Abstract
Background
The prevalence of chronic kidney disease (CKD) is rising in Malaysia. Early detection is necessary to prevent disease progression, especially in terms of cardiovascular (CV) risk, the main cause of death in end-stage renal disease (ESRD). Retinal changes have proven to be a good predictor of CKD whereas cardiac biomarkers are useful in cardiovascular risk stratification. We aimed to demonstrate the correlation between retinal changes and cardiac biomarkers with CKD.
Methods
This single-centre cross-sectional study was conducted among patients with CKD stages 3, 4, and 5 (not on dialysis) from the Nephrology Clinic, Universiti Kebangsaan Malaysia Medical Centre. A total of 84 patients were recruited with an even distribution across all three stages. They underwent fundus photography where images were analysed for vessel calibre (central retinal venular equivalent (CRVE), central retinal arterial equivalent (CRAE), and tortuosity indices. Optical coherence tomography was used to measure macular volume. Blood samples were sent for laboratory measurement of high-sensitivity C-reactive protein (hs-CRP) and asymmetric dimethylarginine (ADMA). These parameters were analysed in relation to CKD.
Results
The mean age was 58.8 ± 11.7 years, with 52.4% male and 47.6% female patients. Among them, 64.3% were diabetics. Retinal vessel tortuosity (r = -0.220, p-value = 0.044) had a negative correlation with the estimated glomerular filtration rate (eGFR). CRVE showed a positive correlation with proteinuria (r = 0.342, p = 0.001) but negative correlation with eGFR (r = -0.236, p = 0.031). Hs-CRP positively correlated with proteinuria (r = 0.313, p = 0.04) and negatively correlated with eGFR (r = -0.370, p = 0.001). Diabetic patients had a higher CRVE compared to non-diabetic patients (p = 0.02). History of ischaemic heart disease was associated with a smaller macula volume (p = 0.038). Male gender (r2 = 0.066, p = 0.031) and HbA1c had a positive influence (r2 = 0.066, p = 0.047) on retinal vessel tortuosity. There was a positive influence of age (r2 = 0.183, p = 0.012) and hs-CRP (r2 = 0.183, p = 0.045) on CRVE. As for macula volume, it negatively correlated with diabetes (r2 = 0.015, p = 0.040) and positively correlated with smoking (r2 = 0.015, p = 0.012).
Conclusion
Our study showed that eGFR value affects retinal vessel tortuosity, CRVE and hs-CRP. These parameters bear potential to be used as non-invasive tools in assessing CKD. However, only macula volume may be associated with CVD risk among the CKD population.
Similar content being viewed by others
Background
The optimal management approach of chronic kidney disease (CKD) should be focused on preventive strategy as there is currently no cure for CKD. Early detection and intervention are required to prevent the progression to end-stage renal disease (ESRD). The prevalence of CKD in Malaysia has drastically risen from 9.07% in 2011 to 15.48% in 2018, likely attributable to the concurrent rise in the prevalence of risk factors such as diabetes mellitus (DM), hypertension, obesity, and increasing age [1].
The recent use of non-invasive methods in detecting early retinal changes such as retinal vessel tortuosity and calibre as well as macula volume has been shown to enhance CKD identification and risk prediction in earlier stages as these retinal changes precede changes such as diabetic retinopathy [2]. With earlier detection, more proactive interventions can be implemented without having to perform additional risky procedures such as renal biopsy, especially in cases whereby the aetiology of CKD is still doubtful.
To date, many studies have indicated that retinal changes are good predictors of CKD outcomes [2, 3]. One of the possible reasons is the fact that the kidney and the retina share several similarities. Anatomically, they share the same developmental pathway. Physiologically, the renin–angiotensin–aldosterone system is present in both these organs. Thus, factors leading to CKD such as atherosclerosis, vascular remodelling, endothelial dysfunction, and oxidative stress can also predispose to many retinal diseases [4].
Additionally, previous studies have shown that increased retinal venular diameter (measured by means of central retinal venular equivalent measurement, CRVE) was associated with an increased risk of CKD [2, 5]. However, this was not replicated in other studies [6,7,8]. In contrast, other publications revealed that smaller retinal arterioles (measured by central retinal arteriolar equivalent, CRAE) were associated with an increased risk of CKD [3, 5, 7, 8]. Other changes such as reduced macula volume [9] and increased tortuosity of retinal vessels [10] were also found in those with CKD.
In addition, the assessment of cardiovascular risk is vital in the early stage of CKD diagnosis as CVD is the leading cause of death in ESRD. Previous studies reported that patients with CKD were found to be in a chronic low-grade pro-inflammatory state which would promote atherosclerosis, thus leading to the conclusion that CKD in itself is a risk factor for CVD [11]. Cardiac biomarkers such as high-sensitivity C-reactive protein (hs-CRP) and asymmetric dimethylarginine (ADMA) have also been shown to be elevated in those with cardiovascular risk [12, 13]. Hs-CRP is a biomarker of inflammation which plays a key role in atherosclerosis. Previous studies have consistently proven that CRP levels independently predict the first episode of cardiovascular event across all Framingham risk groups [14,15,16]. ADMA, on the other hand, is a competitive inhibitor for endothelial nitric oxide synthase, which leads to endothelial dysfunction, and consequently circulating ADMA levels adds prognostic value towards cardiovascular risk [17, 18]. Therefore, these biomarkers were good predictors of CVD, even among CKD patients [11, 19, 20]. In short, these novel non-invasive tools for assessment of CKD and cardiovascular risk represent the future of risk prediction methods. Therefore, we embarked on this study to determine the correlation between retinal changes and cardiac biomarkers with CKD stages.
Methodology
Study population
This was a single-centre cross-sectional study involving patients above 18 years old who attended the Nephrology Clinic of Universiti Kebangsaan Malaysia Medical Centre (UKMMC) between October 2019 and January 2021. They must have an eGFR of < 60 ml/min/1.73m2, stable serum creatinine for three months, and HbA1c of < 10% if diabetic. Those with active malignancy, infection or vasculitis, on dialysis, pregnant, and history of renal transplant or retinal photocoagulation therapy were excluded. Written consent was obtained before the blood sample was taken for cardiac biomarkers. All participants were then sent to the Ophthalmology Department for fundus photography and Optical Coherence Tomography (OCT). Ethical approval was obtained from the Research and Ethics Committee UKM (FF-2019–425).
eGFR was calculated based on the Modification of Diet in Renal Disease (MDRD) Eq. [21]. Four variables were used whereby Glomerular filtration rate (GFR) (mL per minute per 1.73m2) = 175 × SerumCr-1.154 × age-0.203 × 1.212 (if patient is black) × 0.742 (if female).
CKD stage was classified as eGFR: stage 1 (> 90 ml/min/1.73m2), stage 2 (60 to 89 ml/min/1.73m2), stage 3a (45 to 59 ml/min/1.73m2), stage 3b (30 to 44 ml/min/1.73m2), stage 4 (15 to 29 ml/min/1.73m2) and stage 5 (less than 15 ml/min/1.73m2).
Sample size
Based on the sample size calculation using a power of 80%, alpha-value of 0.05, and effect size of 0.3, a total of 82 patients was needed. The effect size of 0.3 indicated a moderate correlation and it was based on a previous study [9].
Demographic data and laboratory parameters assessment
All participants underwent a face-to-face interview to obtain their demographic data, past medical history, smoking status, and current medications. Full blood count, renal profile, liver function test, fasting serum lipid profile, HbA1c, and urine protein creatinine index (UPCI) were collected and processed at the hospital laboratory as part of routine investigations.
Assessment of retinal changes
All participants were sent for fundus photography and OCT at the Ophthalmology Department. Both eyes were assessed but the image of the right eye was chosen by default. If the right eye images were uninterpretable, then the left eye would be used for assessment.
Retinal vessel calibre (CRVE and CRAE)
Colour retinal photographs of both eyes were taken after dilating the pupils with 1% tropicamide and 2.5% phenylephrine hydrochloride with a digital mydriatic retinal camera (Topcon Retinal Camera TRC-50DX [type 1A], Tokyo, Japan). One retinal image of each eye was obtained to assess the retinal vessel calibre. The fundus photos were segmentalised and the four largest arteries and veins were manually selected at the region 0.5 to 1.0 disc diameters away from the disc margin (refer Fig. 1). Retinal arteriolar and venular calibres were summarised as CRAE and CRVE respectively. CRAE and CRVE were calculated by the engineering team based on the revised Knudtson-Parr-Hubbard formula [22] using an automated computer-assisted programme.
Retinal vessel tortuosity
The fundus photos were then sent to the Centre for Integrated Systems Engineering & Advanced Technologies (INTEGRA), Department of Electrical, Electronic and Systems Engineering, Faculty of Engineering and Built Environment, UKM for retinal vessel tortuosity assessment using the MATLAB tool. The formula used for calculating vessel tortuosity is as follows [23, 24]:
where
(xi, yi) = coordinates of ith pixel in the vessel segment
N = vessel segment constituent points
Macula volume
Macula volume was measured via OCT using the OCT Spectralis Machine manufactured by Heidelberg Engineering, Germany. A macular scan was performed in an area of 6 × 6mm2 using the “Fast Macula” protocol, a standard protocol for macular cube scan. Based on the definition provided by the Early Treatment Diabetic Retinopathy Study [25], the built-in software was used to produce retinal thickness maps that were subsequently split into nine average retinal subfields with a 6 mm diameter circle centred at the true fovea location. The overall macular thickness and macular cube volume over the entire grid were derived from the output of the computer software [26]. Refer Fig. 2.
Assessment for cardiac biomarkers
Hs-CRP
Blood samples for hs-CRP were sent to the Pantai Hospital laboratory and analysed using Latex Enhanced Immunoturbidimetric (Roche Diagnostic Corp) using Cobas INTEGRA Instruments. A hs-CRP level of < 1 mg/L signified low cardiovascular risk, 1–3 mg/L indicated moderate cardiovascular risk, and > 3 mg/L showed high cardiovascular risk [27].
ADMA
Peripheral blood taken for ADMA assessment was kept in EDTA tubes at -20ºC. It was then measured using universal ADMA ELISA kits (Novus Biologicals) by the scientific officer (single operator) in the UKM laboratory. Based on Nemeth et al. [28], a normal range was taken as 50–180 ng/ml.
Statistical analysis
Data were analysed using IBM®SPSS software version 26. All missing data were treated by the series mean calculation method. All data were tested for normality. Normally distributed data were expressed as mean ± standard deviation (SD) whereas non-normally distributed data were expressed as median with interquartile range (IQR) (25th and 75th percentile). Non-parametric data were analysed using Kruskal–Wallis or Mann–Whitney U test whereas normally distributed data were analysed using t-test or ANOVA. The correlation (r) between any two parameters was analysed using Pearson test. Multiple linear regression was used for multivariable analysis to test main predictors for each of the five criteria for retinal changes (tortuosity, CRVE, CRAE and macula volume). The enter method of linear regression was used for the analysis. There was no interaction or multicollinearity between the dependent variables. A p-value of less than 0.05 was considered to indicate significance in all tests.
Results
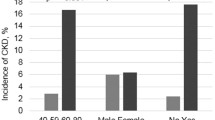
The participants’ baseline characteristics are shown in Tables 1 and 2. The 84 patients were evenly distributed with 28 patients each in CKD groups 3, 4, and 5. The distribution by gender, age, ethnicity, BMI, and blood pressure was similar between the three groups. The mean age of the participants was 58.8 ± 11.2 years. There were 44 males (52.4%) and 40 females (47.6%). Ethnically, the majority of our participants were Malays (76.2%), followed by Chinese (19.0%), and Indians (4.8%).
Among the participants, more than half (n = 54, 64.3%) were diabetic. However, only 43 out of the 54 patients (79.6%) had CKD attributed to DM. Out of these 43 patients, 65% were males. The remaining 11 diabetic patients had CKD attributed to other causes, namely two due to obstructive uropathy, eight due to chronic glomerulonephritis, and one due to longstanding hypertension. From the results, a higher number of CKD with structural aetiologies such as obstructive uropathy, vesicoureteral reflux (VUR), and post nephrectomy were in CKD stage 4 as compared to stages 3 and 5.
In addition, all three groups of CKD had a similar distribution of DM, hypertension, ischaemic heart disease (IHD), stroke, and smoking. However, those in CKD stage 5 had a higher incidence of peripheral vascular disease (p-value = 0.015). There were significantly fewer patients who were on ACE-I (p-value = 0.012) and ARB (p-value = 0.012) in the CKD stage 5 group compared to stages 3 and 4 (Table 1).
Among patients with lower stages of CKD, they showed higher proteinuria levels (p-value = 0.001), lower haemoglobin (p-value < 0.001), higher hs-CRP levels (p-value = 0.037), more tortuous retinal vessels (p-value = 0.032), and higher venular calibre (p-value = 0.01) based on CRVE. However, there was no significant difference between the CKD groups in terms of ADMA, arterial calibre based on CRAE, and macula volume (Table 2).
Retinal vessel tortuosity
The correlational analysis of CKD patients showed that retinal vessel tortuosity had a weak negative correlation with eGFR (r = -0.220, p = 0.044) (Table 3). However, there was no association between tortuosity and diabetes (p = 0.355), IHD (p = 0.592), or diabetic retinopathy (p = 0.361) (Table 4).
After adjustment for age, gender, BMI, smoking status, diabetes status, blood pressure, LDL, HbA1c, haemoglobin, eGFR, proteinuria, hs-CRP, and ADMA in the linear regression model, gender was found to influence retinal vessel tortuosity whereby males had more tortuous vessels by 0.008 (p-value = 0.031, B = -0.008). Also, for every one unit of HbA1c increment, there was a 0.003 increase in tortuosity (p-value = 0.047, B = 0.003) (Table 5).
Retinal CRVE
CRVE showed a weak positive correlation with creatinine (r = 0.280, p = 0.01) and proteinuria (r = 0.342, p = 0.001) as well as a weak negative correlation with eGFR (r = -0.236, p = 0.031) (Table 3). Diabetic patients had higher CRVE values (p-value = 0.02)(Table 4). Age was a significant predictor of CRVE, with every increment of age in years leading to an increase in CRVE by 1.626 µm (p-value = 0.010, B = 1.626). In contrast, for every hs-CRP increment, there was a reduction in CRVE by 1.336 µm (p-value = 0.045, B = -1.336) (Table 5).
Retinal CRAE
CRAE did not correlate with eGFR, creatinine, or proteinuria level (Table 3). There was also no association with diabetes status (p = 0.086), IHD (p = 0.072), or diabetic retinopathy (p = 0.152) (Table 4). No factors were found to influence CRAE based on the linear regression model (Table 5).
Macula volume
There was no correlation between macula volume with eGFR, creatinine, or proteinuria level (Table 3). However, patients with IHD were found to have a reduced macula volume (p = 0.038). Based on the linear regression model, smoking and diabetic status were significant predictors of macula volume; smoking increased macula volume by 1 mm3 (p-value = 0.012, B = 1) while diabetic patients had a lower macula volume by 0.615 mm3 (p-value = 0.041, B = -0.615) (Table 5).
Hs-CRP
Next, hs-CRP showed a weak negative correlation with eGFR (r = -0.370, p = 0.001), a moderate correlation with creatinine (r = 0.624, p = < 0.001), and a weak positive correlation with proteinuria (r = 0.313, p = 0.004) (Table 3). However, there was no association with diabetic status (p = 0.092), IHD (p = 0.298), or diabetic retinopathy (p = 0.097) (Table 4).
ADMA
ADMA levels did not have any correlation with eGFR, creatinine, or proteinuria level (Table 3). There was no association with diabetes status (p = 0.779), IHD (p = 0.465) or diabetic retinopathy (p = 0.854) (Table 4).
Although there were only weak to moderate correlations for eGFR, creatinine, and proteinuria with retinal tortuosity, CRVE, and hs-CRP, the post-hoc analysis showed statistically significant differences in the tortuosity, CRVE, and hs-CRP when grouped into stages i.e. between CKD stages 3 and 5 (p-value: CRVE = 0.015, hs-CRP = 0.032) and CKD stage 4 and 5 (p-value: tortuosity = 0.013, CRVE = 0.007, hs-CRP = 0.023). However, no statistically significant difference was detected in the tortuosity, CRVE and hs-CRP between CKD stages 3 and 4 (p-value: tortuosity = 0.128, CRVE = 0.385, hs-CRP = 0.844) (Table 6). This concurs that the most dramatic changes of retinal vessel tortuosity, CRVE and hs-CRP occur during the late stage of CKD at stage 5.
Discussion
Among the patients with CKD stages 3 and below, diabetic kidney disease (51.2%) was the main cause of CKD. This was consistent with the reported data in the Malaysian National Renal Registry 2018 [29]. Amongst the participants with DM (64.3%), one-third of them had been diagnosed with diabetic retinopathy based on previous eye assessments. Generally, CKD in diabetic patients is usually attributed to DM unless other causes are evident [30]. In this study, 11 out of 54 (20%) of the diabetic patients also had other underlying significant structural abnormalities that caused CKD as shown by their kidney biopsy findings or imaging showing.
Known risk factors for CVD include obesity, smoking, dyslipidaemia, hypertension, DM, family history of premature coronary disease, CKD, and albuminuria [31]. CKD, especially among those with DM, predisposes to a high risk of atherosclerotic CVD (ASCVD), including coronary artery disease, peripheral vascular disease, and stroke. From our observation, 23.8% of our patients had IHD, 15.5% had a stroke before, and 4% had peripheral vascular disease. Only 6% of patients included in the study were smokers, which was low compared to the prevalence of smokers among the general population of Malaysia.
The comparison between patients in CKD stages 3, 4, and 5 showed a significant difference in eGFR, proteinuria level, haemoglobin level, hs-CRP, retinal vessel tortuosity, and CRVE results, as well as the use of ACE-I and ARBs between the groups. For instance, higher proteinuria was found in those with a lower stage of CKD, likely due to the degree of renal injury in these patients. However, it can also be attributable to the low usage of antiproteinuric agents such as ACE-I or ARB due to the risk of hyperkalaemia and reduced eGFR in this group of patients.
Subsequent correlational analysis between retinal changes and CKD stages showed that a lower eGFR, higher creatine level, and proteinuria (all signs of lower stage of CKD) were correlated with a higher CRVE, even though it was a weak correlation. CRVE was also significantly correlated with diabetes status, whereby diabetics were found to have a higher CRVE compared to non-diabetics. These findings concurred with the results from Yip et al. [5] and Liew et al. [2]. Similarly, the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) [32] also highlighted an association between CKD and a higher venular calibre, especially among diabetics.
In addition, patients with lower eGFR were found to have more tortuous retinal vessels. It was proven by Sasongko, MB et al. in which a greater retinal vessel tortuosity was independently associated with retinopathy and early-stage nephropathy in type 1 diabetes [10]. Tortuosity and venular calibre are structural changes of the retina that happen as a result of endothelial dysfunction and haemodynamic instability in CKD patients [10, 33, 34]. This is likely attributed to the increased inflammatory states in CKD which leads to increased permeability of the vessels and thickening of the basement membrane, which in turn increases the venular calibre [33]. The pathophysiology behind retinal vessel tortuosity is postulated to be due to the constant inflammatory state in CKD leading to dysregulation of blood flow [35, 36], tissue hypoxia, endothelial dysfunction [37], and increased levels of vascular endothelial growth factor (VEGF) [38] causing vessel wall weaking and fragility, and consequently remodelling occurs.
In our study population, no correlation was detected between CKD stages with CRAE, possibly due to the small sample size and the difference in the genetic and environmental profile of our patients compared to previous studies. Asians have a different profile of contributing factors to CKD compared to Caucasians. In the literature, two studies by Sabanayagam et al. [7, 8] among Singaporean patients showed an association between retinal arteriolar narrowing and CKD. Even though the two countries were geographically near, the Singaporean study had a bigger sample size but only comprised patients of Chinese ethnicity, as compared to the multi-ethnic patient population in our study.
Furthermore, in our study, patients with IHD had a lower macula volume. A study by Balmforth et al. [9] found that patients with CKD had reduced macula volume and that reduced macula volume was also associated with higher levels of systemic inflammation, as measured by hs-CRP. The elevated level of this inflammatory marker could be representative of a generalised systemic microvascular injury confined not only to the kidneys, but also involving the cardiovascular system. Unfortunately, this was not reflected in our study as no association was found between hs-CRP and IHD, despite hs-CRP being one of the most sensitive markers of inflammation [39]. Macula volume is thought to be affected due to the atrophy of the cells as a result of compromised blood supply caused by microvascular injury [9].
On the other hand, proteinuria was associated with a higher hs-CRP level in our study population. With a higher level of proteinuria as CKD progresses, hs-CRP clearance is likely reduced in these patients, thus resulting in higher hs-CRP levels in the body [40]. It is known that hs-CRP can reduce the expression of nitric oxide synthase, leading to inflammation and subsequent development of atherosclerosis. As a result, the CKD patients remain in a state of chronic low-grade inflammation as observed by Abraham et al. [11]. In short, these findings support the fact that proteinuria is a known risk factor for ASCVD [31].
In contrast to previous studies supporting ADMA association with reduced macula volume and IHD [9, 13], ADMA levels did not show a significant association with any parameters in this study, possibly due to the fact that the majority of our patients did not have IHD. However, ADMA level was elevated in our CKD patients compared to the normal population based on the reference levels in Nemeth et al. [28], thus proving that even CKD stage 3 is a definite risk factor for ASCVD.
Next, no significant association was detected between diabetic retinopathy with retinal vascular and macula changes, although Sasongko et al. [41]. previously demonstrated an increased retinal vessel tortuosity in association with diabetic retinopathy. This could be due to the fact that only one-fifth (21%) of patients in this study were diagnosed with diabetic retinopathy. A significant number of patients with diabetic retinopathy had previously undergone photocoagulation retinal therapy and were excluded from this study as their retinal vessels could not be accurately assessed.
Last but not least, the linear regression analysis found that CKD patients of older age had poorer CRVE outcomes, independent of other factors. This could be explained by older patients having a longer duration of CKD. Furthermore, CKD deteriorates further with age and the prolonged proinflammatory processes on the vessel can lead to further weakening and dilatation. On the other hand, macula volume was affected by smoking status and diabetic status after adjusting for other factors whereby a higher macula volume was found in smokers and non-diabetics. This was in contrast with previous studies that showed smoking either reduced [42] or had no effect [43] on macula volume. Reduced macula volume is thought to be a consequence of the vasoactive effect of smoking and the oxidative stress that produce hypoxia and ischaemia [42], similar to how retinal blood flow becomes impaired in patients with diabetes [44]. Interestingly, the male gender was found to have an increased vessel tortuosity based on this model, a new finding that was not reported elsewhere previously, possibly due to the fact that the majority of patients with CKD due to diabetes in this study were male patients (65%). In a post-hoc analysis, diabetics are found to have more tortuous vessels. The increment in Hba1c also contributed to increased vessel tortuosity as previously reported [41]. Increment in hs-CRP also independently caused an increase in CRVE, likely attributable to an inflammatory state that leads to endothelin dysfunction and increased vessel permeability [33, 34].
One of the main limitations in our study was the convenience sampling that might have contributed to selection bias. In addition, although the storage and testing for ADMA were done manually by a single operator in a consistent manner, it was however performed in two batches, thereby creating potential bias in testing. Additionally, future studies should consider the use of prospective design with the inclusion of a larger number of participants at earlier stages of CKD. This will provide more information to ascertain the degree and the starting point of retinal changes and cardiac biomarkers in CKD.
Conclusion
In conclusion, our study showed that eGFR value affects retinal vessel tortuosity, CRVE and hs-CRP. These parameters bear potential to be used as non-invasive tools in assessing CKD. However, only macula volume may be associated with CVD risk among the CKD population.
Availability of data and materials
The datasets used and analysed are available from the corresponding author on reasonable request.
Abbreviations
- ACEI:
-
Angiotensin converting enzyme inhibitor
- ADMA:
-
Asymmetric dimethylarginine
- ADPKD:
-
Autosomal dominant polycystic kidney disease
- ARB:
-
Angiotensin receptor blocker
- ASCVD:
-
Atherosclerotic cardiovascular disease
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CCB:
-
Calcium channel blocker
- CKD:
-
Chronic kidney disease
- CRAE:
-
Central retinal arteriolar equivalent
- CRVE:
-
Central retinal venular equivalent
- CVD:
-
Cardiovascular disease
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- ELISA:
-
Enzyme linked immunosorbent assay
- ESRD:
-
End stage renal disease
- FSGS:
-
Focal segmental glomerulosclerosis
- hbA1c:
-
Glycosylated haemoglobin
- Hs-CRP:
-
High sensitivity C-reactive protein
- IgAN:
-
Immunoglobulin A nephropathy
- IHD:
-
Ischaemic heart disease
- LDL:
-
Low density lipoprotein
- MDRD:
-
Modification of diet in renal disease
- OCT:
-
Optical coherence tomography
- PVD:
-
Peripheral vascular disease
- UPCI:
-
Urine protein creatinine index
- VUR:
-
Vesicouereteric reflux
References
Saminathan TA, Hooi LS, Mohd Yusoff MF, Ong LM, Bavanandan S, Rodzlan Hasani WS, et al. Prevalence of chronic kidney disease and its associated factors in Malaysia; findings from a nationwide population-based cross-sectional study. BMC Nephrol. 2020;21(1):344.
Liew G, Mitchell P, Wong TY, Wang JJ. Retinal microvascular signs are associated with chronic kidney disease in persons with and without diabetes. Kidney Blood Press Res. 2012;35(6):589–94.
Ooi QL, Tow FK, Deva R, Alias MA, Kawasaki R, Wong TY, et al. The microvasculature in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(8):1872–8.
Wong CW, Wong TY, Cheng CY, Sabanayagam C. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int. 2014;85(6):1290–302.
Yip W, Ong PG, Teo BW, Cheung CY, Tai ES, Cheng CY, et al. Retinal vascular imaging markers and incident chronic kidney disease: a prospective cohort study. Sci Rep. 2017;7(1):9374.
Sabanayagam C, Shankar A, Klein BE, Lee KE, Muntner P, Nieto FJ, et al. Bidirectional association of retinal vessel diameters and estimated GFR decline: the Beaver Dam CKD Study. Am J Kidney Dis. 2011;57(5):682–91.
Sabanayagam C, Shankar A, Koh D, Chia KS, Saw SM, Lim SC, et al. Retinal microvascular caliber and chronic kidney disease in an Asian population. Am J Epidemiol. 2009;169(5):625–32.
Sabanayagam C, Tai ES, Shankar A, Lee J, Sun C, Wong TY. Retinal arteriolar narrowing increases the likelihood of chronic kidney disease in hypertension. J Hypertens. 2009;27(11):2209–17.
Balmforth C, van Bragt JJ, Ruijs T, Cameron JR, Kimmitt R, Moorhouse R, et al. Chorioretinal thinning in chronic kidney disease links to inflammation and endothelial dysfunction. JCI Insight. 2016;1(20):e89173.
Sasongko MB, Wong TY, Donaghue KC, Cheung N, Jenkins AJ, Benitez-Aguirre P, et al. Retinal arteriolar tortuosity is associated with retinopathy and early kidney dysfunction in type 1 diabetes. Am J Ophthalmol. 2012;153(1):176-83 e1.
Abraham G, Sundaram V, Sundaram V, Mathew M, Leslie N, Sathiah V. C-Reactive protein, a valuable predictive marker in chronic kidney disease. Saudi J Kidney Dis Transpl. 2009;20(5):811–5.
Silva D, Pais de Lacerda A. High-sensitivity C-reactive protein as a biomarker of risk in coronary artery disease. Rev Port Cardiol. 2012;31(11):733–45.
Bouras G, Deftereos S, Tousoulis D, Giannopoulos G, Chatzis G, Tsounis D, et al. Asymmetric Dimethylarginine (ADMA): a promising biomarker for cardiovascular disease? Curr Top Med Chem. 2013;13(2):180–200.
Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004;109(11):1349–53.
Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109(16):1955–9.
Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–65.
Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97(5):e53–9.
Boger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med. 2006;38(2):126–36.
Ueda S, Yamagishi S, Kaida Y, Okuda S. Asymmetric dimethylarginine may be a missing link between cardiovascular disease and chronic kidney disease. Nephrology (Carlton). 2007;12(6):582–90.
Quoc Hoang TA, Tam V, Thang HV. Plasma asymmetric dimethylarginine and its association with some of cardiovascular disease risk factors in chronic kidney disease. Med J Malaysia. 2019;74(3):209–14.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70.
Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106(12):2269–80.
Heneghan C, Flynn J, O’Keefe M, Cahill M. Characterization of changes in blood vessel width and tortuosity in retinopathy of prematurity using image analysis. Med Image Anal. 2002;6(4):407–29.
Fraz MM, Welikala RA, Rudnicka AR, Owen CG, Strachan DP, Barman SA. QUARTZ: quantitative analysis of retinal vessel topology and size - an automated system for quantification of retinal vessel morphology. Int J Exp Syst Appl. 2015;20(42):7221–34.
Rice TA. The early treatment diabetic retinopathy study. Trans Pa Acad Ophthalmol Otolaryngol. 1982;35(1):24–30.
Liu X, Kale AU, Capewell N, Talbot N, Ahmed S, Keane PA, et al. Optical coherence tomography (OCT) in unconscious and systemically unwell patients using a mobile OCT device: a pilot study. BMJ Open. 2019;9(11):e030882.
Li R, Xue Y, Wang T, Gong L, Peng P, Xiong P, et al. A comparison study between wide-range and high-sensitivity C-reactive protein assays (Roche Cobas c702) for low C-reactive protein concentration in patients with cardiovascular risk. J Clin Lab Anal. 2019;33(8):e22957.
Nemeth B, Ajtay Z, Hejjel L, Ferenci T, Abram Z, Muranyi E, et al. The issue of plasma asymmetric dimethylarginine reference range - A systematic review and meta-analysis. PLoS ONE. 2017;12(5): e0177493.
Registry NR. 26th Report of The Malaysian Dialysis and Transplant Registry 2018. 2018.
Kidney Disease: Improving Global Outcomes Diabetes Work G. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4S):S1–115.
American Diabetes A. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103–23.
Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115(11):1859–68.
Ikram MK, de Jong FJ, Vingerling JR, Witteman JC, Hofman A, Breteler MM, et al. Are retinal arteriolar or venular diameters associated with markers for cardiovascular disorders? The Rotterdam Study. Invest Ophthalmol Vis Sci. 2004;45(7):2129–34.
Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA). Invest Ophthalmol Vis Sci. 2006;47(6):2341–50.
Kristinsson JK, Gottfredsdottir MS, Stefansson E. Retinal vessel dilatation and elongation precedes diabetic macular oedema. Br J Ophthalmol. 1997;81(4):274–8.
Kylstra JA, Wierzbicki T, Wolbarsht ML, Landers MB 3rd, Stefansson E. The relationship between retinal vessel tortuosity, diameter, and transmural pressure. Graefes Arch Clin Exp Ophthalmol. 1986;224(5):477–80.
Witt N, Wong TY, Hughes AD, Chaturvedi N, Klein BE, Evans R, et al. Abnormalities of retinal microvascular structure and risk of mortality from ischemic heart disease and stroke. Hypertension. 2006;47(5):975–81.
Hartnett ME, Martiniuk D, Byfield G, Geisen P, Zeng G, Bautch VL. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in a rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008;49(7):3107–14.
Conde-Sanchez M, Roldan-Fontana E, Chueca-Porcuna N, Pardo S, Porras-Gracia J. Analytical performance evaluation of a particle-enhanced turbidimetric cystatin C assay on the Roche COBAS 6000 analyzer. Clin Biochem. 2010;43(10–11):921–5.
Descamps-Latscha B, Witko-Sarsat V. Importance of oxidatively modified proteins in chronic renal failure. Kidney Int Suppl. 2001;78:S108–13.
Sasongko MB, Wong TY, Nguyen TT, Cheung CY, Shaw JE, Wang JJ. Retinal vascular tortuosity in persons with diabetes and diabetic retinopathy. Diabetologia. 2011;54(9):2409–16.
Moschos MM, Nitoda E, Laios K, Ladas DS, Chatziralli IP. The Impact of Chronic Tobacco Smoking on Retinal and Choroidal Thickness in Greek Population. Oxid Med Cell Longev. 2016;2016:2905789.
Ciesielski M, Rakowicz P, Stopa M. Immediate effects of smoking on optic nerve and macular perfusion measured by optical coherence tomography angiography. Sci Rep. 2019;9(1):10161.
Farias LB, Lavinsky D, Schneider WM, Guimaraes L, Lavinsky J, Canani LH. Choroidal thickness in patients with diabetes and microalbuminuria. Ophthalmology. 2014;121(10):2071–3.
Acknowledgements
Special mention to the late Professor Dr Syed Zulkifli Syed Zakaria (1962-2022) for his continuous support and statistical guidance throughout the study.
Funding
Research & Ethics Committee, Universiti Kebangsaan Malaysia: no role in study design, collection, analysis, interpretation of data or write-up of study; Malaysian Society of Nephrology, Malaysia: no role in study design, collection, analysis, interpretation of data or write-up of study.
Author information
Authors and Affiliations
Contributions
KAMH recruited patients, analysed and interpreted all patient data and was the major contributor in writing the manuscript; RMU contributed in the study design and is the overall supervisor and major contributor in writing the manuscript; RMO contributed in the study design and the write-up process in regards to nephrology; LK contributed in the write-up process in regards to nephrology; WHWAH was the main ophthalmology advisor and contributed in the write-up from ophthalmology perspective; YMH recruited patients and helped in data analysis from ophthalmology perspective; TKS helped in data extraction and analysis from ophthalmology perspective; WMDWZ contributed to producing the formula for image interpretation and contributed to manuscript write-up; AA contributed to producing the formula for image interpretation and contributed to manuscript write-up; AB contributed in laboratory testing and contributed in manuscript write-up.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was given by the Research and Ethics Committee, Universiti Kebangsaan Malaysia and all methods were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from all patients to the use of their data and images.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mustafar, R., Hishamuddin, K.A.M., Mohd, R. et al. Retinal changes and cardiac biomarker assessment in relation to chronic kidney disease: a single centre study. BMC Nephrol 24, 338 (2023). https://doi.org/10.1186/s12882-023-03386-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-023-03386-w