Abstract
Background
In this study, we aimed to clarify the beneficial effects of urate-lowering treatment with the novel agent dotinurad on renal function in patients with chronic kidney disease (CKD) and hyperuricemia (HUA).
Methods
Thirty-five patients with CKD (mean age 65.4 ± 14.8 years, 23 men) diagnosed with HUA were recruited. Changes in eGFR before and after dotinurad administration were assessed. Patients first underwent a 3-month observation period and then 3 months treatment with dotinurad.
Results
During the observation period, mean eGFR (mL/min/1.73 m2) declined significantly. The baseline eGFR was 31.8 ± 16.4 and the serum urate level (sUA, mg/dL) was 8.1 ± 1.7. During the treatment period, eGFR recovered to 36.5 ± 17.5 and sUA decreased to 6.7 ± 1.0. The increase in eGFR after dotinurad administration was correlated with a decrease in sUA (R = 0.375, p = 0.0263).
Conclusion
Dotinurad administration to patients with CKD and HUA appears to be beneficial in restoring kidney function. Dotinurad may represent a potential medication for the prevention of kidney function decline caused by HUA.
Similar content being viewed by others
Introduction
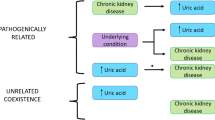
Hyperuricemia (HUA) is a noncommunicative disease whose prevalence has rapidly increased since World War II [1]. HUA causes gout, including gouty arthritis, and also increases the risk of kidney dysfunction or chronic kidney disease (CKD) [2,3,4]. For instance, we previously demonstrated through a cohort study that HUA increases the risk of CKD occurrence 3.99-fold among the general population [4]. Therefore, HUA is considered to be a cause of CKD.
On the other hand, the necessity of urate-lowering therapy for CKD patients with HUA is uncertain. In other words, whether HUA is an aggravating factor for CKD or not is unclear.
In this study, we aimed to investigate whether urate-lowering therapy would improve the course of kidney function in patients with CKD and HUA using dotinurad, a novel selective urate reabsorption inhibitor (SURI) that selectively inhibits the urate transporter 1 (URAT1 / SLC22A12), as an antihyperuricemic agent.
Methods
Study design, participants, and intervention (Fig. 1)
The study participants were outpatients who visited the Division of Nephrology at Teikyo University Chiba Medical Center Hospital between April 2021 and November 2021 and met the criteria for CKD [5] and HUA [serum uric acid (sUA) level > 7 mg/dL, symptomatic gout, or both] [6]. Patients with a history of systemic diseases such as malignancy and collagen diseases were excluded.
The patients first underwent a 3-month observation period and then 3 months of dotinurad treatment. To be specific, after a 3-month observation period before intervention, 0.5 mg/day of dotinurad was administered once a day. In most cases, the dose of dotinurad was increased to 1.0 mg/day to achieve sUA levels < 6.0 mg/dL [6]. Other medications – including irbesartan (1 case) and losartan (2 cases) that have a urate-lowering effect and hydrochlorothiazide (1 case) that have a urate-elevating effect—were not changed during the study period.
Data collection
Demographic, clinical, and laboratory data were reviewed. Levels of sUA, serum creatinine (sCr), hemoglobin, serum albumin, serum cholinesterase, urinary protein, urinary creatinine, and urinary uric acid were measured at our hospital using standardized reagents and methods. The degree of proteinuria was quantified using the spot urine protein-creatinine ratio (UPCR, g/gCr). The fractional excretion of uric acid (FEUA) was also calculated before dotinurad administration and 3 months after.
Kidney function was expressed as the estimated glomerular filtration rate (eGFR), which was determined using the following formula proposed by the Japanese Society of Nephrology [7]:
Statistical analysis
Continuous data are expressed as means ± standard deviation or medians with 25th and 75th percentiles, and categorical data are expressed as percentages. Changes in various parameters before and after dotinurad administration were determined using paired Student’s t-test.
eGFR-pre represents the difference in eGFR at the start of treatment (baseline) and before 3 months of treatment, and ΔeGFR-post represents the difference in eGFR after 3 months of treatment and at baseline. Changes in eGFR before and after dotinurad administration (difference between eGFR-pre and ΔeGFR-post) were also determined using paired Student’s t-test. Stratified analyses were performed on the basis of gender and age.
Differences with a p-value < 0.05 were considered statistically significant. All statistical analyses were performed using EZR Version 1.33 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of the R commander, which is designed to add statistical functions that are frequently used in biostatistics [8].
Results
Baseline characteristics and laboratory data of the participants are presented in Table 1. At starting of treatment, mean age was 65.4 ± 14.8 years and 71.4% of the patients were male. Mean eGFR was 31.7 ± 16.4 and mean sUA was 8.1 ± 1.7. Of the 35 participants, 17 patients classified as CKD stage G3, 13 as CKD stage G4, and 5 as CKD stage G5. None of the participants complained of adverse effects of dotinurad during the study period.
Changes in sUA and various parameters other than eGFR before and after dotinurad use (baseline and after 3 Mo) are shown in Table 2. After 3 months of dotinurad administration, the mean sUA decreased significantly from 8.1 ± 1.7 to 6.7 ± 1.0. Regarding FEUA, no significant difference was observed between baseline and after 3 Mo, suggesting that the decrease in sUA via inhibition of URAT1 by dotinurad probably had already reached equilibrium.
Figure 2 presents a comparison of eGFR values before 3 months, at baseline, and after 3 months. During 3 months of observation, mean eGFR declined significantly from 35.5 ± 16.8 to 31.8 ± 16.4, suggesting that kidney dysfunction was progressive in these patients. Additionally, during 3 months of intervention, mean eGFR increased from 31.8 ± 16.4 to 36.5 ± 17.5, suggesting that kidney function was recovered by urate-lowering treatment using dotinurad, and accordingly, mean ΔeGFR-pre showed a negative value (-3.7 ± 5.6), whereas mean ΔeGFR-post showed a positive value (4.7 ± 9.5), and the difference between them was statistically significant. Such beneficial effects of dotinurad administration on kidney function were observed regardless of gender, age, eGFR at starting of treatment, or primary CKD diagnosis (Table 3).
To evaluate whether the difference in kidney function or primary disease may influence the nephroprotective effect of dotinurad, we performed a multiway analysis of variance, in which ΔeGFR-post was adopted as the objective variable and CKD stage or primary disease as explanatory variable. The result is shown in Fig. 3. Neither CKD stage nor primary disease influenced the degree of improvement in kidney function after the administration of dotinurad.
Increase in eGFR after dotinurad administration (ΔeGFR-post) stratified by CKD stage and primary disease. The difference in CKD stage or primary disease did not affect the nephroprotective effect of dotinurad. GK, gout kidney; DKD, diabetic kidney disease; Nscl, nephrosclerosis; CGN, chronic glomerulonephritis
Figure 4 shows the relationship between the decrease in sUA and GFR-post (increase in eGFR) during 3 months of intervention. A significant positive correlation was observed between them, suggesting that the improvement in kidney function after dotinurad usage was caused not by a profound pleiotropic effect of this drug but by the decrease in the reabsorption of urate per se.
Discussion
In the present single-center study of CKD patients with HUA, changes in kidney function before and after administration of dotinurad were investigated. Kidney function declined during the 3 months of observation and then increased after dotinurad administration. This result suggested two possible explanations.
-
(1)
HUA could contribute to the exacerbation of kidney dysfunction in patients with CKD, at least in certain settings.
-
(2)
Dotinurad restored kidney dysfunction caused by HUA.
The number of patients with CKD has been increasing worldwide, and the disease is estimated to affect 200 million individuals worldwide [9]. Furthermore, the number of patients with CKD is expected to increase. CKD creates a considerable burden and is recognized as an important problem for both individuals and society. CKD is a risk factor for not only end-stage kidney disease (ESKD) but also cardiovascular disease, which is the main cause of death worldwide [10, 11]. In addition, worldwide medical expenses associated with hemodialysis for ESKD are estimated to increase to US$1,000 billion within the next 10 years [12]. Therefore, establishing an effective strategy for CKD remission is an important public and national health issue.
Accumulating evidence has shown that HUA is associated with CKD progression [2,3,4]; however, most trials to clarify whether urate-lowering treatment can attenuate the decline in renal function in patients with CKD have failed to achieve these goals [13,14,15]. Although such studies adopted xanthine oxidoreductase inhibitor as a urate-lowering drug, adaptation of SURI as a urate-lowering drug might demonstrate reno-protective effects on progressive kidney damage related to HUA. Histologic findings of renal injury directly related to HUA include deposition of monosodium urate monohydrate in the renal interstitium [16,17,18], and SURI might prevent the passive inflow of urate from the internal lumen of proximal collecting tubule to the renal interstitum via URAT1 and the tubular cell matrix. This possibility is supported by the clear relationship between decreased sUA and increased eGFR observed in the present study (Fig. 4).
Of course, it is important to review diet and lifestyle in hyperuricemia, as in diabetes, which is a lifestyle-related disease such as hyperuricemia. On the other hand, as in diabetes, the nephroprotective effect of dietary and lifestyle changes in hyperuricemia is unclear. Our results suggest that SURI, which acts directly on the renal proximal tubules of the kidney, has a distinct nephroprotective effect, as do SGLT2 inhibitors, which also act directly on the renal proximal tubules.
The result of present study, however, should be interpreted with caution, for patients with CKD with apparent HUA – like present study population—may not teem in the real world, and the beneficial effect of dotinurad is supposed to be limited to patients with apparent HUA. This study had certain limitations. First, it is unclear whether the findings could be generalized to other ethnic or age groups because the subjects analyzed were all outpatients of one hospital, and the possibility of sampling bias cannot be denied. Second, the number of participants was small, preventing us from performing a stratified analysis. Third, the present study was a single-arm observational study, and we only compared post-treatment and pre-treatment phases and did not compare changes in kidney function between patients who did and did not use dotinurad. Fourth, pathological findings from renal biopsies were not examined in this study. Fifth, the reduction of deposed monosodium urate monohydrate in the renal interstitium through appropriate diagnostic images such as dual energy CT was not demonstrated in the present study. To overcome these limitations, prospective randomized studies with larger numbers of patients are required.
In conclusion, in the present study, the use of dotinurad in CKD patients with HUA appeared to be beneficial for preserving kidney function, and these results further indicate that this novel SURI might be a potential key medication for preventing kidney function decline in CKD patients with HUA.
Availability of data and materials
The data presented in this study are available on request from the corresponding author.
Abbreviations
- CKD:
-
Chronic kidney disease
- HUA:
-
Hyperuricemia
- eGFR:
-
Estimated glomerular filtration rate
- SURI:
-
Selective urate reabsorption inhibitor
- URAT:
-
Urate transporter
- SLC:
-
Solute carrier
- sUA:
-
Serum uric acid
- sCr:
-
Serum creatinine
- UPCR:
-
Urine protein-creatinine ratio
- ESKD:
-
End-stage kidney disease
References
Hakoda M, Kasagi F. Future trends for the number of gout patients in Japan. Gout Uric Nucleic Acids. 2020;44:33–9. https://doi.org/10.14867/gnamtsunyo.44.1_33. (in Japanese).
Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–50. https://doi.org/10.1053/j.ajkd.2004.06.006.
Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008;19:2407–13. https://doi.org/10.1681/ASN.2008010080.
Kawashima M, Wada K, Ohta H, et al. Association between asymptomatic hyperuricemia and new-onset chronic kidney disease in Japanese male workers: a long-term retrospective cohort study. BMC Nephrol. 2011;12:31. https://doi.org/10.1186/1471-2369-12-31.
Japanese Society of Nephrology. Essential points from evidence-based clinical practice guidelines for chronic kidney disease 2018. Clin Exp Nephrol. 2019;23:1–15. https://doi.org/10.1007/s10157-018-1648-1.
Hisatome I, Ichida K, Mineo I, et al. Japanese Society of Gout and Uric & Nucleic Acids 2019 guidelines for management of hyperuricemia and gout 3rd edition. Gout Uric Nucleic Acids. 2020;44(suppl):S1–40. https://doi.org/10.14867/gnamtsunyo.44.Supplement_sp-1.
Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92. https://doi.org/10.1053/j.ajkd.2008.12.034.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244.
Perico N, Remuzzi G. Chronic kidney disease: a research and public health priority. Nephrol Dial Transplant. 2012;27(suppl 3):iii19–26. https://doi.org/10.1093/ndt/gfs284.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. https://doi.org/10.1056/NEJMoa041031.
Nakayama M, Metoki H, Terawaki H, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population: the Ohasama study. Nephrol Dial Transplant. 2007;22:1910–5. https://doi.org/10.1093/ndt/gfm051.
Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–82. https://doi.org/10.1016/S0140-6736(14)61601-9.
Badve SV, Pascoe EM, Tiku A, et al. Effects of allopurinol on the progression of chronic kidney disease. N Engl J Med. 2020;382:2504–13. https://doi.org/10.1056/NEJMoa1915833.
Kimura K, Hosoya T, Uchida S, et al. Febuxostat therapy for patients with Stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. 2018;72:798–810. https://doi.org/10.1053/j.ajkd.2018.06.028.
Doria A, Galecki AT, Spino C, et al. Serum urate lowering with allopurinol and kidney function in Type 1 diabetes. N Engl J Med. 2020;382:2493–503. https://doi.org/10.1056/NEJMoa1916624.
Seegmiller JE, Erazier PD. Biochemical considerations of the renal damage of gout. Ann Rheum Dis. 1966;25(6 Suppl):668–72. https://doi.org/10.1136/ard.25.Suppl_6.668.
Sellmayr M, Petzsche MRH, Ma Q, et al. Only hyperuricemia with crystalluria, but not asymptomatic hyperuricemia, drives progression of chronic kidney disease. J Am Soc Nephrol. 2020;31:2773–92. https://doi.org/10.1681/ASN.2020040523.
Bardin T, Nguyen QD, Tran KM, et al. A cross-sectional study of 502 patients found a diffuse hyperechoic kidney medulla pattern in patients with severe gout. Kidney Int. 2021;99:218–26. https://doi.org/10.1016/j.kint.2020.08.024.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception of this study. Hoichi Amano and Hiroyuki Terawaki designed the study and performed statistical analyses. The first draft of the manuscript was written by Hoichi Amano, and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript, which was modified by Hiroyuki Terawaki.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the principles of the Declaration of Helsinki. This study was approved by the Review Board of Teikyo University (approval No. 21–196). Informed consent was not obtained from individual patients because the laboratory data used in this study were extracted from routine examination files and analyzed retrospectively. We posted the research content at the hospital and gave all participants the opportunity to refuse to participate according to the instruction by the Review Board of Teikyo University (approval No. 21–196).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Amano, H., Kobayashi, S. & Terawaki, H. Dotinurad restores exacerbated kidney dysfunction in hyperuricemic patients with chronic kidney disease. BMC Nephrol 25, 97 (2024). https://doi.org/10.1186/s12882-024-03535-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03535-9