Abstract
Background
The single-pool model urea clearance index (single-pool Kt/Vurea; spKt/V) is the most commonly used method for dialysis adequacy assessment. However, only a few studies have examined the relationship between spKt/V values and parameters related to sarcopenia and nutritional status. This study aimed to evaluate whether the spKt/V is an indicator of sarcopenia and nutritional status in patients undergoing maintenance hemodialysis (MHD).
Methods
A total of 142 patients were included in this single-center, cross-sectional study. Venous blood samples were collected shortly before the hemodialysis session. The adequacy of dialysis in patients receiving MHD was assessed using spKt/V. Sarcopenia was identified according to the Asian Working Group for Sarcopenia (2019) definition. Receiver operating characteristic curve and area under the curve were used to evaluate the predictive value of spKt/V in sarcopenia. Univariate and multivariate binary logistic regression analyses were used to determine the association between spKt/V and sarcopenia and nutritional status.
Results
The mean spKt/V level was 1.3 ± 0.2, the prevalence of sarcopenia was 15.5% in patients on MHD. The best cutoff value of spKt/V in sarcopenia was 1.45 for both sexes, 1.33 and 1.45 for men and women, respectively (P < 0.05). The multivariate binary logistic regression shown that the spKt/V was independently positively associated with sarcopenia (OR = 122.88, 95% CI = 0.64–0.87, P = 0.002). Grouping spKt/V by the best cutoff value, when spKt/V ≥ 1.45, the OR of sarcopenia was 11.75 (95% CI = 3.16–43.67, P < 0.001). Subgroup analyses showed that when spKt/V ≥ 1.33 in men and spKt/V ≥ 1.45 in woman, the OR of sarcopenia was 9.73 (95% CI = 2.25–42.11, P = 0.002) and 14.52 (95% CI = 1.06–199.67, P = 0.045), respectively.
Conclusions
The present study showed that spKt/V was an important influencing factor of sarcopenia and malnutrition in Asian patients on MHD.
Similar content being viewed by others
Introduction
Patients with end-stage kidney disease (ESKD) on maintenance hemodialysis (MHD) often experience various metabolic disorders, among which sarcopenia is a serious complication characterized by muscle protein loss, decreased muscle strength, and impaired functionality. Sarcopenia in patients on dialysis increases the risk of cardiovascular events, infections, and all-cause mortality [1] while imposing a strain on healthcare insurance systems. Patients with CKD are prone to malnutrition or protein-energy wasting (PEW), which places them at risk of poor prognoses [2]. The worldwide prevalence of PEW ranges from 11 to 54% in patients with CKD stages 3–5 [3] and 28–54% in those undergoing dialysis [4].
Currently, the single-pool model urea clearance index (urea clearance multiply by dialysis session duration/volume of urea distribution [Vurea]; spKt/V) is the most commonly used method for dialysis doses assessment [5]. According to the National Kidney Foundation-Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) Clinical Practice Guidelines, the recommended minimum level for patients with little or no residual renal function (RKF) is set at 1.2 spKt/V (equal to 65% urea reduction rate [URR]) [6]. Studies indicate that Vurea can serve as an indicator of skeletal muscle mass and nutritional health. Patients with low muscle mass or malnutrition typically have higher Kt/V values, which ultimately leads to poor prognosis [7]. Moreover, spKt/V values ≥ 1.4 are negatively correlates with albumin, body mass index (BMI) and Geriatric Nutrition Risk Index (GNRI) in patients on hemodialysis [8]. These findings demonstrate that dialysis dose is intricately linked to skeletal muscle and nutritional status. Interpretation of the available data on the relationship between Kt/V and patient survival suggests that increasing the dialysis dose appears to be beneficial to the patient, but reaching the “overdialysis” threshold may lead to higher mortality [9]; hence, the optimal threshold dose of dialysis is still debatable.
To our knowledge, studies have not utilized the spKt/V to diagnose and evaluate sarcopenia in patients on MHD, and only a few quantitative studies examined the relationship between spKt/V values and parameters related to sarcopenia and nutritional status. Therefore, this cross-sectional study aimed to examine the relationship between spKt/V and sarcopenia and nutritional status in patients on MHD.
Methods and materials
Population and study design
Patients who regularly received MHD at our hemodialysis center in September 2022 were enrolled in this single-center, cross-sectional study. The inclusion criteria were: (1) performed maintenance hemodialysis regularly for more than 3 months, 3 times a week, and for four hours each time, (2) aged ≥ 18 years, (3) treatment with bicarbonate dialysate and polysulfone membranes, and (4) provision of informed consent. The non-inclusion criteria were: (1) patients with pacemakers; (2) those with comorbid malignant tumors, decompensated cirrhosis, heart failure, acute myocardial infarction, cerebrovascular accident, or severe infections within 3 months; or (3) those with physical disabilities or who could not cooperate with the study for other reasons. This study adhered to the principles of the Declaration of Helsinki and was approved by the appropriate ethics committee (No. 2023-040-01). Venous blood samples were collected shortly before the hemodialysis session, and demographic, clinical, biochemical, and body composition measurement data were recorded.
Demographic, clinical, and laboratory parameters
Data on the history of diabetes, sex, age, and dialysis vintage were collected. The blood samples obtained before dialysis were analyzed at the Clinical Laboratory Department of our hospital using biochemical tests. Subsequently, serum creatinine, serum albumin, proalbumin, and highly sensitive C-reactive protein. According to the modification of diet in renal disease (MDRD) to calculate the estimated glomerularfiltration rate (eGFR) [10]:
Assessment of dialysis adequacy
The adequacy of dialysis in patients receiving MHD was assessed using spKt/V and URR. The spKt/V was calculated according to second-generation logarithmic estimates of spKt/V:
Where R is the ratio of pre- to posthemodialysis concentrations of BUN, t is the dialysis session duration (in hours), UF is the amount of ultrafiltration (L) during the given hemodialysis session, and W is the post-hemodialysis weight (kg) [11]. Pre- and posthemodialysis urea levels were obtained using the slow-flow technique described in the NKF-KDOQI guidelines [6]. The URR was calculated according to the following formula [12]:
Pinch strength and grip strength measurement
Pinch strength and grip strength were measured using the BASELINE digital Pinch Force Tester (12–0081, Fabrication Enterprises Inc., USA) and the BASELINE digital Grip Force Tester (12–0091, Fabrication Enterprises Inc., USA). The pinch force tester and grip force tester were placed in the hand of MHD patents without a fistulae or the dominant hand before dialysis. Participants were asked to apply as much pinch and grip strength as possible to the instrument. The measurements were repeated three times and their maximum values were taken.
Body composition measurement
The patients’ body composition was measured using a body composition analyzer (Multiscan 5000; Bodystat, Isle of Man, UK) by bioimpedance spectroscopy analysis (BIS). Measurements were performed with patients in the supine position, with electrode sheets attached to the hands and feet on the side without a fistula for hemodialysis, and the measurements were performed after inputting the relevant information. Lean body mass, phase angle (PhA), body cell mass, extracellular water (ECW), intracellular water (ICW), and the ECW/ICW ratio were measured. The equations for calculating appendicular skeletal muscle (ASM) and appendicular skeletal muscle index (ASMI) are as follows:
for men; and
for women.
Where Z is the reactance at different frequencies in Ω, height in m, and ASM in kg; and ASMI was calculated as ASM/height2 (kg/m2) [13]. BMI was calculated as weight/height2 (kg/m2).
Assessment of Sarcopenia
Sarcopenia was defined in accordance with the criteria of the 2019 Asian Sarcopenia Working Group on Sarcopenia: (1) ASMI < 7.0 kg/m2 for men and ASMI < 5.7 kg/m2 for women; (2) Grip strength < 28 kg for men and < 18 kg for women [14].
Calculation of nutritional indices
The GNRI was calculated according to the following formula:
Where ideal body weight was calculated as 22 (kg/m2) × height. If the actual body weight was greater than the ideal body weight, the value of “(actual body weight/ideal body weight)” was set to 1 [15]. Currently, several studies have shown that a GNRI < 91.2 in patients on MHD can be defined as a risk of malnutrition [16]. This definition was adapted in the present study.
The modified Creatinine Index (mCI) was calculated according to the following formula [17]:
Statistical analyses
SPSS (version 25.0; IBM Corp, Armonk, NY, USA) and R software (version 4.2.1) was used for statistical analyses. Continuous variables with a normal distribution were described as mean ± standard deviation, and those with non-normal distribution as median (interquartile range [IQR]). Categorical variables were described using percentages. Participants were divided into non-sarcopenic and sarcopenic groups. Comparisons between two groups were performed by two independent-samples t test, χ2 test, or Mann-Whitney U test. Receiver operating characteristic (ROC) curve and its respective area under the curve (AUC) were used to evaluate the predictive value of spKt/V in sarcopenia and GNRI. The cutoff value was defined as the maximum value of (sensitivity - [1-specificity]). Univariate analyses were conducted to verify the correlation between spKt/V and sarcopenia parameters and nutritional parameters. In multivariate binary logistic regression analyses, potentially relevant variables or known to be important in the physiology of sarcopenia: sex, age, diabetes, dialysis vintage and BMI, were included to determine whether the spKt/V and the best cutoff value of spKt/V values for both sexes, men and women was independently associated with sarcopenia. A two-tailed P-value < 0.05 was considered statistically significant.
Results
General clinical characteristics of the participants
The screening process is shown in Fig. 1. We reported in Table 1 the characteristics of the overall population as well as the comparison between Non-sarcopenia and Sarcopenia groups. The partial indicators of muscle strength, body composition, and nutritional indices were significantly lower in the sarcopenia group compared to the non-sarcopenia group. The age, spKt/V in the sarcopenia group were significantly higher than those of the non-sarcopenia group (P < 0.05).
Correlations between spKt/V and sarcopenia parameters and nutritional parameters
The results of univariate binary logistic regression analysis are shown in Table 2. We found that much of the lower levels indicators of muscle strength, body composition, and nutritional indices were related to spKt/V (P < 0.05).
spKt/V in Sarcopenia and GNRI diagnosis
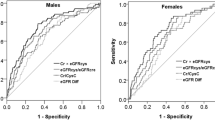
The ROC curves showed the predicted probability of sarcopenia based on spKt/V. The AUC was 0.739 and the best cutoff value of spKt/V on sarcopenia was 1.45 for both sexes with a sensitivity of 63.64% and specificity of 82.64% (Fig. 2; P < 0.001). The AUC was 0.793 and the best cutoff value of spKt/V on sarcopenia was 1.33 for men with a sensitivity of 73.33% and specificity of 74.36% (Fig. 3; P < 0.001). The AUC was 0.744 and the best cutoff value of spKt/V on sarcopenia was 1.45 for women with a sensitivity of 87.5% and specificity of 66.67% (Fig. 3; P = 0.03). In evaluating GNRI with spKt/V, the AUC in the ROC curve analysis was 0.628 (95% CI = 0.48–0.77, P = 0.09). The results indicated that spKt/V had a higher predictive capacity in the diagnosis of sarcopenia (Supplementary Fig. S1).
Associations between spKt/V and Sarcopenia
Models were fitted using multivariate binary logistic regression with sarcopenia as a categorical variable to analyse the associations between spKt/V and sarcopenia, considering the best cutoff value of spKt/V and group by gender. After adjusted for sex, age, diabetes mellitus, dialysis vintage, and BMI, we found that the spKt/V was independently positively associated with sarcopenia (Tables 3 and 4).
Discussion
In this cross-sectional study, we hypothesized that spKt/V is associated with the muscular and nutritional status of patients during thrice-weekly hemodialysis. Primarily, the spKt/V value was found to be a sensitive indicator for sarcopenia in patients on MHD. Furthermore, higher levels of spKt/V were independently associated with a high risk of sarcopenia in MHD patients.
Urea clearance, an indicator of dialysis dose, is expressed as Kt/V [18]. The determination of Kt/V is based on a kinetic model of urea per dialysis, which can be estimated using either a single-pool Kt/V (spKt/V) or a double-pool Kt/V; the latter explains the post-dialysis urea rebound (equilibrium Kt/V [eKt/V]) [19].
The NKF-KDOQI guidelines recommend that for patients with low residual natural renal clearance (KRU, 2 mL/min), the target dialysis dose for thrice-weekly HD is 1.2–1.4 spKt/V per dialysis [6]. It has been shown that spKt/V is a significant predictor of morbidity and mortality in patients receiving HD [20, 21]. Studies have shown a strong correlation between Kt/V and mortality in HD patients [22]. It has been shown that lower than recommended Kt/V (< 1.2) may increase mortality, especially in HD patients among women [23]. In one study, the greatest survival gain of higher HD dose was associated with a Kt/V approaching the 1.6 to 1.8 range [24]. In another study, researchers found that increasing the dialysis dose did not improve mortality in MHD patients, but rather increased the relative risk of death, especially at higher doses ( spKt/V > 1.6) [25]. Therefore, the optimal dialysis dose is still debatable.
Wang et al. found that patients with sarcopenia had higher spKt/V levels than healthy individuals [26]. A study by Kaya et al. found that patients on dialysis with weight and muscle mass loss may experience severe PEW when spKt/V is greater than the target value [8]. Higher Kt/V values are associated with low lean body mass index and a high risk of death in patients on dialysis, ultimately leading to a poor prognosis [27]. However, no study has used dialysis doses to predict skeletal muscle damage in patients.
In patients on MHD, malnutrition is prevalent, and HD survival is associated with the delivered dialysis dose [28]. This may be related to the deleterious effects of malnutrition (manifested as lower V), Which is a well-known risk factor for adverse outcomes and mortality in HD patients [29]. Chertow et al. suggest that patients’ nutritional status should be carefully assessed when spKt/V > 1.6 [7]. Similarly, Owen et al. concluded that mortality in patients on HD was strongly and negatively correlated with dialysis dose, irrespective of whether Kt/V or URR was measured [12], suggesting that increasing the level of dialysis dose is a practical and effective way of reducing mortality and improving clinical outcome [20]. However, the HEMO study by Rocco et al. concluded that dialysis dose interventions were unlikely to have a significant impact on nutritional outcomes [30]. But none of these studies mentioned the effect of a reasonable spKt/V cutoff value on sarcopenia and malnutrition in MHD patients.
In our study, We found that the spKt/V was independently positively associated with sarcopenia in MHD patients, the best cutoff value of spKt/V in sarcopenia was 1.45 for both sexes, 1.33 and 1.45 for men and women, respectively; conversely, in evaluating GNRI with spKt/V, the AUC in the ROC curve analysis was 0.628 (95% CI = 0.48–0.77, P = 0.09). These results showed that the cutoff value for women was greater than that for men. In hemodialysis, Kt/V varies between men and women (because of their different body sizes and compositions); Kt depends mainly on the effective clearance of the dialyser and the duration of dialysis [31]. Malgorzata et al. revealed that Kt was similar for males and females and do not depend on V (total body water), which means that the overall capacity of the transport system is similar in females and males, and that the effects of body size and composition are significantly stronger in men than in women [31]. Hence, the body size of men is likely to account for the lower spKt/V than women in this study. Thus the therapeutic target value of spKt/V should be considered for body size and gender.
GNRI is a method of nutritional screening for patients on MHD and an important predictor of mortality in HD patients [32]. However, this study indicated that spKt/V may have a higher optimistic predictive value to identify sarcopenia than the GNRI in these population. Thus, spKt/V may be a clinically useful marker of sarcopenia.
Studies have shown that anthropometry and body composition provide important information about the nutritional status of patients on dialysis, with muscle mass being a marker of protein nutritional status [33]. For example, lean body mass represents the “fat-free” muscle mass in HD patients and is a crucial and useful marker of nutritional assessment in HD patients [34]. Body cell mass constitutes the metabolically active body mass responsible for energy exchange (e.g., muscle mass). Low body cell mass have been identified as nutritional and prognosticator markers [35]. Additionally, ECW and ICW measured using BIS have been introduced as markers of cellular health, and the ECW/ICW ratio has been shown to correlate with malnutrition [36]. And previous studies have found that mCI is a reliable marker of nutritious status in patients receiving HD [37]. Our findings revealed that the lower levels of grip strength, pinch strength, lean body mass, body cell mass, ECW, ICW, BMI, ASMI, and mCI values were related to spKt/V. Higher levels of ECW/ICW values were related to spKt/V. This suggests that there may be more nutrient loss as spKt/V increases. Patients undergoing MHD lose about 1–8 g of protein per hemodialysis, resulting in fewer nutrients to synthesize muscle proteins [38]. Prolonged MHD, therefore, results in patients being exposed to its negative effects, including nutrient loss and increased energy expenditure, which may subsequently lead to malnutrition [39].
Currently, the scientific efforts to reduce morbidity and mortality in patients undergoing HD are focused on three major themes: dialysis dosage, nutrition, and the biocompatibility of dialysis procedures [40]. Adequate assessment and follow-up of the dialysis dose and nutritional status of patients with renal failure may be critical in slowing disease progression and preventing malnutrition. Therefore, spKt/V might be useful as a screening indicator based on medical record information, as an alternative to the Simplified 5-item Rating Questionnaire (SARC-F) scale for estimating sarcopenia versus dystrophy, as described in the consensus [14].
To our knowledge, this was the first study to evaluate the association of spKt/V with sarcopenia in patients on MHD. The present study explored the best cutoff value of spKt/V on sarcopenia, so it can be used as a indicator to identify the patients at risk for developing sarcopenia in advance and malnutrition, according to spKt/V and accordingly initiate interventions to improve the prognosis and quality of life of patients on MHD.
This study had a few limitations. First, the causality could not be determined due to its observational nature of. Second, we cannot exclude the possibility of residual confounders such as inadequate predialysis care. Third, we included patients from the same center, which makes the conclusions less representative. Lastly, we did not evaluate the dietary intake of these patients in a comprehensive nutritional assessment for malnutrition risk. In the future, multicenter studies covering a wider range of confounders should be conducted to establish the association and increase the generalizability of the findings.
Conclusion
Our study highlighted spKt/V as an independent predictor of sarcopenia in patients on MHD. It demonstrates the importance of spKt/V ≥ 1.45 as an indicator of skeletal muscle wasting. Nutritional status plays an important role in improving the quality of life of patients on dialysis. Therefore, the combination of dialysis dose with muscle mass and nutritional status should be considered as an additional indicator of prognosis in the clinical management of patients on HD. Furthermore, longitudinal studies are needed to further assess the role of dialysis doses on the muscular and nutritional aspects of patients undergoing HD.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- MHD:
-
Maintenance hemodialysis
- CKD:
-
Chronic kidney disease
- ESRD:
-
End-stage renal disease
- PEW:
-
Protein-energy wasting
- spKt/V:
-
Single-pool model urea clearance index
- NKF-KDOQI:
-
National Kidney Foundation-Kidney Disease Outcomes Quality Initiative
- RKF:
-
Residual renal function
- URR:
-
Urea reduction rate
- BMI:
-
Body mass index
- GNRI:
-
Geriatric Nutrition Risk Index
- MDRD:
-
Modification of diet in renal disease
- eGFR:
-
Estimated glomerularfiltration rate
- BIS:
-
Bioimpedance spectroscopy analysis
- PhA:
-
Phase angle
- ECW:
-
Extracellular water
- ICW:
-
Intracellular water
- ASM:
-
Appendicular skeletal muscle
- ASMI:
-
Appendicular skeletal muscle index
- mCI:
-
Modified Creatinine Index
References
Wang XH, Mitch WE, Price SR. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. NAT REV NEPHROL. 2022;18(3):138–52.
Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18(3):254–62.
Koppe L, Fouque D, Kalantar-Zadeh K. Kidney cachexia or protein-energy wasting in chronic kidney disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2019;10(3):479–84.
Carrero JJ, Thomas F, Nagy K, Arogundade F, Avesani CM, Chan M, Chmielewski M, Cordeiro AC, Espinosa-Cuevas A, Fiaccadori E, et al. Global prevalence of protein-energy wasting in kidney disease: a Meta-analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr. 2018;28(6):380–92.
Wang M, Obi Y, Streja E, Rhee CM, Chen J, Hao C, Kovesdy CP, Kalantar-Zadeh K. Impact of residual kidney function on hemodialysis adequacy and patient survival. Nephrol Dial Transpl. 2018;33(10):1823–31.
KDOQI Clinical Practice Guideline for Hemodialysis Adequacy. 2015 update. AM J KIDNEY DIS. 2015;66(5):884–930.
Chertow GM, Owen WF, Lazarus JM, Lew NL, Lowrie EG. Exploring the reverse J-shaped curve between urea reduction ratio and mortality. KIDNEY INT. 1999;56(5):1872–8.
Kaya T, Sipahi S, Cinemre H, Karacaer C, Varim C, Nalbant A, Tamer A. Relationship between the target dose for hemodialysis adequacy and nutritional assessment. ANN SAUDI MED. 2016;36(2):121–7.
Salahudeen AK, Dykes P, May W. Risk factors for higher mortality at the highest levels of spKt/V in haemodialysis patients. Nephrol Dial Transpl. 2003;18(7):1339–44.
Inker LA, Titan S. Measurement and estimation of GFR for Use in Clinical Practice: Core Curriculum 2021. Am J Kidney Dis. 2021;78(5):736–49.
Daugirdas JT. Second generation logarithmic estimates of single-pool variable volume Kt/V: an analysis of error. J AM SOC NEPHROL. 1993;4(5):1205–13.
Owen WJ, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med. 1993;329(14):1001–6.
Yamada Y, Nishizawa M, Uchiyama T, Kasahara Y, Shindo M, Miyachi M, Tanaka S. Developing and validating an age-independent equation using Multi-frequency Bioelectrical Impedance Analysis for Estimation of Appendicular Skeletal Muscle Mass and establishing a cutoff for Sarcopenia. Int J Environ Res Public Health 2017;14(7).
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia diagnosis and treatment. J AM MED DIR ASSOC. 2020;21(3):300–7.
Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, Cynober L, Aussel C. Geriatric nutritional risk index: a New Index for evaluating At-Risk Elderly Medical patients. Am J Clin Nutr. 2005;82:777–83.
Yamada K, Furuya R, Takita T, Maruyama Y, Yamaguchi Y, Ohkawa S, Kumagai H. Simplified nutritional screening tools for patients on maintenance hemodialysis. AM J CLIN NUTR. 2008;87(1):106–13.
Canaud B, Granger VA, Molinari N, Chenine L, Leray-Moragues H, Rodriguez A, Chalabi L, Morena M, Cristol JP. Creatinine index as a surrogate of lean body mass derived from urea Kt/V, pre-dialysis serum levels and anthropometric characteristics of haemodialysis patients. PLoS ONE. 2014;9(3):e93286.
Vanholder R, Van Biesen W, Lameire N. A swan song for Kt/Vurea. Semin Dial. 2019;32(5):424–37.
Churchill BM, Patri P. The nitty-gritties of Kt/Vurea calculations in Hemodialysis and Peritoneal Dialysis. Indian J Nephrol. 2021;31(2):97–110.
Sun Y, Wang Y, Yu W, Zhuo Y, Yuan Q, Wu X. Association of dose and frequency on the survival of patients on maintenance of hemodialysis in China: A Kaplan–Meier and cox proportional hazard model analysis. Med Sci Monit. 2018;24:5329–37.
Liu SX, Wang ZH, Zhang S, Xiao J, You LL, Zhang Y, Dong C, et al. The association between dose of hemodialysis and patients mortality in a prospective cohort study. Sci Rep. 2022;12(1):13708.
Lu J, Zhu M, Liu S, Zhu M, Pang H, Lin X, et al. The relationship between survival rate and intradialytic blood pressure changes in maintenance hemodialysis patients. Ren Fail. 2017;39(1):417–22.
AlSahow A, Muenz D, Al-Ghonaim MA, Al SI, Hassan M, Al AA, et al. Kt/V: achievement, predictors and relationship to mortality in hemodialysis patients in the Gulf Cooperation Council countries: results from DOPPS (2012–18) clin. Kidney J. 2021;14(3):820–30.
Miller JE, Kovesdy CP, Nissenson AR, Mehrotra R, Streja E, Van Wyck D, et al. Association of hemodialysis treatment time and dose with mortality and the role of race and sex. Am J Kidney Dis. 2010;55(1):100–12.
Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–9.
Wang M, Liu L, Shen X, Li Y, He Q. Assessing lean tissue by bioelectrical impedance analysis pre hemodialysis underestimates the prevalence of Sarcopenia in maintenance hemodialysis patients. EUR J CLIN NUTR. 2021;75(9):1407–13.
Perez-Garcia R, Jaldo M, Alcazar R, de Sequera P, Albalate M, Puerta M, Ortega M, Ruiz MC, Corchete E. Unlike kt, high Kt/V is associated with greater mortality: the importance of low V. Nefrologia (Engl Ed). 2019;39(1):58–66.
Beguin L, Krummel T, Longlune N, Galland R, Couchoud C, Hannedouche T. Dialysis dose and mortality in haemodialysis: is higher better? Nephrol Dial Transpl. 2021;36(12):2300–7.
Piccoli GB, Lippi F, Fois A, Gendrot L, Nielsen L, Vigreux J, Chatrenet A, D’Alessandro C, Cabiddu G, Cupisti A. Intradialytic Nutrition and Hemodialysis prescriptions: a personalized Stepwise Approach. Nutrients. 2020;12(3):785.
Rocco MV, Dwyer JT, Larive B, Greene T, Cockram DB, Chumlea WC, Kusek JW, Leung J, Burrowes JD, McLeroy SL, et al. The effect of dialysis dose and membrane flux on nutritional parameters in hemodialysis patients: results of the HEMO Study. KIDNEY INT. 2004;65(6):2321–34.
Debowska M, Paniagua R, Ventura MD, Avila-Diaz M, Prado-Uribe C, Mora C, Garcia-Lopez E, Qureshi AR, Lindholm B, Waniewski J. Dialysis adequacy indices and body composition in male and female patients on peritoneal dialysis. Perit Dial Int. 2014;34(4):417–25.
Xiong J, Wang M, Zhang Y, Nie L, He T, Wang Y, Huang Y, Feng B, Zhang J, Zhao J. Association of Geriatric Nutritional Risk Index with Mortality in Hemodialysis patients: a Meta-analysis of Cohort studies. Kidney Blood Press Res. 2018;43(6):1878–89.
Guo Y, Zhang M, Ye T, Wang Z, Yao Y. Application of Bioelectrical Impedance Analysis in Nutritional Management of patients with chronic kidney disease. Nutrients. 2023;15(18):3941.
Zhou DC, Yang XH, Zhan XL, Gu YH, Guo LL, Jin HM. Association of lean body mass with nutritional parameters and mortality in hemodialysis patients: a long-term follow-up clinical study. Int J Artif Organs. 2018;41(6):297–305.
Oliveira T, Garagarza C, Valente A, Caetano C. Low body cell mass index in hemodialysis patients: Association with clinical parameters and survival. Hemodial Int. 2020;24(2):228–36.
Yajima T, Yajima K, Takahashi H, Yasuda K. Combined Predictive Value of Extracellular Fluid/Intracellular Fluid Ratio and the Geriatric Nutritional Risk Index for Mortality in Patients Undergoing Hemodialysis. NUTRIENTS 2019;11(11).
Fujioka H, Koike T, Imamura T, Tomoda F, Kakeshita K, Yamazaki H, Kinugawa K. Impact of Geriatric Nutritional Risk Index and Modified Creatinine Index Combination on Mortality in Hemodialysis Patients. NUTRIENTS 2022;14(4).
Rippe B, Oberg CM. Albumin turnover in peritoneal and Hemodialysis. Semin Dial. 2016;29(6):458–62.
Sabatino A, Regolisti G, Karupaiah T, Sahathevan S, Sadu Singh BK, Khor BH, Salhab N, Karavetian M, Cupisti A, Fiaccadori E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin Nutr. 2017;36(3):663–71.
Movilli E. Adequacy, nutrition, and biocompatibility: their relevance on clinical outcome in haemodialysis patients. Blood Purif. 1999;17(2–3):159–65.
Acknowledgements
The authors are indebted to all the nephrologists and nurses in our hemodialysis center for their excellent management of hemodialysis patients. The authors also thank the patients and staff involved in the cross-sectional study.
Funding
This study was supported by the Guangzhou Science and Technology Project (202201020033), and Research-oriented Hospital Program of Guangzhou (2022RHPG05).
Author information
Authors and Affiliations
Contributions
Y.L. and T.X. contributed to the study concept and design, acquisition of data, data analysis, and drafting of the manuscript; R.X. and Y.L. was involved in the analysis and interpretation of the data; X.Z. and Y.L. contributed to the study concept and design, acquisition of data, partial data analysis, and critical revision of the manuscript for important intellectual content; R.T. was involved in the study design, critical revision of the manuscript for important intellectual content, final approval of the submitted manuscript, and study supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board at the Guangzhou Red Cross Hospital of Jinan University [No. 2023-040-01], and written informed consent was obtained from all participants. This study adhered to the tenets of the Declaration of Helsinki and the Guidance on Sample Collection of Human Genetic Diseases by the Ministry of Public Health of China and does not contain any studies with animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Xing, T., Xu, R. et al. Single-pool model urea clearance index is associated with sarcopenia and nutritional status in patients undergoing maintenance hemodialysis: a cross-sectional study. BMC Nephrol 25, 80 (2024). https://doi.org/10.1186/s12882-024-03510-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03510-4