Abstract
Background
Antiepileptic drugs (AEDs) are effective medications available for epilepsy. However, many patients do not respond to this treatment and become resistant. Genetic polymorphisms may be involved in the variation of AEDs response. Therefore, we conducted an updated systematic review and a meta-analysis to investigate the contribution of the genetic profile on epilepsy drug resistance.
Methods
We proceeded to the selection of eligible studies related to the associations of polymorphisms with resistance to AEDs therapy in epilepsy, published from January 1980 until November 2016, using Pubmed and Cochrane Library databases. The association analysis was based on pooled odds ratios (ORs) and 95% confidence intervals (CIs).
Results
From 640 articles, we retained 13 articles to evaluate the relationship between ATP-binding cassette sub-family C member 1 (ABCB1) C3435T polymorphism and AEDs responsiveness in a total of 454 epileptic AEDs-resistant cases and 282 AEDs-responsive cases. We found a significant association with an OR of 1.877, 95% CI 1.213–2.905. Subanalysis by genotype model showed a more significant association between the recessive model of ABCB1 C3435T polymorphism (TT vs. CC) and the risk of AEDs resistance with an OR of 2.375, 95% CI 1.775–3.178 than in the dominant one (CC vs. TT) with an OR of 1.686, 95% CI 0.877–3.242.
Conclusion
Our results indicate that ABCB1 C3435T polymorphism, especially TT genotype, plays an important role in refractory epilepsy. As genetic screening of this genotype may be useful to predict AEDs response before starting the treatment, further investigations should validate the association.
Similar content being viewed by others
Background
Epilepsy is a chronic neurological worldwide disorder [1]. Most cases of epileptic patients respond to antiepileptic drugs (AEDs). However, about one-third of epileptic patients develop recurrent seizures, despite the efficacy of treatment at the optimal dose regimen. They are then, considered resistant to antiepileptic treatment [2]. The international league against epilepsy (ILAE) redefined refractory epilepsy in 2010 as the persistence of seizures after two adequate trials of appropriate and tolerated AEDs [3].
The exact mechanism of refractory epilepsy is not well understood. Two main hypotheses are potentially involved in the biological mechanism of AEDs resistance: transporter and target hypotheses. The transporter hypothesis supports the overexpression of drug efflux transporters at the blood–brain barrier (BBB) reducing AEDs access to the brain. The target hypothesis contends that the changes in drug intracellular target sites (receptors) result in decreased sensitivity of AEDs [4, 5]. Therefore, the two mechanisms prevent pharmacological effects of antiepileptic at cerebral sites initiating seizures. It seems that genetic polymorphisms of drug transporter and target genes have a potential impact on the resistance to treatment: they may be responsible for the mechanisms of intractable epilepsy [5,6,7] by changing the function of genes products [8,9,10] and leading to the AEDs failure [4,12,13,, 11–14]. Moreover, other authors have suggested that they may involve the prognosis of newly treated epilepsy [15]. Since drug-resistant epilepsy represents a major problem in the control of seizures, the researchers focused on the genetic profile to try to better understand the pharmacoresistance for a more effective treatment.
Since drug resistance often occurs in patients with multiple AEDs, the multidrug transporter hypothesis is considered better than the target hypothesis to explain the phenomenon of AEDs resistant epilepsy. However, the two hypotheses may complement each other. Given that drug transport mechanisms are the candidate mechanisms underlying AEDs resistance [16], many studies took significantly into consideration the association between efflux transporters overexpression inducing recurrent seizures.
Bioavailability and response to medication in epilepsy are mainly influenced by atp-binding cassette (ABC) transporter superfamily. The atp-binding cassette sub-family b member 1 (ABCB1) and the atp-binding cassette sub-family c member 2 (ABCC2) also known as multidrug resistance protein 1 (MDR1) and multidrug resistance protein 2 (MDR2), located at the membrane of BBB endothelial cells, are members of the ABC superfamily. They are the most studied candidate genes in pharmacoresistant epilepsy [5]. P-glycoprotein (P-gp) was the first human ABC protein that has been discovered [17]. ABCB1 gene encodes it and it affects a wide range of drugs distribution in target compartments [18,19,20]. The C3435T polymorphism is the most investigated polymorphism in the ABCB1 gene (single nucleotide polymorphism (SNP) in exon 26) and it has received the most attention. It has been associated with the variations in the expression levels of P-gp [21]. Previous studies focusing on the association between ABCB1 C3435T polymorphism and drug-resistant epilepsy showed discordant findings. Several studies have supported the hypothesis of this association (alleles, genotypes or haplotypes) to AEDs resistance [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. However, a number of studies conducted on epileptic patients from different regions and ethnicities failed to confirm this result [38,39,40,41,42]. Subsequently, the opposed findings stimulated some previous meta-analyses of which the majority indicated that no association existed [43,44,45,46,47,48,49]. Besides, G1249A polymorphism is one of the common polymorphisms in the ABCC2 gene (SNP in exon 10). The overexpression of the ABCC2 transporter protein reduces AEDs levels in brain tissues, which is a risk factor for pharmacoresistant epilepsy. A genotypic association between this polymorphism and responsiveness to AEDs has been suggested in Asian populations [50, 51]. However, other studies published contradictory results and they did not find any association [42,53,54,55,, 52–56]. Furthermore, only two meta-analyses investigated its role in drug-resistant epilepsy and found that ABCC2 G1249A polymorphism was significantly associated with the decreased risk of AED resistance [57, 58].
Among their pharmacological effects, some AEDs may block voltage-dependent sodium channels [59, 60], which stimulate the researchers to investigate the potential link between drug-resistant epilepsy and polymorphisms in channels genes like SCN1A gene. This gene is the most studied drug target gene in epilepsy and it exhibits an intronic polymorphism IVS5-91G > A, one of the most common polymorphisms (SNP at intron splice donor site of exon 5). It alters the proportion of human brain NaV1.1-5N (exon 5N) and NaV1.1-5A (exon 5A) proteins, but the functional impact of the splicing on NaV1.1 is unknown. The correlation between SCN1A IVS5-91G > A polymorphism and maximum doses of Oxcarbazepine (OXC) may have a potential effect on resistant to epilepsy. The same study found the same correlation for ABCC2 G1249A polymorphism [61]. An additional study reported a genotypic association of SCN1A IVS5-91G > A polymorphism with the response to Carbamazepine (CBZ)/OXC [51, 62], and another one showed its role on pharmacoresponse to CBZ via an effect on GABAergic cortical interneurons [63]. However, other studies [64,65,66] and only one meta-analysis [67] were unable to replicate this association.
Overall, even the most considered polymorphisms that may explain mechanisms of pharmacoresistant epilepsy, showed contradictory and inclusive results. Therefore, we assembled pharmacogenetics (PGt) and pharmacogenomics (PGx) studies reporting associations between AEDs resistant epilepsy and eventual polymorphisms. Then, we performed an updated meta-analysis to clarify their role in response to AEDs.
Methods
We defined search strategy, study selection criteria, data elements and methods for study quality assessment.
Data sources and literature searches
We conducted a literature search using Pubmed and Cochrane Library with English-language restriction from January 1980 to November 2016. The key words used in the search strategy were: “anti-epileptic drug(s)”, “antiepileptic drug(s)”, “anti epileptic drug(s)” and “epilepsy” and “efficacy”, “intractable”, “refractory”, “resistance”, “resistant”, “response to treatment”, “pharmacoresistance”, “pharmacoresistant” and “genetic factor(s)”, “genotype(s)”, “pharmacogenetic(s)”, “pharmacogenomic(s)”, “polymorphism(s)”, “variant(s)”, “variation(s)”, “SNP(s)”. We did not search of additional publications. The reported results followed the preferred reporting items for systematic reviews and meta-analyses guidelines (PRISMA).
Eligibility and inclusion criteria
For eligibility, we retained full-text publications showing a relationship between genetic polymorphisms and responsiveness of AEDs in epilepsy (monotherapy or polytherapy).
The included studies met the following criteria: 1) Original research articles reported a genotypic evaluation of polymorphisms and resistant epilepsy to antiepileptic treatment. 2) Studies compared AEDs-resistant cases with AEDs-responsive cases. 3) Studies showed sufficient individual genotype frequencies for specific genotype model. 4) At least three studies on the same polymorphism were available in order to avoid the non-pertinence of the results and the high risk of bias.
Data extraction
Two independent authors performed the data eligibility, they extracted the following information from each included study: first author, publication year, ethnicity of the study population, the number of cases and controls, genotype model for each polymorphism, age, gender, aetiology, type of epilepsy, and AEDs administered.
Data synthesis and analysis
We calculated the association between polymorphisms and AEDs resistant epilepsy using individual and overall odds ratios (OR) with corresponding 95% confidence intervals (CIs) by Forest Plot (Comprehensive Meta-Analysis Version 3, USA). The P-value determined the significance of the combined ORs. If the P-value (P) < 0.05, we considered the pooled ORs statistically significant [68]. The Z-value showed uniformisation of values and their position in the full distribution of values in the program. The I 2 statistic test assessed statistical heterogeneity among included studies; if I 2 < 50%, fixed-effects model pooled study data and if I 2 ≥ 50%, random-effects model pooled it [69]. Additionally, we performed subgroup analysis using genotype model to quantify the reported association between polymorphisms and AEDs resistant epilepsy in each reported genotypic model. To identify publication bias between the included studies, we applied Funnel plot and Egger’s regression tests. The graph of Funnel plot reflected publication bias. Egger’s test assessed and confirmed funnel plot’s results: P < 0.05 determined the existence of bias [70].
Results
Evidence base
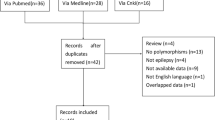
We identified a total of 640 potentially relevant articles. We excluded a total of 591 publications from the further analysis: abstract, articles showing absence of associations between polymorphisms and AEDs resistant epilepsy for insufficient data, case reports, duplicated articles, letter to the editors, meta-analysis, not epileptic studies, not human reports, researches about other treatments than AEDs, review articles and studies not related to associations between polymorphisms and AEDs resistant epilepsy (Fig. 1).
Among the 49 reports that met eligibility requirements], 39 reviewed an association between polymorphisms and epilepsy drug resistance [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,37, 50, 51, 62, 71–90]. We identified the majority of polymorphisms in AEDs transporter genes: ABCB1 and ABCC2. We also found other polymorphisms in AEDs target genes: gamma-aminobutyric acid-a receptor alpha1-subunit (GABRA1), gamma-aminobutyric acid-a receptor alpha2-subunit (GABRA2), gamma-aminobutyric acid-a receptor alpha3-subunit (GABRA3), sodium channel nav1.1 (SCN1A), sodium channel nav1.2 (SCN2A), in other potential genes as apolipoprotein e (ApoE), cytochrome p450 1a1 (CYP1A1), cytochrome p450 family member 2c9 (CYP2C9), gamma-aminobutyric acid transporter 3 (GAT3), glutathione s-transferases mu 1 (GSTM1) and solute ligand carrier family 6 member a4 (SLC6A4). We summarized the characteristics of polymorphisms implicated in AEDs resistance in different ethnic groups (Table 1). We excluded 10 full-text studies for insufficient data (Fig. 1). Only 13 met the inclusion criteria and constituted the data set for this analysis [22,23,24,25,26,27,28,34,35,29, 31, 33–36] (Table 2).
Data analysis
We carried out a meta-analysis to evaluate the relationship between ABCB1 C3435T polymorphism and AEDs resistance among AEDs-resistant patients vs. AEDs-responsive patients. The included studies were heterogeneous for the study characteristics. The analysis of data showed that 454 of 1653 AEDs-resistant patients (27.465%) and 282 of 1732 AEDs-responsive patients (16.282%) were included in the statistical analysis [22,23,24,25,26,27,28,34,35,29, 31, 33–36]. The frequency of AEDs-resistant cases was higher than AEDs-responsive patients. We divided the age of cases and controls into three subgroups: >20 years, 20–40 years, and <40 years. We divided the gender of cases and controls into two subgroups: males >50% and males <50%. A total of eight included studies were conducted in Asia [22,28,35,, 23, 27–29, 34–36], three studies in Europe [25, 26, 31], one study in Egypt [24] and one another in Australia [33]. We classified the cases by epilepsy syndrome (idiopathic, cryptogenic or symptomatic epilepsy) [22, 23, 28, 31, 34, 36] or by seizure types (generalized or partial seizures) [22,23,24, 28, 29, 31, 33, 35, 36]. However, the classifications of cases by epilepsy syndrome were not mentioned in seven studies [24,25,26,27, 29, 33, 35] and the classifications of cases by seizure types were not mentioned in three studies [26, 27, 34]. Two studies were stratified by epilepsy syndrome [28, 31] and three studies were stratified by seizure types [29, 33, 35]. Cases were treated with AEDs polytherapy in seven studies [23,27,28,, 26–29, 35, 36]. Only one study reported association between ABCB1 C3435T polymorphism and cases with Phenytoin (PHT) therapy, the administration of PHT as monotherapy or polytherapy was not mentioned [24]. However, AEDs were not specified in five studies [22, 25, 27, 31, 33]. We summarized the characteristics of the available included studies in Table 2.
Association of ABCB1 C3435T polymorphism with the susceptibility to AEDs resistance
The heterogeneity among the included studies was high (I 2 = 82.961%, P < 10-3) and we used a random-effects model [22,23,24,25,26,27,28,34,35,29, 31, 33–36]. The summary OR was 1.877, 95% CI 1.213–2.905, P = 0.005 showing that ABCB1 C3435T was significantly associated with AEDs resistance (Fig. 2).
Association between ABCB1 C3435T polymorphism and AEDs resistant epilepsy. Forest plot showed individual and overall ORs (black squares) with corresponding 95% CIs (horizontal bars) by individual report. P-value showed statistical significance of ORs and Z-value showed uniformisation of values and its position in the full distribution of values. Heterogeneity between the studies was mentioned
For the robustness of our findings, we used subanalysis by dominant (CC vs. TT) and recessive (TT vs. CC) genotype models. The heterogeneity among the nine included studies was high (I 2 = 87.843%, P < 10-3) in the dominant model [22,23,24,25,26,27,28,29, 31]. The summary OR was 1.686, 95% CI 0.877–3.242, P = 0.117 under a random-effects model (Fig. 3). The analysis of the recessive model revealed that the heterogeneity was absent (I 2 = 0.000%, P = 0.727) among the four included studies [33,34,35,36]. The summary OR was 2.375, 95% CI 1.775–3178, P < 10-3 under a fixed-effects model (Fig. 4). Therefore, the results of our present meta-analysis indicates that the association of ABCB1 C3435T polymorphism with the risk of AEDs resistance, exists and it is more significant in ABCB1 3435TT genotype than in 3435CC genotype.
Association between ABCB1 3435CC genotype and AEDs resistant epilepsy. Forest plot showed individual and overall ORs (black squares) with corresponding 95% CIs (horizontal bars) by individual report. P-value showed statistical significance of ORs and Z-value showed uniformisation of values and its position in the full distribution of values. Heterogeneity between the studies was mentioned
Association between ABCB1 3435TT genotype and AEDs resistant epilepsy. Forest plot showed individual and overall ORs (black squares) with corresponding 95% CIs (horizontal bars) by individual report. P-value showed statistical significance of ORs and Z-value showed uniformisation of values and its position in the full distribution of values. Heterogeneity between the studies was mentioned
Analysis of publication bias
For the association between ABCB1 C3435T polymorphism, ABCB1 3435CC, and 3435TT genotype models with AEDs resistance, Funnel Plot showed asymmetrical appearances (Figs. 5, 6 and 7) and Egger’s regression test showed that P = 0.413, P = 0.492, and P = 0.085, respectively, were more than 0.05. The two tests demonstrated a significant publication bias.
Discussion
Epilepsy is a serious health problem affecting about 65 million people worldwide and manifesting many syndromes and types of seizures [60]. Since uncontrollable seizures increase morbidity and mortality, drug-resistant epilepsy is one of the major problems that physicians encounter. Recurrent seizures can devastate patients and their families. Therefore, drug-resistant epilepsy still remains one of the main challenges for epileptologists.
Since that genetic polymorphisms may play a role in response to AEDs [10], we conducted an updated systematic review in order to summarize the impact of polymorphisms in ABCB1, ABCC2, ApoE, CYP1A1, CYP2C9, GABRA1, GABRA2, GABRA3, GAT3, GSTM1, SCN1A, SCN2A, and SLC6A4 genes on AEDs resistant epilepsy. Our meta-analysis concerned only the association between ABCB1 C3435T polymorphism and drug-resistant epilepsy, which revealed a significant risk to pharmacoresistance (OR = 1.877, 95% CI 1.213–2.905, P = 0.005) (Fig. 2). Some studies confirmed our results [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Nevertheless, many other reports failed to prove an association between ABCB1 C3435T polymorphism and refractory epilepsy [38,39,40,41,92,93,94,95,42, 91–96].
The first publication showed that drug-resistant patients compared to drug-responsive patients, were more likely to have the CC genotype than the TT genotype (P = 0.006) [25]. Zimprich et al. confirmed the result [97]. Moreover, many studies indicated that the CC genotype were more prevalent in drug-resistant epilepsy [12,17,18,19,20,21,22,, 16–23]. However, three Asian studies [34,35,36] and one Australian study [33] showed the opposite association of TT genotype high frequency. In addition, our meta-analysis showed that patients resistant to AEDs were more likely to have ABCB1 3435TT genotype (OR = 2.375, 95% CI 1.775–3.178, P < 10-3) than 3435CC genotype (OR = 1.686, 95% CI 0.877–3.242, P = 0.117) (Figs. 3 and 4).
Due to these controversial results, meta-analyses were made in order to clarify the association between ABCB1 C3435T polymorphism and drug-resistant epilepsy. The majority suggest that the ABCB1 C3435T polymorphism may not be involved in the response to AEDs [58,59,60,61,62]. The study of Bournissen et al. showed no association of ABCB1 C3435T polymorphism with risk of drug resistance in overall and in the subgroup analysis by ethnicity (Asian and Caucasian populations) (n = 3371 subjects) [43]. The first study of Haerian et al. demonstrated the lack of allelic association with the risk of drug resistance under fixed and random effects models (n = 6755 subjects) [44] and the second study of Haerian et al. showed no significant association of ABCB1 alleles, genotypes, and haplotypes with recurrent seizures (n = 7067 patients) [45]. In the two studies, subanalysis of studies by ethnicity (Asian and Caucasian populations) yielded similar findings. Nurmohamed et al. failed to find a statistical significance between genotypes of ABCB1 C3435T polymorphism in cases and controls (n = 3996 subjects) [46]. No allelic neither genotypic association of ABCB1 C3435T polymorphism with childhood risk of drug resistance was found in overall and in the subgroup analysis by ethnicity (Asian and Caucasian populations) (n = 1249 subjects) in the study of Sun et al. [47]. Recently, two meta-analyses have indicated that CC genotype was associated with recurrent seizures in Caucasians. However, none of the genetic comparisons exhibited a significant association in Asians [63, 64]. In our knowledge, no another meta-analysis showed the same result as ours. Overall, meta-analyses stratified by genotype genetic models in the overall studies, indicate that the polymorphism may not play a major role in drug resistance to AEDs [46] and similar results are found in the subgroup analysis for the Asian and the Caucasian populations [43,44,45, 47]. However, other meta-analyses show a significant association in a specific ethnic subgroup [63, 64]. These discrepant results are mainly due to the small sample size, which is a common problem in association studies leading to underpowered genotypic results. Worldwide collaboration between different centers is then necessary to increase the sample size. In addition, ethnicity is another factor that may affect the results. An allele may become more common in ethnic subgroup but not in another, which may affect the response to AEDs [45]. However, four meta-analyses show no evidence that the ABCB1 C3435T polymorphism is associated with the risk of resistance to AEDs in Asians and Caucasians [43,44,45, 47]. Therefore, meta-analysis startified by ethnicity are needed to increase in order to confirm the ethnic-dependence of AEDs resistant epilepsy.
AEDs transporters have contribute in pharmacoresistant epilepsy. In fact, the most studied AEDs transporter proteins like membrane proteins, are ABC transporter superfamily members. They are ATP-dependent drug efflux pumps for specific AED and are mainly encoded by ABCB1 gene. ABCB1 protein or P-gp was transporte AED in the BBB [72]. P-gp activity can be affected by ABCB1 polymorphisms reducing plasmatic levels of AEDs and minimizing antiepileptic treatment efficiency in epileptic patients [98, 99]. If genetic background affects the expression of P-gp, then penetration of AEDs in the brain might depend on the patient’s genotype [16, 18]. Homozygous TT genotype is associated with decreased P-gp expression [4, 100].
Compared to literature search supporting conflicting results, our results show a higher contribution of ABCB1 3435TT genotype on response to AEDs. Our findings may contribute to exhibit the implication of genetic markers in refractory epilepsy before starting the treatment. In order to have a better AEDs therapeutic response, the identification of new potential genetic markers become necessary against pharmcoresistance in epilepsy. This will lead to a better understanding of drug resistance mechanisms in epilepsy. Furthermore, it will be extremely important for individual AEDs selection, early surgery feasibility and development of new efficacious treatments.
Limitations
Our analysis is consistent to our strategy search, inclusion criteria and statistical parameters. However, it may be limited due to several factors: 1) Few number of included studies is insufficient to carry out a subgroup analysis by ethnicity. In addition, the ethnicities in the included studies are heterogeneous. PGt and PGx studies of AEDs resistance should be performed by ethnicity. 2) Publication bias and heterogeneity might have an impact on the meta-analysis results. 3) Most of the included studies match different types of epilepsy with different AEDs. The affinity of each AED for ABC transporters is variable. In fact, Valproic acid (VPA) is a widely used AED and it is not transported by P-gp [101]. Thereby, the association between ABCB1 C3435T polymorphism and drug resistance epilepsy could be affected. Correlation between PGt and PGx results with specific AED should be required. 4) Different inclusion criteria are used to classify AEDs-resistant patients in the included studies, subsequently, the interpretation of the meta-analysis results become very complex. In fact, AEDs-resistant patients were defined as patients who had at least one seizure per month or 10 seizures over the previous year, despite two or more AEDs at therapeutic dosages and/or serum drug concentrations in three studies [22, 28, 34]. In other reports, drug resistance was defined as the occurrence of at least four seizures over the year despite more than three appropriate and tolerated AEDs for the epilepsy syndrome [25, 31, 33]. In some studies, it was defined as the failure of two appropriate and tolerated AEDs trials [27, 29], with a poor clinical outcome and recurrent seizures [35], or the occurrence of any types of seizures for a minimum of one year at the same dose of AEDs [36], or any seizures during the past three months [24] and more than 10 seizures over the year [23].
Conclusions
Various studies have yielded contradictory findings regarding the relationship between ABCB1 C3435T polymorphism and AEDs resistance in epilepsy. In the current meta-analysis, we demonstrate the existence of a statistical significant association between ABCB1 3435TT genotype and refractory epilepsy. Therefore, the screening of ABCB1 gene for this polymorphism in the future might be useful to decide the best treatment option for each patient and to predict the treatment outcome for new epileptic patients. However, considering the few number of included studies and the significant publication bias found in this meta-analysis, further investigations should be helpful to validate the use of this polymorphism in treatment decisions.
Abbreviations
- ABC:
-
atp-binding cassette
- ABCB1 :
-
atp-binding cassette sub-family b member 1
- ABCC2 :
-
atp-binding cassette sub-family c member 2
- AEDs:
-
Antiepileptic drugs
- ApoE :
-
Apolipoprotein e
- BBB:
-
Blood–brain barrier
- CBZ:
-
Carbamazepine
- CIs:
-
Confidence intervals
- CYP1A1 :
-
Cytochrome p450 1a1
- CYP2C9 :
-
Cytochrome p450 family member 2c9
- GABRA1 :
-
Gamma-aminobutyric acid-a receptor alpha1-subunit
- GABRA2 :
-
Gamma-aminobutyric acid-a receptor alpha2-subunit
- GABRA3 :
-
Gamma-aminobutyric acid-a receptor alpha3-subunit
- GAT3 :
-
Gamma-aminobutyric acid transporter 3
- GSTM1 :
-
Glutathione s-transferases mu 1
- ILAE:
-
International league against epilepsy
- MDR1:
-
Multidrug resistance protein 1
- MDR2 :
-
Multidrug resistance protein 2
- ORs:
-
Odds ratios
- OXC:
-
Oxcarbazepine
- P :
-
P-value
- P-gp:
-
P-glycoprotein
- PGt:
-
Pharmacogenetics
- PGx:
-
Pharmacogenomics
- PHT:
-
Phenytoin
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses guidelines
- SCN1A :
-
Sodium channel nav1.1
- SCN2A :
-
Sodium channel nav1.2
- SLC6A4 :
-
Solute ligand carrier family 6 member a4
- SNP:
-
Single nucleotide polymorphism
- VPA:
-
Valproic acid
References
Depont C. The potential of pharmacogenetics in the treatment of epilepsy. Eur J Pedaetric Neurol. 2006;10(2):57–65.
Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. Lancet. 2015;385(9971):884–98.
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser WA, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51(6):1069–77.
Lazarowski A, Czornyj L, Lubienieki F, Girardi E, Vazquez S, D'Giano C. ABC transporters during epilepsy and mechanisms underlying multidrug resistance in refractory epilepsy. Epilepsia. 2007;48 (Suppl 5):140–9.
Tishler DM, Weinberg KI, Hinton DR, Barbaro N, Annett GM, Raffel C. MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia. 1995;36(1):1–6.
Loscher W, Klotz U, Zimprich F, et al. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia. 2009;50(1):1–23.
Cascorbi I. ABC transporters in drug-refractory epilepsy: limited clinical significance of pharmacogenetics? Clin Pharmacol Ther. 2010;87(1):15.
Rau T, Erney B, Gores R, Eschenhagen T, Beck J, Langer T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clin Pharmacol Ther. 2006;80(5):468–76.
de Jong FA, Scott-Horton TJ, Kroetz DL, McLeod HL, Friberg LE, Mathijssen RH, et al. Irinotecan-induced diarrhea: functional significance of the polymorphic ABCC2 transporter protein. Clin Pharmacol Ther. 2007;81:42–9.
Haenisch S, Zimmermann U, Dazert E, Wruck CJ, Dazert P, Siegmund W, et al. Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharmacogenomics J. 2007;7(1):56–65.
Sisodiya SM, Martinian L, Scheffer GL, van der Valk P, Cross JH, Scheper RJ, et al. Major vault protein, a marker of drug resistance, is upregulated in refractory epilepsy. Epilepsia. 2003;44(11):1388–96.
Schmidt D, Loscher W. Drug resistance in epilepsy: putative neurobiologic and clinical mechanisms. Epilepsia. 2005;46(6):858–77.
Kubota H, Ishihara H, Langmann T, Schmitz G, Stieger B, Wieser HG, et al. Distribution and functional activity of P-glycoprotein and multidrug resistanceassociated proteins in human brain microvascular endothelial cells in hippocampal sclerosis. Epilepsy Res. 2006;68(3):213–28.
Remy S, Beck H. Molecular and cellular mechanisms of pharmacoresistance in epilepsy. Brain. 2006;129(Pt 1):18–35.
Speed D, Hoggart C, Petrovski S, Tachmazidou I, Coffey A, Jorgensen A, et al. A genome-wide association study and biological pathway analysis of epilepsy prognosis in a prospective cohort of newly treated epilepsy. Hum Mol Genet. 2013;23(1):247–58.
Kwan P, Brodie MJ. Potential role of drug transporters in the pathogenesis of medically intractable epilepsy. Epilepsia. 2005;46(2):224–35.
Potschka H, Fedrowitz M, Löscher W. P-glycoprotein and multidrug resistance-associated protein are involved in the regulation of extracellular levels of the major antiepileptic drug carbamazepine in the brain. Neuroreport. 2001;12(16):3557–60.
Loscher W, Potschka H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx. 2005;2(1):86–98.
Fromm MF. Importance of P-glycoprotein at blood-tissue barriers. Trends Pharmacol Sci. 2004;25(8):423–9.
Sarkadi B, Homolya L, Szakács G, Váradi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86(4):1179–236.
Tate SK, Sisodiya SM. Multidrug resistance in epilepsy: a pharmacogenomic update (review). Exp Opin Pharmacother. 2007;8(10):1441–9.
Hung CC, Tai JJ, Lin CJ, Lee MJ, Liou HH. Complex haplotypic effects of the ABCB1 gene on epilepsy treatment response. Pharmacogenomics. 2005;6(4):411–7.
Hung CC, Jen Tai J, Kao PJ, Lin MS, Liou HH. Association of polymorphisms in NR1I2 and ABCB1 genes with epilepsy treatment responses. Pharmacogenomics. 2007;8(9):1151–8.
Ebid AH, Ahmed MM, Mohammed SA. Therapeutic drug monitoring and clinical outcomes in epileptic Egyptian patients: a gene polymorphism perspective study. Ther Drug Monit. 2007;29(3):305–12.
Siddiqui A, Kerb R, Weale ME, Brinkmann U, Smith A, Goldstein DB, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348(15):1442–8.
Stasiołek M, Romanowicz H, Połatyńska K, Chamielec M, Skalski D, Makowska M, et al. Association between C3435T polymorphism of MDR1 gene and the incidence of drug-resistant epilepsy in the population of Polish children. Behav Brain Funct. 2016;12(1):21.
Taur SR, Kulkarni NB, Gandhe PP, Thelma BK, Ravat SH, Gogtay NJ, et al. Association of polymorphism of CYP2C9, CYP2C19, and ABCB1, and activity of P-glycoprotein with response to anti-epileptic drugs. J Postgrad Med. 2014;60(3):265–9.
Sayyah M, Kamgarpour F, Maleki M, Karimipoor M, Gharagozli K, Shamshiri AR. Association analysis of intractable epilepsy with C3435T and G2677T/A ABCB1 gene polymorphisms in Iranian patients. Epileptic Disord. 2011;13(2):155–65.
Keangpraphun T, Towanabut S, Chinvarun Y, Kijsanayotin P. Association of ABCB1 C3435T polymorphism with phenobarbital resistance in Thai patients with epilepsy. J Clin Pharm Ther. 2015;40(3):315–9.
Basic S, Hajnsek S, Bozina N, Filipcic I, Sporis D, Mislov D, et al. The influence of C3435T polymorphism of ABCB1 gene on penetration of Phenobarbital across the blood-brain barrier in patients with generalized epilepsy. Seizure. 2008;17(6):524–30.
Sánchez MB, Herranz JL, Leno C, Arteaga R, Oterino A, Valdizán EM, et al. Genetic factors associated with drug-resistance of epilepsy: relevance of stratification by patient age and aetiology of epilepsy. Seizure. 2010;19(2):93–101.
Soranzo N, Cavalleri GL, Weale ME, Wood NW, Depondt C, Marguerie R, et al. Identifying candidate causal variants responsible for altered activity of the ABCB1 multidrug resistance gene. Genome Res. 2004;14(7):1333–44.
Tan NC, Heron SE, Scheffer IE, Pelekanos JT, McMahon JM, Vears DF, et al. Failure to confirm association of a polymorphism in ABCB1 with multidrug-resistant epilepsy. Neurology. 2004;63(6):1090–2.
Kwan P, Baum L, Wong V, Ng PW, Lui CH, Sin NC, et al. Association between ABCB1 C3435T polymorphism and drug-resistant epilepsy in Han Chinese. Epilepsy Behav. 2007;11(1):112–7.
Shaheen U, Prasad DK, Sharma V, Suryaprabha T, Ahuja YR, Jyothy A, et al. Significance of MDR1 gene polymorphism C3435T in predicting drug response in epilepsy. Epilepsy Res. 2014;108(2):251–6.
Seo T, Ishitsu T, Ueda N, Nakada N, Yurube K, Ueda K, et al. ABCB1 polymorphisms influence the response to antiepileptic drugs in Japanese epilepsy patients. Pharmacogenomics. 2006;7(4):551–61.
Subenthiran S, Abdullah NR, Joseph JP, Muniandy PK, Mok BT, Kee CC, et al. Linkage disequilibrium between polymorphisms of ABCB1 and ABCC2 to predict the treatment outcome of Malaysians with complex partial seizures on treatment with carbamazepine mono-therapy at the Kuala Lumpur Hospital. PLoS One. 2013;8(5), e64827.
Chen L, Liu CQ, Hu Y, Xiao ZT, Chen Y, Liao JX. Association of a polymorphism in MDR1 C3435T with response to antiepileptic drug treatment in ethic Han Chinese children with epilepsy. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9(1):11–4.
Grover S, Bala K, Sharma S, Gourie-Devi M, Baghel R, Kaur H, et al. Absence of a general association between ABCB1 genetic variants and response to antiepileptic drugs in epilepsy patients. Biochimie. 2010;92(9):1207–12.
Ozgon GO, Bebek N, Gul G, Cine N. Association of MDR1 (C3435T) polymorphism and resistance to carbamazepine in epileptic patients from Turkey. Eur Neurol. 2009;59(1-2):67–70.
Sills GJ, Mohanraj R, Butler E, McCrindle S, Collier L, Wilson EA, et al. Lack of association between the C3435T polymorphism in the human multidrug resistance (MDR1) gene and response to antiepileptic drug treatment. Epilepsia. 2005;46(5):643–7.
Ufer M, Mosyagin I, Muhle H, Jacobsen T, Haenisch S, Hasler R, et al. Nonresponse to antiepileptic pharmacotherapy is associated with the ABCC2-24C > T polymorphism in young and adult patients with epilepsy. Pharmacogenet Genomics. 2009;19(5):353–62.
Bournissen FG, Moretti ME, Juurlink DN, Koren G, Walker M, Finkelstein Y. Polymorphism of the MDR1/ABCB1 C3435T drug-transporter and resistance to anticonvulsant drugs: a meta-analysis. Epilepsia. 2009;50(4):898–903.
Haerian BS, Roslan H, Raymond AA, Tan CT, Lim KS, Zulkifli SZ, et al. ABCB1 C3435T polymorphism and the risk of resistance to antiepileptic drugs in epilepsy: a systematic review and meta-analysis. Seizure. 2010;19(6):339–46.
Haerian BS, Lim KS, Tan CT, Raymond AA, Mohamed Z. Association of ABCB1 gene polymorphisms and their haplotypes with response to antiepileptic drugs: a systematic review and meta-analysis. Pharmacogenomics. 2011;12(5):713–25.
Nurmohamed L, Garcia-Bournissen F, Buono RJ, Shannon MW, Finkelstein Y. Predisposition to epilepsy--does the ABCB1 gene play a role? Epilepsia. 2010;51(9):1882–5.
Sun G, Sun X, Guan L. Association of MDR1 gene C3435T polymorphism with childhood intractable epilepsy: a meta-analysis. J Neural Transm (Vienna). 2014;121(7):717–24.
Li SX, Liu YY, Wang QB. ABCB1 gene C3435T polymorphism and drug resistance in epilepsy: evidence based on 8,604 subjects. Med Sci Monit. 2015;21:861–8.
Lv WP, Han RF, Shu ZR. Associations between the C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy: a meta-analysis. Int J Clin Exp Med. 2014;7(11):3924–32.
Sha'ari HM, Haerian BS, Baum L, Saruwatari J, Tan HJ, Rafia MH, et al. ABCC2 rs2273697 and rs3740066 polymorphisms and resistance to antiepileptic drugs in Asia Pacific epilepsy cohorts. Pharmacogenomics. 2014;15(4):459–66.
Ma CL, Wu XY, Zheng J, Wu ZY, Hong Z, Zhong MK. Association of SCN1A, SCN2A and ABCC2 gene polymorphisms with the response to antiepileptic drugs in Chinese Han patients with epilepsy. Pharmacogenomics. 2014;15(10):1323–36.
Ufer M, von Stulpnagel C, Muhle H, Haenisch S, Remmler C, Majed A, et al. Impact of ABCC2 genotype on antiepileptic drug response in Caucasian patients with childhood epilepsy. Pharmacogenet Genomics. 2011;21:624–30.
Seo T, Ishitsu T, Oniki K, Abe T, Shuto T, Nakagawa K. ABCC2 haplotype is not associated with drug-resistant epilepsy. J Pharm Pharmacol. 2008;60(5):631–5.
Kim DW, Lee SK, Chu K, Jang IJ, Yu KS, Cho JY, et al. Lack of association between ABCB1, ABCG2, and ABCC2 genetic polymorphisms and multidrug resistance in partial epilepsy. Epilepsy Res. 2009;84(1):86–90.
Kwan P, Wong V, Ng PW, Lui CH, Sin NC, Wong KS, et al. Gene-wide tagging study of the association between ABCC2, ABCC5 and ABCG2 genetic polymorphisms and multidrug resistance in epilepsy. Pharmacogenomics. 2011;12(3):319–25.
Hilger E, Reinthaler EM, Stogmann E, Hotzy C, Pataraia E, Baumgartner C, et al. Lack of association between ABCC2 gene variants and treatment response in epilepsy. Pharmacogenomics. 2012;13(2):185–90.
Chen P, Yan Q, Xu H, Lu A, Zhao P. The effects of ABCC2 G1249A polymorphism on the risk of resistance to antiepileptic drugs: a meta-analysis of the literature. Genet Test Mol Biomarkers. 2014;18(2):106–11.
Wang Y, Tang L, Pan J, Li J, Zhang Q, Chen B. The recessive model of MRP2 G1249A polymorphism decrease the risk of drug-resistant in Asian Epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2015;112:56–63.
Pi Piana C, Antunes Nde J, Della PO. Implications of pharmacogenetics for the therapeutic use of antiepileptic drugs. Expert Opin Drug Metab Toxicol. 2014;10(3):341–58.
Franco V, Perucca E. The pharmacogenomics of epilepsy. Expert Rev Neurother. 2015;15(10):1161–70.
Ma CL, Wu XY, Jiao Z, Hong Z, Wu ZY, Zhong MK, et al. SCN1A, ABCC2 and UGT2B7 gene polymorphisms in association with individualized oxcarbazepine therapy. Pharmacogenomics. 2015;16(4):347–60.
Abe T, Seo T, Ishitsu T, Nakagawa T, Hori M, Nakagawa K. Association between SCN1A polymorphism and carbamazepine-resistant epilepsy. Br J Clin Pharmacol. 2008;66(2):304–7.
Menzler K, Hermsen A, Balkenhol K, Duddek C, Bugiel H, Bauer S, et al. A common SCN1A splice-site polymorphism modifies the effect of carbamazepine on cortical excitability–a pharmacogenetic transcranial magnetic stimulation study. Epilepsia. 2014;55(2):362–9.
Zimprich F, Stogmann E, Bonelli S, Baumgartner C, Mueller JC, Meitinger T, et al. A functional polymorphism in the SCN1A gene is not associated with carbamazepine dosages in Austrian patients with epilepsy. Epilepsia. 2008;49(6):1108–9.
Manna I, Gambardella A, Bianchi A, Striano P, Tozzi R, Aguglia U, et al. A functional polymorphism in the SCN1A gene does not influence antiepileptic drug responsiveness in Italian patients with focal epilepsy. Epilepsia. 2011;52(5):e40–4.
Yip TS, O’Doherty C, Tan NC, Dibbens LM, Suppiah V, et al. SCN1A variations and response to multiple antiepileptic drugs. Pharmacogenomics J. 2014;14(4):385–9.
Haerian BS, Baum L, Kwan P, Tan HJ, Raymond AA, Mohamed Z, et al. SCN1A, SCN2A and SCN3A gene polymorphisms and responsiveness to antiepileptic drugs: a multicenter cohort study and meta-analysis. Pharmacogenomics. 2013;14(10):1153–66.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–29.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst. 1989;81(2):107–15.
Escalante-Santiago D, Feria-Romero IA, Ribas-Aparicio RM, Rayo-Mares D, Fagiolino P, Vázquez M, et al. MDR-1 and MRP2 Gene Polymorphisms in Mexican Epileptic Pediatric Patients with Complex Partial Seizures. Front Neurol. 2014;5:184.
Maleki M, Sayyah M, Kamgarpour F, Karimipoor M, Arab A, Rajabi A, Gharagozli K, et al. Association between ABCB1-T1236C polymorphism and drug-resistant epilepsy in Iranian female patients. Iran Biomed J. 2010;14(3):89–96.
Subenthiran S, Abdullah NR, Muniandy PK, Joseph JP, Cheong KC, Ismail Z, et al. G2677T polymorphism can predict treatment outcome of Malaysians with complex partial seizures being treated with Carbamazepine. Genet Mol Res. 2013;12(4):5937–44.
Qu J, Zhou BT, Yin JY, Xu XJ, Zhao YC, Lei GH, et al. ABCC2 polymorphisms and haplotype are associated with drug resistance in Chinese epileptic patients. CNS Neurosci Ther. 2012;18(8):647–51.
Grover S, Gourie-Devi M, Bala K, Sharma S, Kukreti R. Genetic association analysis of transporters identifies ABCC2 loci for seizure control in women with epilepsy on first-line antiepileptic drugs. Pharmacogenet Genomics. 2012;22(6):447–65.
Sporis D, Sertic J, Henigsberg N, Mahovic D, Bogdanovic N, Babic T. Association of refractory complex partial seizures with a polymorphism of ApoE genotype. J Cell Mol Med. 2005;9(3):698–703.
Gong JE, Qu J, Long HY, Long LL, Qu Q, Li XM, et al. Common variants of APOE are associated with anti-epileptic drugs resistance in Han Chinese patients. Int J Neurosci. 2016;2:1–6.
Grover S, Talwar P, Gourie-Devi M, Gupta M, Bala K, Sharma S, et al. Genetic polymorphisms in sex hormone metabolizing genes and drug response in women with epilepsy. Pharmacogenomics. 2010;11(11):1525–34.
Seven M, Batar B, Unal S, Yesil G, Yuksel A, Guven M. The effect of genetic polymorphisms of cytochrome P450 CYP2C9, CYP2C19, and CYP2D6 on drug-resistant epilepsy in Turkish children. Mol Diagn Ther. 2014;18(2):229–36.
Kumari R, Lakhan R, Kalita J, Misra UK, Mittal B. Association of alpha subunit of GABAA receptor subtype gene polymorphisms with epilepsy susceptibility and drug resistance in north Indian population. Seizure. 2010;19(4):237–41.
Kumari R, Lakhan R, Garg RK, Kalita J, Misra UK, Mittal B. Pharmacogenomic association study on the role of drug metabolizing, drug transporters and drug target gene polymorphisms in drug-resistant epilepsy in a north Indian population. Indian J Hum Genet. 2011;17(Supp1):S32–40.
Hung CC, Chen PL, Huang WM, Tai JJ, Hsieh TJ, Ding ST, et al. Gene-wide tagging study of the effects of common genetic polymorphisms in the α subunits of the GABA(A) receptor on epilepsy treatment response. Pharmacogenomics. 2013;14(15):1849–56.
Kim DU, Kim MK, Cho YW, Kim YS, Kim WJ, Lee MG, et al. Association of a synonymous GAT3 polymorphism with antiepileptic drug pharmacoresistance. J Hum Genet. 2011;56(9):640–6.
Liu CS, Tsai CS. Enhanced lipid peroxidation in epileptics with null genotype of glutathione S-transferase M1 and intractable seizure. Jpn J Pharmacol. 2002;90(3):291–4.
Wang P, Zhou Q, Sheng Y, Tang B, Liu Z, Zhou B. Association between two functional SNPs of SCN1A gene and efficacy of carbamazepine monotherapy for focal seizures in Chinese Han epileptic patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39(5):433–41.
Zhou BT, Zhou QH, Yin JY, Li GL, Qu J, Xu XJ, et al. Effects of SCN1A and GABA receptor genetic polymorphisms on carbamazepine tolerability and efficacy in Chinese patients with partial seizures: 2-year longitudinal clinical follow-up. CNS Neurosci Ther. 2012;18(7):566–72.
Abo El Fotoh WM, Abd El Naby SA, Habib MS, ALrefai AA, Kasemy ZA. The potential implication of SCN1A and CYP3A5 genetic variants on antiepileptic drug resistance among Egyptian epileptic children. Seizure. 2016;41:75–80.
Kwan P, Poon WS, Ng HK, Kang DE, Wong V, Ng PW, et al. Multidrug resistance in epilepsy and polymorphisms in the voltage-gated sodium channel genes SCN1A, SCN2A, and SCN3A: correlation among phenotype, genotype, and mRNA expression. Pharmacogenet Genomics. 2008;18(11):989–98.
Hecimovic H, Stefulj J, Cicin-Sain L, Demarin V, Jernej B. Association of serotonin transporter promoter (5-HTTLPR) and intron 2 (VNTR-2) polymorphisms with treatment response in temporal lobe epilepsy. Epilepsy Res. 2010;91(1):35–8.
Kauffman MA, Consalvo D, Gonzalez-Morón D, Aguirre F, D'Alessio L, Kochen S. Serotonin transporter gene variation and refractory mesial temporal epilepsy with hippocampal sclerosis. Epilepsy Res. 2009;85(2-3):231–4.
Lakhan R, Misra UK, Kalita J, Pradhan S, Gogtay NJ, Singh MK, et al. No association of ABCB1 polymorphisms with drug-refractory epilepsy in a north Indian population. Epilepsy Behav. 2009;14(1):78–82.
Kim DW, Kim M, Lee SK, Kang R, Lee SY. Lack of association between C3435T nucleotide MDR1 genetic polymorphism and multidrug-resistant epilepsy. Seizure. 2006;15(5):344–7.
Leschziner GD, Andrew T, Leach JP, Chadwick D, Coffey AJ, Balding DJ, et al. Common ABCB1 polymorphisms are not associated with multidrug resistance in epilepsy using a gene-wide tagging approach. Pharmacogenet Genomics. 2007;17(3):217–20.
Shahwan A, Murphy K, Doherty C, Cavalleri GL, Muckian C, Dicker P, et al. The controversial association of ABCB1 polymorphisms in refractory epilepsy: an analysis of multiple SNPs in an Irish population. Epilepsy Res. 2007;73(2):192–8.
Vahab SA, Sen S, Ravindran N, Mony S, Mathew A, Vijayan N, et al. Analysis of genotype and haplotype effects of ABCB1 (MDR1) polymorphisms in the risk of medically refractory epilepsy in an Indian population. Drug Metab Pharmacokinet. 2009;24(3):255–60.
Zhou L, Cao Y, Long H, Long L, Xu L, Liu Z, et al. ABCB1, ABCC2, SCN1A, SCN2A, GABRA1 gene polymorphisms and drug resistant epilepsy in the Chinese Han population. Pharmazie. 2015;70(6):416–20.
Zimprich F, Sunder-Plassmann R, Stogmann E, Gleiss A, Dal-Bianco A, Zimprich A, et al. Association of an ABCB1 gene haplotype with pharmacoresistance in temporal lobe epilepsy. Neurology. 2004;63(6):1087–9.
Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101(3):563–70.
Lasoń W, Chlebicka M, Rejdak K. Research advances in basic mechanisms of seizures and antiepileptic drug action. Pharmacol Rep. 2013;65(4):787–801.
Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–8.
Baltes S, Fedrowitz M, Tortós CL, Potschka H, Löscher W. Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J Pharmacol Exp Ther. 2007;320(1):331–43.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
This study included articles which are available via PubMed. All information analysed in this study was collected in a dataset and this is available from the corresponding author on reasonable request.
Authors’ contributions
M.C. and W.K. contributed equally to this work: designed the study, collected the data, conducted the analyses and wrote the manuscript. K.T. helped to perform the outcome analyses. H.K., I.B.Y.T. and L.H. revised the manuscript. All authors read and approved the final document.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chouchi, M., Kaabachi, W., Klaa, H. et al. Relationship between ABCB1 3435TT genotype and antiepileptic drugs resistance in Epilepsy: updated systematic review and meta-analysis. BMC Neurol 17, 32 (2017). https://doi.org/10.1186/s12883-017-0801-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-017-0801-x