistics
- TSE:
-
Turbo Spin Echo
- VBM:
-
Voxel-based Morphometry
- W:
-
Watt
- WM:
-
White Matter
- WMV:
-
White Matter Volume
- WR:
-
Work Rate
- WTAR:
-
Wechsler Test of Adult Reading
- 6-MWT:
-
Six Minute Walk Test
References
Feinstein A, Freeman J, Lo AC. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. Lancet Neurol. 2015;14(2):194–207.
Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–51.
Heesen C, Bohm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Multiple sclerosis (Houndmills, Basingstoke, England). 2008;14(7):988–91.
Chiaravalloti ND, Moore NB, DeLuca J. The efficacy of the modified Story Memory Technique in progressive MS. Mult Scler. 2020;26(3):354–62. https://doi.org/10.1177/1352458519826463.
Goverover Y, Chiaravalloti N, Genova H, DeLuca J. A randomized controlled trial to treat impaired learning and memory in multiple sclerosis: The self-GEN trial. Multiple sclerosis (Houndmills, Basingstoke, England). 2018;24(8):1096–104.
Messinis L, Nasios G, Kosmidis MH, Zampakis P, Malefaki S, Ntoskou K, et al. Efficacy of a computer-assisted cognitive rehabilitation intervention in relapsing-remitting multiple sclerosis patients: a multicenter randomized controlled trial. Behav Neurol. 2017;2017:5919841.
Chiaravalloti ND, Goverover Y, Costa SL, DeLuca J. A pilot study examining speed of processing training (SPT) to improve processing speed in persons with multiple sclerosis. Front Neurol. 2018;9:685.
Campbell J, Langdon D, Cercignani M, Rashid W. A randomised controlled trial of efficacy of cognitive rehabilitation in multiple sclerosis: a cognitive, Behavioural, and MRI study. Neural plasticity. 2016;2016:4292585.
Pedulla L, Brichetto G, Tacchino A, Vassallo C, Zaratin P, Battaglia MA, et al. Adaptive vs. non-adaptive cognitive training by means of a personalized App: a randomized trial in people with multiple sclerosis. J Neuroengineering Rehab. 2016;13(1):88.
Mani A, Chohedri E, Ravanfar P, Mowla A, Nikseresht A. Efficacy of group cognitive rehabilitation therapy in multiple sclerosis. Acta Neurol Scand. 2018;137(6):589–97.
Rilo O, Pena J, Ojeda N, Rodriguez-Antiguedad A, Mendibe-Bilbao M, Gomez-Gastiasoro A, et al. Integrative group-based cognitive rehabilitation efficacy in multiple sclerosis: a randomized clinical trial. Disabil Rehabil. 2018;40(2):208–16.
Briken S, Gold SM, Patra S, Vettorazzi E, Harbs D, Tallner A, et al. Effects of exercise on fitness and cognition in progressive MS: a randomized, controlled pilot trial. Multiple sclerosis (Houndmills, Basingstoke, England). 2014;20(3):382–90.
Sandroff BM, Bollaert RE, Pilutti LA, Peterson ML, Baynard T, Fernhall B, et al. Multimodal exercise training in multiple sclerosis: a randomized controlled trial in persons with substantial mobility disability. Contemp Clin Trials. 2017;61:39–47.
Romberg A, Virtanen A, Ruutiainen J. Long-term exercise improves functional impairment but not quality of life in multiple sclerosis. J Neurol. 2005;252(7):839–45.
Jimenez-Morales RM, Herrera-Jimenez LF, Macias-Delgado Y, Perez-Medinilla YT, Diaz-Diaz SM, Forn C. Cognitive training combined with aerobic exercises in multiple sclerosis patients: a pilot study. Rev Neurol. 2017;64(11):489–95.
Jonsdottir J, Gervasoni E, Bowman T, Bertoni R, Tavazzi E, Rovaris M, et al. Intensive multimodal training to improve gait resistance, mobility, Balance and Cognitive Function in Persons With Multiple Sclerosis: A Pilot Randomized Controlled Trial. Front Neurol. 2018;9:800.
Barbarulo AM, Lus G, Signoriello E, Trojano L, Grossi D, Esposito M, et al. Integrated cognitive and Neuromotor rehabilitation in multiple sclerosis: a pragmatic study. Front Behav Neurosci. 2018;12:196.
Filippi M, Riccitelli G, Mattioli F, Capra R, Stampatori C, Pagani E, et al. Multiple sclerosis: effects of cognitive rehabilitation on structural and functional MR imaging measures--an explorative study. Radiology. 2012;262(3):932–40.
Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL, Ball KK. The impact of speed of processing training on cognitive and everyday performance. Aging Ment Health. 2005;9(3):262–71.
Langeskov-Christensen M, Hvid L, Boye-Jensen H, Nielsen HH, Petersen T, Stenager E, et al. High-intensity aerobic exercise does not improve cognitive performance in people with multiple sclerosis: a randomised controlled trial. Mult Scler J. 2019;25(S2):P1608.
Hubbard EA, Motl RW, Fernhall BO. Acute high-intensity interval exercise in multiple sclerosis with mobility disability. Med Sci Sports Exerc. 2019;51(5):858–67.
Pilutti LA, Paulseth JE, Dove C, Jiang S, Rathbone MP, Hicks AL. Exercise training in progressive multiple sclerosis: a comparison of recumbent stepping and body weight-supported treadmill training. Int J MS Care. 2016;18(5):221–9.
Zimmer P, Bloch W, Schenk A, Oberste M, Riedel S, Kool J, et al. High-intensity interval exercise improves cognitive performance and reduces matrix metalloproteinases-2 serum levels in persons with multiple sclerosis: A randomized controlled trial. Mult Scler. 2018;24(12):1635–44. https://doi.org/10.1177/1352458517728342.
Kierkegaard M, Lundberg IE, Olsson T, Johansson S, Ygberg S, Opava C, et al. High-intensity resistance training in multiple sclerosis - an exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J Neurol Sci. 2016;362:251–7.
Barrett CL, Mann GE, Taylor PN, Strike P. A randomized trial to investigate the effects of functional electrical stimulation and therapeutic exercise on walking performance for people with multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). 2009;15(4):493–504.
Motl RW, Pekmezi D, Wingo BC. Promotion of physical activity and exercise in multiple sclerosis: Importance of behavioral science and theory. Mult Scler J Exp Transl Clin. 2018;4(3):2055217318786745.
Roar M, Illes Z, Sejbaek T. Practice effect in symbol digit modalities test in multiple sclerosis patients treated with natalizumab. Mult Scler Related Disord. 2016;10:116–22.
Costa SL, Genova HM, DeLuca J, Chiaravalloti ND. Information processing speed in multiple sclerosis: Past, present, and future. Multiple sclerosis (Houndmills, Basingstoke, England). 2017;23(6):772–89.
Leavitt VM, Wylie G, Krch D, Chiaravalloti N, DeLuca J, Sumowski JF. Does slowed processing speed account for executive deficits in multiple sclerosis? Evidence from neuropsychological performance and structural neuroimaging. Rehab Psychol. 2014;59(4):422–8.
Sandroff BM, Klaren RE, Pilutti LA, Dlugonski D, Benedict RH, Motl RW. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. J Neurol. 2014;261(2):363–72.
Beier M, Bombardier CH, Hartoonian N, Motl RW, Kraft GH. Improved Physical Fitness Correlates with Improved Cognition in Multiple Sclerosis. Arch Phys Med Rehabil. 2014;95(7):1328–34.
Sangelaji B, Estebsari F, Nabavi SM, Jamshidi E, Morsali D, Dastoorpoor M. The effect of exercise therapy on cognitive functions in multiple sclerosis patients: a pilot study. Med J Islam Repub Iran. 2015;29:205.
Mattioli F, Stampatori C, Zanotti D, Parrinello G, Capra R. Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in multiple sclerosis. J Neurol Sci. 2010;288(1–2):101–5.
Mattioli F, Stampatori C, Scarpazza C, Parrinello G, Capra R. Persistence of the effects of attention and executive functions intensive rehabilitation in relapsing remitting multiple sclerosis. Multiple Scler Related Disord. 2012;1(4):168–73.
Parisi L, Rocca MA, Valsasina P, Panicari L, Mattioli F, Filippi M. Cognitive rehabilitation correlates with the functional connectivity of the anterior cingulate cortex in patients with multiple sclerosis. Brain Imaging Behav. 2014;8(3):387–93.
Bonavita S, Sacco R, Della Corte M, Esposito S, Sparaco M, d'Ambrosio A, et al. Computer-aided cognitive rehabilitation improves cognitive performances and induces brain functional connectivity changes in relapsing remitting multiple sclerosis patients: an exploratory study. J Neurol. 2015;262(1):91–100.
Benedict RH, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). 2017;23(5):721–33.
LaRocca NG, Hudson LD, Rudick R, Amtmann D, Balcer L, Benedict R, et al. The MSOAC approach to developing performance outcomes to measure and monitor multiple sclerosis disability. Multiple sclerosis (Houndmills, Basingstoke, England). 2018;24(11):1469–84.
Langdon DW, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Multiple sclerosis (Houndmills, Basingstoke, England). 2012;18(6):891–8.
Costers L, Gielen J, Eelen PL, Schependom JV, Laton J, Remoortel AV, et al. Does including the full CVLT-II and BVMT-R improve BICAMS? Evidence from a Belgian (Dutch) validation study. Multiple Scler Related Disord. 2017;18:33–40.
Walker LA, Osman L, Berard JA, Rees LM, Freedman MS, MacLean H, et al. Brief international cognitive assessment for multiple sclerosis (BICAMS): Canadian contribution to the international validation project. J Neurol Sci. 2016;362:147–52.
Goretti B, Niccolai C, Hakiki B, Sturchio A, Falautano M, Minacapelli E, et al. The Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS): normative values with gender, age and education corrections in the Italian population. BMC Neurol. 2014;14:171.
Parmenter BA, Testa SM, Schretlen DJ, Weinstock-Guttman B, Benedict RH. The utility of regression-based norms in interpreting the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc. 2010;16(1):6–16.
Wechsler D. Wechsler test of adult Reading: WTAR. Psychological Corporation; 2001.
Nelson H. National Adult Reading Test. Manual NFER-Nelson: Windsor: NFER-Nelso; 1982.
Pilutti LA, Sandroff BM, Klaren RE, Learmonth YC, Platta ME, Hubbard EA, et al. Physical fitness assessment across the disability Spectrum in persons with multiple sclerosis: a comparison of testing modalities. J Neurol Phys Ther. 2015;39(4):241–9.
Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Multiple sclerosis (Houndmills, Basingstoke, England). 2008;14(3):383–90.
Sandroff BM, Pilutti LA, Dlugonski D, Learmonth YC, Pula JH, Motl RW. Comparing two conditions of administering the six-minute walk test in people with multiple sclerosis. Int J MS Care. 2014;16(1):48–54.
Leone C, Patti F, Feys P. Measuring the cost of cognitive-motor dual tasking during walking in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). 2015;21(2):123–31.
Learmonth YC, Ensari I, Motl RW. Cognitive motor interference in multiple sclerosis: insights from a systematic quantitative review. Arch Phys Med Rehabil. 2017;98(6):1229–40.
Wajda DA, Sosnoff JJ. Cognitive-motor interference in multiple sclerosis: a systematic review of evidence, correlates, and consequences. Biomed Res Int. 2015;2015:720856.
Learmonth YC, Sandroff BM, Pilutti LA, Klaren RE, Ensari I, Riskin BJ, et al. Cognitive motor interference during walking in multiple sclerosis using an alternate-letter alphabet task. Arch Phys Med Rehabil. 2014;95(8):1498–503.
Klaren RE, Hubbard EA, Zhu W, Motl RW. Reliability of accelerometer scores for measuring sedentary and physical activity behaviors in persons with multiple sclerosis. Adapt Phys Activ Q. 2016;33(2):195–204.
Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The multiple sclerosis impact scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(Pt 5):962–73.
Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-item MS walking scale (MSWS-12). Neurology. 2003;60(1):31–6.
Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. How responsive is the multiple sclerosis impact scale (MSIS-29)? A comparison with some other self report scales. J Neurol Neurosurg Psychiatry. 2005;76(11):1539–43.
McGuigan C, Hutchinson M. Confirming the validity and responsiveness of the multiple sclerosis walking Scale-12 (MSWS-12). Neurology. 2004;62(11):2103–5.
Baert I, Freeman J, Smedal T, Dalgas U, Romberg A, Kalron A, et al. Responsiveness and clinically meaningful improvement, according to disability level, of five walking measures after rehabilitation in multiple sclerosis: a European multicenter study. Neurorehabil Neural Repair. 2014;28(7):621–31.
Goodwin E, Green C, Spencer A. Estimating a preference-based index for an eight-dimensional health state classification system for multiple sclerosis. Value Health. 2015;18(8):1025–36.
Goodwin E, Green C. A quality-adjusted life-year measure for multiple sclerosis: developing a patient-reported health state classification system for a multiple sclerosis-specific preference-based measure. Value Health. 2015;18(8):1016–24.
Cella DF, Dineen K, Arnason B, Reder A, Webster KA. Karabatsos G, et al. validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology. 1996;47(1):129–39.
Chiaravalloti ND, Moore NB, Nikelshpur OM, DeLuca J. An RCT to treat learning impairment in multiple sclerosis: the MEMREHAB trial. Neurology. 2013;81(24):2066–72.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Kos D, Kerckhofs E, Carrea I, Verza R, Ramos M, Jansa J. Evaluation of the Modified Fatigue Impact Scale in four different European countries. Multiple sclerosis (Houndmills, Basingstoke, England). 2005;11(1):76–80.
Sullivan MJ, Edgley K, Dehoux E. A survey of multiple sclerosis: I. perceived cognitive problems and compensatory strategy use. Canadian J Rehab. 1990;4(2):99–105.
Koini M, Filippi M, Rocca MA, Yousry T, Ciccarelli O, Tedeschi G, et al. Correlates of executive functions in multiple sclerosis based on structural and functional MR imaging: insights from a multicenter study. Radiology. 2016;280(3):869–79.
Loitfelder M, Fazekas F, Petrovic K, Fuchs S, Ropele S, Wallner-Blazek M, et al. Reorganization in cognitive networks with progression of multiple sclerosis: insights from fMRI. Neurology. 2011;76(6):526–33.
Loitfelder M, Fazekas F, Koschutnig K, Fuchs S, Petrovic K, Ropele S, et al. Brain activity changes in cognitive networks in relapsing-remitting multiple sclerosis - insights from a longitudinal FMRI study. PLoS One. 2014;9(4):e93715.
Leow AD, Klunder AD, Jack CR Jr, Toga AW, Dale AM, Bernstein MA, et al. Longitudinal stability of MRI for mapping brain change using tensor-based morphometry. NeuroImage. 2006;31(2):627–40.
Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson Series B. 1994;103(3):247–54.
Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14(3):140–51.
Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M. Modulation of brain resting-state networks by sad mood induction. PLoS One. 2008;3(3):e1794.
Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral cortex (New York, NY : 1991). 2008;18(8):1856–64.
Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–53.
Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond Ser B Biol Sci. 2005;360(1457):1001–13.
Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–5.
De Giglio L, Tona F, De Luca F, Petsas N, Prosperini L, Bianchi V, et al. Multiple sclerosis: changes in thalamic resting-state functional connectivity induced by a home-based cognitive rehabilitation program. Radiology. 2016;280(1):202–11.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Smith A. Symbol digit modalities test. Los Angeles: Western Psychological Services; 1991.
Barr WB. Methodologic issues in neuropsychological testing. J Athl Train. 2001;36(3):297–302.
Camp SJ, Stevenson VL, Thompson AJ, Ingle GT, Miller DH, Borras C, et al. A longitudinal study of cognition in primary progressive multiple sclerosis. Brain. 2005;128(Pt 12):2891–8.
Sonder JM, Burggraaff J, Knol DL, Polman CH, Uitdehaag BM. Comparing long-term results of PASAT and SDMT scores in relation to neuropsychological testing in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). 2014;20(4):481–8.
Rosti-Otajarvi EM, Hamalainen PI. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst Rev. 2014;(2):Cd009131.
Mohr DC, Spring B, Freedland KE, Beckner V, Arean P, Hollon SD, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom. 2009;78(5):275–84.
Bamman MM, Cutter GR, Brienza DM, Chae J, Corcos DM, DeLuca S, et al. Medical rehabilitation: guidelines to advance the field with high-impact clinical trials. Arch Phys Med Rehabil. 2018;99(12):2637–48.
El-Sayes J, Harasym D, Turco CV, Locke MB, Nelson AJ. Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist. 2019;25(1):65–85.
Tomassini V, Johansen-Berg H, Jbabdi S, Wise RG, Pozzilli C, Palace J, et al. Relating brain damage to brain plasticity in patients with multiple sclerosis. Neurorehabil Neural Repair. 2012;26(6):581–93.
Sandroff BM, Wylie GR, Sutton BP, Johnson CL, DeLuca J, Motl RW. Treadmill walking exercise training and brain function in multiple sclerosis: Preliminary evidence setting the stage for a network-based approach to rehabilitation. Mult Scler J Exp Transl Clin. 2018;4(1):2055217318760641.
Cerasa A, Gioia MC, Valentino P, Nistico R, Chiriaco C, Pirritano D, et al. Computer-assisted cognitive rehabilitation of attention deficits for multiple sclerosis: a randomized trial with fMRI correlates. Neurorehabil Neural Repair. 2013;27(4):284–95.
Chiaravalloti ND, Wylie G, Leavitt V, Deluca J. Increased cerebral activation after behavioral treatment for memory deficits in MS. J Neurol. 2012;259(7):1337–46.
Dobryakova E, Wylie GR, DeLuca J, Chiaravalloti ND. A pilot study examining functional brain activity 6 months after memory retraining in MS: the MEMREHAB trial. Brain Imaging Behav. 2014;8(3):403–6.
Leavitt VM, Wylie GR, Girgis PA, DeLuca J, Chiaravalloti ND. Increased functional connectivity within memory networks following memory rehabilitation in multiple sclerosis. Brain Imaging Behav. 2014;8(3):394–402.
Benedict RHB, DeLuca J, Enzinger C, Geurts JJG, Krupp LB, Rao SM. Neuropsychology of multiple sclerosis: looking Back and moving forward. J Int Neuropsychol Soc. 2017;23(9–10):832–42.
Acknowledgements
The authors wish to acknowledge the Canadian MS Society for providing funding for the present study. The National MS Society (USA) provided supplemental funding for MRI at UAB.
The paper has been presented as a poster at the ECTRIMS conference, 2019.
Funding
The present study is funded by the Multiple Sclerosis Society of Canada grant no. EGID 3185. The grant underwent extensive peer-review over two rounds as part of the grant award process. There is further supplemental funding from National Multiple Sclerosis Society (SI-1905-34071). REDCap at Washington University is supported by Clinical and Translational Science Award (CTSA) Grant [UL1 TR000448] and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842. None of the sponsors played a role in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Consortia
Contributions
The manuscript was drafted by AF, UD and JF. All other co-authors contributed significantly to previous application drafts of which some has been used in the present draft, to the design of the present study protocol and commented upon and approved the present manuscript before submission. During the execution of the study, the following roles and responsibilities will be undertaken by the participating authors: Primary investigator of the study is AF and the steering center of the study is University of Toronto and Sunnybrook Health Sciences Centre, Toronto, Canada. CM is the on site coordinator. Data management and analysis is led by AS and assisted by GC. The data coordinating center is Division of Biostatistics, Washington University School of Medicine, St. Louis, MO, USA. The cognitive interventions were selected by JDL, NC, MPA and AF, and all training and quality control monitoring of participating centers is undertaken by NC. The exercise interventions were developed by UD, RWM, BMS, PF, JF and GB. Training of participating centers for the aerobic intervention is undertaken by RWM and BMS, with quality control by UD. Training and quality control of the non-aerobic intervention is undertaken by JF. The applied MRI battery was designed and coordinated by MAR, MF and MI. Issues related to study design, recruitment and national legislation were supervised by JC. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participating centers have obtained required ethics approval and patients will not be enrolled before they have provided signed informed consent. People with MS and dementia are excluded from the protocol. Consent from next of kin is not required as this implies a level of cognitive dysfunction that would exclude a person with MS from the study. Ethics approvals have been obtained from:
-
1.
The ethical committee in Region Midt, Denmark (journal no: 1–10–72-300-18)
-
2.
The Kessler Foundation Institutional Review Board (Protocol #R-1017-18)
-
3.
The Sunnybrook Health Sciences Centre Research Ethics Board (Protocol Identification Number 348–2008)
-
4.
The United Kingdom National Health Service Health Research Authority Committee - Wales REC 6 (18/WA/0309)
-
5.
University of Alabama Birmingham IRB (IRB300002637)
-
6.
Comitato Etico Regionale N. Registro CER Liguria 294bis/2018
-
7.
Comitato Etico Regionale per la Sperimentazione Clinica, Regione Toscana, Sezione Area Vasta Centro (13912-spe)
-
8.
Hospital San Raffaele Ethical committee (124/INT/2018)
Furthermore, approvals from national data protection agencies will be obtained if needed to allow data storage and transfer.
Consent for publication
Not applicable.
Competing interests
No competing interests are reported.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Feinstein, A., Amato, M.P., Brichetto, G. et al. Study protocol: improving cognition in people with progressive multiple sclerosis: a multi-arm, randomized, blinded, sham-controlled trial of cognitive rehabilitation and aerobic exercise (COGEx). BMC Neurol 20, 204 (2020). https://doi.org/10.1186/s12883-020-01772-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-020-01772-7