Abstract
Objective
Investigate the longitudinal association of use and time of use of proton pump inhibitors (PPI) with incidence of chronic kidney disease (CKD) and kidney function change.
Methods
Prospective study with 13,909 participants from baseline (2008–2010) and second wave (2012–2014) of the ELSA-Brasil (mean interval between visits = 3.9 years (1.7–6.0)). Participants answered about use and time use of the PPI in the two weeks prior the interview. Renal function was assessed by glomerular filtration rate estimated by the Collaboration Equation for the Epidemiology of Chronic Kidney Disease. Values below 60ml/min/1.73 m² in wave 2 were considered incident CKD. Associations between PPI use and time of use at baseline and incident CKD and decline in renal function were estimated, respectively, by logistic regression and linear models with mixed effects, after adjusting for confounders.
Results
After adjustments, PPI users for more than six months had an increased risk of CKD compared to non-users. Compared to non-users, users PPIs for up to six months and above six months had greater decline in kidney function over time.
Conclusion
This cohort of adults and elderly, after a mean interval of 3.9 years, PPI use and initial duration were associated with kidney function change between visits.
Similar content being viewed by others
Introduction
Proton pump inhibitors (PPIs) are drugs used for gastric disorders. Because of their low toxicity, they are overprescribed and not always used rationally [1, 2]. Although PPIs are safe, observational studies suggest that PPI use is associated with an increased risk of several adverse health events [3, 4], including acute kidney injury (AKI) [5], development and progression of chronic kidney disease (CKD) [6,7,8] and end-stage renal disease (ESRD) [7, 8].
According to the Kidney Disease Improvement Global Outcomes (KDIGO) [9], CKD is characterized by persistent kidney damage (more than three months), usually identified by a glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 or urinary albumin creatinine ratio ≥ 30 mg/g. In developed countries, the estimated prevalence of CKD ranges from 5 to 15% [10, 11]. In Latin America, these data are still scarce, although some studies show an increasing number of people on renal replacement therapy [12, 13]. In a large epidemiological study in Brazil, the prevalence of CKD was estimated at 6.6% (95%CI 6.0–7.4%) among adults [14]. The prevalence of CKD was determined in baseline exams (2008–2010) of the Longitudinal Health Study (ELSA-Brasil) and 8.9% had a glomerular filtration rate < 60 mL/min/1.73 m2 [15].
The mechanisms supporting the association between PPI use and loss of kidney function resulting in CKD are still unclear. Studies suggest the association between these drugs and acute interstitial nephritis (AIN) [16,17,18,19]. About 30–70% of individuals with AIN do not fully recover kidney function [17]. Incomplete recovery of renal function associated with PPI-induced chronic interstitial nephritis can lead to the development of AKI and subsequent CKD [16, 20]. The relationship between AKI and subsequent CKD development is supported by several studies, suggesting an important role in the global CKD epidemiology and ESRD on a bidirectional axis between AKI and CKD [21]. PPI use can also cause severe hypomagnesemia [22, 23], which is associated with a declining GFR rate in individuals with CKD [24], type II diabetes mellitus [25] and incidence of CKD [26].
Although the mechanisms are uncertain, the evidence linking PPI use and negative renal outcomes is consistent and would not be explained by confounding [26]. CKD is a major public health problem, as it is associated with a higher risk of cardiovascular and general mortality, in addition to social and individual costs [27]. Presenting evidence on the association between PPIs and incidence of CKD is important, as well as evaluating the decline in renal function associated with the use of PPIs, provides fundamental information for early treatments. Furthermore, evidence on the PPI use and kidney function association varies according to the duration of medication use is still scarce and controversial [27,28,29]. Thus, this study aimed to prospectively investigate whether regular PPI use at baseline is associated with change in GFR and incident kidney disease after a follow-up of about four years in a sample of middle-aged and elderly adults in the cohort. ELSA-Brazil.
Methods
Study design
A prospective cohort study with baseline (2008–2010) and follow-up (wave 2: 2012–2014) from ELSA-Brasil. The ELSA-Brasil comprises 15,105 active or retired employees, 35–74 years of age at baseline, from universities or research institutions located in six Brazilian capitals (Belo Horizonte, Porto Alegre, Rio de Janeiro, Salvador, Sao Paulo, Vitoria). ELSA-Brasil includes volunteers (76% of the final sample) and actively recruited participants (24%), the latter being recruited from listings of civil servants. Other publications contain detailed information on the ELSA-Brasil design and baseline data [30, 31].
Study population
Of the 15,105 baseline participants, 1,091 (7.2%) did not attend the research center for the second wave of measurements, of which 223 (20.4%) died. Thus, 14,014 subjects (94% of the eligible population) completed the second wave and 95 subjects with missing GFR data at baseline or wave 2 were excluded. For the analysis of drug use time, 313 PPI users with use time missing data were also excluded in the analysis of change in Glomerular Filtration Rate and 297 PPI users with use time missing data in the analysis of the Incidence of Chronic Kidney Disease. Sample selection is described in Fig. 1.
Study variables
Outcomes
We used the continuous measurements of estimated glomerular filtration rate (eGFR) obtained in the baseline and wave 2, and the incident CKD (defined as GFR < 60 ml/min/1.73 m2 in wave 2, according with KDIGO 2012 (Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease) [9]. The GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI) [32] without correction for races, as detailed in Barreto et al. [15].
Creatinine was evaluated in serum samples by the kinetic method, according to Jaffé (Advia 1200; Siemens, Munich, Germany), by applying a conversion factor derived from the calibration sample for isotope dilution mass spectrometry, as recommended by the National Kidney Disease Education Program [33].
Exposure
All participants were instructed to take medication prescriptions, packages, and inserts used in the last two weeks before the interview and report the time of use of each medication. All medicine trade names were converted either to the Brazilian common denomination (BCD) or international common denomination (INN) and the time of use to months. PPI users were individuals who, at baseline, reported using PPI class drugs (omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole) regularly in the last two weeks prior to the interview.
According to the guidelines, the duration of PPI use should be short-term (2 to 12 weeks), after which PPI therapy should be discontinued unless maintenance therapy is clearly indicated. Thus, the time of use was stratified into non-users (no PPI use at baseline), users up to six months and above six months [34,35,36].
Covariates
The covariates were obtained through standardized face-to-face interviews and clinical procedures at the ELSA-Brasil baseline [30].
Sociodemographic characteristics included sex and per capita household income (distributed by quintiles). The variable age was used to index time. Thus, the baseline age of each individual was the starting point, and the age at wave 2 corresponded to the baseline age plus the interval in years between the two visits ([date of wave 2 visit - date of baseline visit] / 365.25). Confounding factors were excessive alcohol consumption, defined as intake of ≥ 210 g of ethanol per week for men and ≥ 140 g per week for women [37], smoking was assessed by the following questions: “Are you or have you ever been a smoker, that is, have you smoked at least 100 cigarettes (five packs of cigarettes) throughout your life?” and “Do you currently smoke cigarettes?,” they were classified as never smokers, ex-smokers, and current smokers, obesity (body mass index ≥ 30 kg/m2), diabetes and hypertension (self-reported); cardiovascular disease (CVD) (self-reported medical diagnosis of the following comorbidities: acute myocardial infarction, angina, congestive heart failure, stroke or myocardial revascularization) and use of non-steroidal anti-inflammatory drugs (NSAIDs), Angiotensin II receptor blockers (ARBs) and Angiotensin-converting enzyme inhibitors (ACE).
Statistical analysis
Categorical variables were described as proportions and continuous variables either as medians and interquartile ranges or means and standard deviation (SD).
The association between PPI use and duration of use (both at baseline) with the incidence of CKD was investigated using binary logistic regression with no CKD incident as reference category. Crude and adjusted Odds Ratio (OR) and respective 95% confidence intervals (95%CI), were estimated. First, we estimated the crude OR of the correlation between PPI use and CKD (Model 0), then we adjust to age, sex, and per capita household income (Model 1). Subsequently, Model 1 was adjusted for excessive alcohol consumption, smoking and obesity (Model 2). Finally, CVD, diabetes, and hypertension data were added to model 2 (Model 3). The adjustment for NSAIDs, ARBs and ACEs use was added to the final model (Model 4). In model 4, all variables defined according to the literature that, a priori, would be potential confounding factors, regardless of the p-value, were kept. The significance level adopted was p < 0.05.
In the analysis of kidney function change, mixed linear regression models with random intercept and slope were used to assess longitudinal changes in GFR between baseline and wave 2. Such models are suitable for unbalanced and/or unevenly distributed data throughout time and data where the inter-subject variability is greater than the intra-subject one [38,39,40]. The fixed effects (β) and variance components (α) of the mixed linear models were estimated using restricted maximum likelihood methods.
In linear regression models with mixed effects, the exposure regression coefficients indicate the mean variation of the result at baseline and each moment (wave 2 in this paper). The interaction terms between a fixed-effect variable (PPI use and duration of use) and time determine whether that variable predicts longitudinal changes in the dependent variable over time. Therefore, we evaluated the interaction terms between age and the explanatory variables of interest, but only statistically significant terms (p < 0.05) were kept in the models.
We included the explanatory variables (PPI use and duration of use) and all covariates in the models as fixed effects, and age was modeled a random effect to index the time. All models included random effects on the age intercept and slope, allowing the individual’s initial value and longitudinal trajectory to vary with the population trajectory and average [38].
First, the GFR analysis was conducted using the explanatory variables of interest. We entered the covariates (sex, per-capita-household income, excessive alcohol consumption, smoking, obesity, cardiovascular disease, diabetes, hypertension, and NSAIDs, ARBs and ACEs use) into the models, step by step. We maintained in the final variables considered as confounding factors, according to the literature, regardless of the p-value. Finally, the interaction terms were added: PPI use x age and duration of PPI use x age. We presented the results only from the final models.
We conducted analyzes on Stata 14.0 (Stata Corporation, College Station, TX, USA).
Results
The mean age of the participants without CKD was 51.4 (SD = 8.7) years and 57.4% were women. The PPI users were older, had lower per capita household income, were less smokers and more obese. Moreover, the prevalence of CVD, diabetes, hypertension, and use of NSAIDs, ARBs and ACEs was higher among PPI users (Table 1). Additionally, 7.6% (N = 1,005) reported regularly used of PPIs, with 1.2% (N = 161) using for up to six months and 6.4% (N = 844) above six months.
Compared to non-users, PPI users had a lower mean glomerular filtration rate at baseline and wave 2. In addition, the prevalence of eGFR < 60 ml/min/1.73 m2 was higher among PPI users in wave 2 (Table 2).
In univariate logistic regression analysis, individuals who used PPIs at baseline had a risk of CKD 1.85 times greater than non-users (95% CI 1.41–2.41, p < 0.001). After adjusting for several confounding factors, the association showed no difference in significance and strength of correlation (Model 2, Table 3), but after adjusting particularly for hypertension the correlation remained borderline (OR = 1.29, 95%CI 0.97–1.71, p = 0.079).
In the univariate analysis, PPI users over six months had 2.33 times the risk of incident CKD compared to non-users (95% CI 1.69–3.22, p = 0.001) (Table 3). After adjusting for several confounders, the association remained borderline (Model 4; Table 4).
In the analysis of change in glomerular filtration, the average age of participants was 51.8 years old (SD = 9.0), and 54.7% were women. PPI users were older, had lower per capita household income, were less smoking and more obese. The prevalence of CVD, diabetes, hypertension and of NSAIDs, ARBs and ACEs use was also higher among PPI users (Table 5). At baseline, 7.9% (N = 1,096) of participants used PPIs, with 1.2% (N = 173) using for up to six months and 6.7% (N = 923) above six months. The average interval between visits was 3.9 years (range: 1.7 to 6.0 years).
After adjusting for all covariates, the interaction PPI use x age was statistically significant at -0.165 (95%CI -0.246; -0.084), indicating that PPI users had a more pronounced decline in eGFR between visits than non-users (Table 6).
The interaction term duration of PPI use x age was statistically significant in the categories of duration of drug use up to six months − 0.196 (95%CI -0.370; -0.021) and over six months − 0.149 (95%CI -0.248; -0.049), indicating that participants who, at baseline, used PPIs for six months or more had a more pronounced decline in eGFR between the two visits compared to non-users (Table 7).
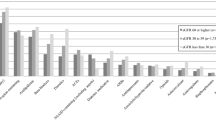
We plotted an illustrative graphical representation of the estimated average for predicted eGFR levels concerning the explanatory variable time of PPI use (Fig. 2). Since age was modeled as a random effect in the analysis, the slopes in the figure indicate eGFR mean, stratified by exposure groups, as individuals aged during the follow up period.
Longitudinal trajectories* of estimated glomerular filtration rate (eGFR) according to time of use of proton pump inhibitors (PPI). ELSA-Brasil (2008–2010 and 2012–2014). *As age was modeled as a random effect in the data analysis, this figure shows changes in estimated glomerular filtration rate over time (i.e., as individuals age). †Predicted numbers are dependent variable values based on estimated regression coefficients and a prediction of independent variable values after adjustments (age, sex, per capita household income, excessive alcohol consumption, obesity, cardiovascular disease, diabetes, hypertension, use of NSAIDs, BRAs, ACEs and interaction: time of PPI use × age)
Discussion
Results from this study suggest a correlation between PPI use and renal function decline. Regular PPI use at baseline was associated with a more pronounced decline in eGFR between visits 1 and 2 in ELSA-Brasil and a higher incidence of CKD (borderline) after adjusting the confounders. The borderline result suggests a correlation since there was no change in the association strength after adjusting for several confounding factors (especially chronic diseases).
In a prospective cohort, Xie et al. evaluated 144,032 participants and after a median follow-up of 5 years that PPI users had a risk of reduced eGFR 1.22 (95%CI 1.16–1.28) times compared to PPI users of histamine H2 receptor antagonist (H2RA) [28]. In another prospective cohort, evaluating 173,321 PPI users and 20,270 H2RA users followed for over 5 years, Xie and colleagues found that PPI users had a risk of 1.32 (95%CI 1.28–1.37) times in the decrease in eGFR compared to H2RA users [29]. Klatte et al., in a retrospective cohort, evaluating 105,305 PPI users and 9,578 H2RA users after a median follow-up of 2.7 years (range 1.5 to 3.8), found a risk of 1.26 (95%CI 1.16–1.36) times for eGFR decline among PPI users [41].
Recent studies have suggested that PPI use is an independent risk factor for CKD development [27,28,29, 38, 41,42,43,44], but our study displayed a borderline association between PPI use and CKD incidence. Some differences between this study and others deserve to be highlighted. First, the follow-up time in our study (mean 3.9 years) was shorter than in the others (5 to 13 years) [29, 41,42,43]. Second, our study compared PPI users and non-users, unlike other studies that evaluated PPI users and H2RA users [41, 43]. Finally, the measurement of PPI exposure differs between studies.
We investigated time of use as a modifier of the effect of the association between PPI use and increased risk of incident CKD [29] and impaired renal function [28, 29, 44]. Rodriguez-Poncelas et al., evaluating 5,636 individuals, found an association between exposure to PPIs and the risk of incident CKD among PPI users from three to six months (HR = 1.42; 95%CI 1.11–1.80) and over six months (HR = 1.16; 95%CI 1.08–1.57) compared to non-users [36]. Xie et al. evaluated the association between PPI exposure time and the risk of renal outcomes among new PPI users (N = 173,321) and found that compared to individuals exposed for ≤ 30 days, there was a gradient between the duration of exposure and the risk of renal outcomes among those exposed for 31–90, 91–180, 181–360, and 361–720 days [29]. The association decreased with an exposure longer than 720 days, which is likely a reflection of a survival bias, an effect commonly referred to as “depletion of susceptibles” in pharmacoepidemiology, where individuals resistant to PPI effects on renal function remain in the cohort [29, 41, 45].
Our results suggest that association between drug use and loss of renal function may varies according to duration of PPI use. We found an association between PPI use over six months and incident CKD and PPI use up to six months and over six months and decline of renal function. These results corroborate the findings of recent studies on the association between PPI use and renal outcomes; and the change of association by the continuity of PPI use [29, 41]. There is no standardization in the literature for the categorization of the time of use of the PPI, which becomes a challenge for the comparison of our results.
Although the mechanisms that support the association between PPI use and CKD are not yet established, the relationship between PPI exposure and the risk of AKI and AIN is well established [16,17,18, 46], with AKI being associated with an increased risk of CKD [20]. And although the association between CKD and PPI exposure is postulated to be intervened by AKI [27, 42], a significant association between PPI use and CKD independent of AKI has been reported, which suggests that monitoring AKI and AIN in PPI users is not enough to protect against CKD [41].
Another possible mechanism is related to severe hypomagnesemia [22] that may be associated with the use of PPIs. Hypomagnesemia is associated with a more pronounced decline in eGFR in individuals with CKD, type II diabetes mellitus, progression to ESRD, and incidence of CKD [23,24,25]. Chronic PPI use can cause endothelial dysfunction leading to CKD through a variety of mechanisms, causing accelerated endothelial aging [47].
Our results corroborate other studies in the literature, showing an association between PPI use and decline in renal function. Nonetheless, we need to address some limitations. First, there may have been an error in classifying non-users of PPIs since any participant who used PPIs and was not using them only in the last two weeks (reference time of drug use) were classified as non-users. Thus, the PPI use prevalence may have been underestimated, underestimating the association. Second, the retention rate in the second wave was very high (94%), eligible individuals for this study who did not participate in wave 2 were older, with less education, higher prevalence of hypertension, diabetes and PPI use according to the analyzes performed (data not shown). Although the losses are small, these factors are associated with the decline in renal function, which may have contributed to overestimate the association found. In addition, it is important to note that, unlike clinical trials, observational study participants who use PPIs may have a higher risk of decline in eGFR for reasons unrelated to drug use. For example, our PPI users were more likely to be older, have lower per capita household income, be obese, and have a higher prevalence of cardiovascular disease, diabetes, and hypertension. Third, the individuals had their creatinine measured only once, which is inconsistent with the definition of CKD, because, although this does not meet the KDIGO definition, most large epidemiological studies have used a single eGFR definition for CKD [42]. Fourth, self-reported information about PPI use and comorbidities may have contributed to a recall bias, as some participants may have forgotten to mention a medication they were using or comorbidity. However, it is worth mentioning that the day before the visit, the participants received calls and were instructed to take the packages and prescriptions of all the medications they were using. Recognizing this potential bias, we adjusted for several confounding factors and the association remained. Besides, there is the possibility that variables not included in study and those not controlled could cause indication bias, as in all observational studies.
Strengths in our study that deserve to be highlighted are the studied population which included a large sample of relatively young individuals from a middle-income country, and the comprehensive data source that with laboratory information on GFR values, medication use and comorbidities. The ELSA-Brasil database represents an excellent tool for studying pharmacoepidemiology and pharmacovigilance because of the information collected on the participant’s medication use at each follow-up visit every three years on average [30]. This is particularly important for Brazil, which still has a very scarce and fragmented drug information system [48]. We also used a statistical model that considers the hierarchical structure of the data and allows the analysis of unbalanced or unevenly distributed longitudinal data.
Conclusion
This study showed that PPI use and duration of its use of up to six months and above are associated with reduced eGFR. We also showed that PPI use for over six months is associated with an increase in the risk of developing CKD in a large sample of adult and elderly Brazilian people. Although observational studies do not have the best design to determine cause and effect, due to the large number of individuals currently using PPIs, healthcare professionals need to be cautious when prescribing, as well as monitoring the use of these drugs, due to the potential effects adverse.
Data availability
Data Sharing The datasets generated during analysed during the current study are available from the corresponding author on reasonable request.
References
Al-Aly Z, Maddukuri G, Xie Y. Proton pump inhibitors and the kidney: implications of current evidence for clinical practice and when and how to deprescribe. Am J Kidney Dis. 2020;75:4:497–507. https://doi.org/10.1053/j.ajkd.2019.07.012.
Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur j intern med. 2017;37:19–24. https://doi.org/10.1016/j.ejim.2016.10.007.
Fossmark R, Martinsen TC, Waldum HL. Adverse effects of proton pump inhibitors—evidence and plausibility. Int j mol Sci. 2019;20:5203. 10.3390%2Fijms20205203.
Yibirin M, Oliveira D, Valera R, Plitt AE, Lutgen S. Adverse effects associated with proton pump inhibitor use. Cureus. 2021;13:e12759. 10.7759%2Fcureus.12759.
Yang Y, George KC, Shang WF, Zeng R, Ge SW, Xu G. Proton-pump inhibitors use, and risk of acute kidney injury: a meta-analysis of observational studies. Drug des dev ther. 2017;11:1291. https://doi.org/10.2147/dddt.s130568.
Wijarnpreecha K, Thongprayoon C, Chesdachai S, Panjawatanana P, Ungprasert P, Cheungpasitporn W. Associations of proton-pump inhibitors and H2 receptor antagonists with chronic kidney disease: a meta-analysis. Dig dis sci. 2017;62:2821–7. https://doi.org/10.1007/s10620-017-4725-5.
Sun J, Sun H, Cui M, Sun Z, Li W, Wei J, et al. The use of anti-ulcer agents and the risk of chronic kidney disease: a meta-analysis. Int urol nephrol. 2018;50:1835–43. https://doi.org/10.1007/s11255-018-1908-8.
Hussain S, Singh A, Habib A, Najmi AK. Proton pump inhibitors use and risk of chronic kidney disease: evidence-based meta-analysis of observational studies. Clin Epidemiol Global Health. 2019;7:46–52. https://doi.org/10.1016/j.cegh.2017.12.008.
Kdigo, CKD Work Group. Kidney Disease: Improving Global Outcomes. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney int. 2013; S3:1–150.
Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–69. https://doi.org/10.1016/s0140-6736(13)60439-0.
Gasparini A, Evans M, Coresh J, Grams ME, Norin O, Qureshi AR, et al. Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol dial transplant. 2016;31:2086–94. https://doi.org/10.1093/ndt/gfw354.
Cusumano AM, Gonzalez-Bedat MC, García-García G, Maury Fernandez S, Lugon JR, Poblete Badal H, et al. Latin american dialysis and renal transplant registry: 2008 report (data 2006). Clin Nephrol. 2010;74:3–8.
Lugon JR, Matos JPSde. Disparities in end-stage renal disease care in South America. Clin nephrol. 2010;74:66–71.
Pinheiro PC, Barros MBDA, Szwarcwald CL, Machado ÍE, Malta DC. Diferenças entre medidas autorreferidas e laboratoriais de diabetes, doença renal crônica e hipercolesterolemia. Ciênc Saúde Colet. 2021;26:1207–19. https://doi.org/10.1590/1413-81232021264.44582020.
Barreto SM, Ladeira RM, Duncan BB, Schmidt MI, Lopes AA, Benseñor IM, et al. Chronic kidney disease among adult participants of the ELSA-Brazil cohort: association with race and socioeconomic position. J epidemiol community health. 2015;70:380–9. https://doi.org/10.1136/jech-2015-205834.
Praga M, Gonzalez E. Acute interstitial nephritis. Kidney int. 2010;77:956–61. https://doi.org/10.1038/ki.2010.89.
Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case–control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney int. 2014;86:837–44. https://doi.org/10.1038/ki.2014.74.
Antoniou T, MacDonald EM, Hollands S, Gomes T, Mamdani MM, Garg AX, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. CMAJ Open. 2015;3:e166–171. https://doi.org/10.9778/cmajo.20140074.
Perazella MA, Luciano RL. Review of select causes of drug-induced AKI. Expert rev clin pharmacol. 2015;8:367–71. https://doi.org/10.1586/17512433.2015.1045489.
Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney int. 2012;81:442–8. https://doi.org/10.1038/ki.2011.379.
Danziger J, William JH, Scott DJ, Lee J, Lehman L, Mark RG, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney int. 2013;83:692–9. https://doi.org/10.1038/ki.2012.452.
Kieboom BCT, Kiefte JJC, Eijgelsheim M, Franco OH, Kuipers EJ, Hofman A, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am j kidney dis. 2015;66:775–82. https://doi.org/10.1053/j.ajkd.2015.05.012.
Van Laecke S, Nagler EV, Verbeke F, Van Biesen W, Vanholder R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am j med. 2013;126:825–31. https://doi.org/10.1016/j.amjmed.2013.02.036.
Pham PC, Pham PM, Pham PT, Pham SV, Pham PA, Pham PT. The link between lower serum magnesium and kidney function in patients with diabetes mellitus type 2 deserves a closer look. Clin nephrol. 2009;71:375–9. https://doi.org/10.5414/cnp71375.
Tin A, Grams ME, Maruthur NM, Astor BC, Couper D, Mosley TH, et al. Results from the atherosclerosis risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney int. 2015;87:820–7. https://doi.org/10.1038/ki.2014.331.
Li T, Xie Y, Al-Aly Z. The association of proton pump inhibitors and chronic kidney disease: cause or confounding? Curr Opin Nephrol Hypertens. 2018;27:182–7. https://doi.org/10.1097/mnh.0000000000000406.
Lazarus B, Chen Y, Wilson FP, Sang Y, Chang AR, Coresh J, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238–46. https://doi.org/10.1001/jamainternmed.2015.7193.
Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Long-term kidney outcomes among users of proton pump inhibitors without intervening acute kidney injury. Kidney int. 2017;91:1482–94. https://doi.org/10.1016/j.kint.2016.12.021.
Xie Y, Bowe B, Li T, Xian H, Balasubramanian S, Al-Aly Z. Proton pump inhibitors and risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2016;27:3153–63. https://doi.org/10.1681/asn.2015121377.
Aquino EM, Barreto SM, Benseñor IM, Carvalho MS, Chor D, Duncan BB, et al. Brazilian longitudinal study of Adult Health (ELSA-Brasil): objectives and design. Am J Epidemiol. 2012;175:315–24. https://doi.org/10.1093/aje/kwr294.
Schmidt MI, Ducan BB, Mill JG, Lotufo PA, Chor D, Barreto SM, et al. Cohort Profile: longitudinal study of Adult Health (ELSA-Brasil). Intern j epidemiol. 2015;44:68–75. https://doi.org/10.1093/ije/dyu027.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of Internal Medice. 2009;150:604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, et al. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am j kidney dis. 2002;39:920–9. https://doi.org/10.1053/ajkd.2002.32765.
Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal refux disease. Am J Gastroenterol. 2013;108:308–28. https://doi.org/10.1038/ajg.2012.444.
Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlpati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2021;117:27–56. https://doi.org/10.14309/ajg.0000000000001538.
Rodriguez-Poncelas A, Barceló MA, Saez M, Coll-de-Tuero G. Duration and dosing of proton pump inhibitors associated with high incidence of chronic kidney disease in population-based cohort. PLoS ONE. 2018;13:e0204231. 10.1371%2Fjournal.pone.0204231.
National Institute on Alcohol Abuse and Alcoholism. What Is a Standard Drink? Available in: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/what-standard-drink. Accessed May 15, 2020.
Fausto MA, Carneiro M, Antunes CM, Pinto JA, Colosimo EA. Mixed linear regression model for longitudinal data: application to an unbalanced anthropometric data set. Cad Saúde Pública. 2008;24:513–24. https://doi.org/10.1590/S0102-311X2008000300005.
Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–80. 10.1002/. (sici)1097-0258(19971030)16:20%3C2349::aid-sim667%3E3.0.co;2-e.
Molenberghs G, Verbeke G. A review on linear mixed models for longitudinal data, possibly subject to dropout. Stat Modelling. 2001;1:235–69. 10.1177%2F1471082X0100100402.
Klatte DCF, Gasparini A, Xu H, Deco P, Trevisan M, Johansson ALV, et al. Association between Proton pump inhibitor use and risk of progression of chronic kidney disease. Gastroenterology. 2017;153:702–10. https://doi.org/10.1053/j.gastro.2017.05.046.
Arora P, Gupta A, Golzy M, Patel N, Carter RL, Jalal K, et al. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. 2016;17:1–8. https://doi.org/10.1186/s12882-016-0325-4.
Cho NJ, Choi CY, Park S, Park Sh, Lee EY, Gil HW. Association of proton pump inhibitor use with renal outcomes in patients with coronary artery disease. Kidney Res Clin Pract. 2018;37:59–68. https://doi.org/10.23876/j.krcp.2018.37.1.59.
Hung SC, Liao KF, Hung HC, Lin CL, Lai SW, Lee PC, et al. Using proton pump inhibitors correlates with an increased risk of chronic kidney disease: a nationwide database-derived case-controlled study. Fam Pract. 2018;35:166–71. https://doi.org/10.1093/fampra/cmx102.
Moride Y, Abenhaim L. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. J Clin Epidemiol. 1994;47:731–7. https://doi.org/10.1016/0895-4356(94)90170-8.
Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nature reviews. Nephrology. 2010;6:461–70.
Yepuri G, Sukhovershin R, Nazari-Shafti TZ, Petrascheck M, Ghebre YT, Cooke JP. Proton pump inhibitors accelerate endothelial senescence. Circ Res. 2016;118:e36–e42. https://doi.org/10.1161/circresaha.116.308807.
Bermudez JAZ, Esher A, Osorio-De-Castro CGS, De Vasconcelos DMM, Chaves GC, Oliveira MA, et al. Pharmaceutical services and comprehensiveness 30 years after the advent of Brazil’s unified health system. Cienc e Saude Coletiva. 2018;23:1937–51. https://doi.org/10.1590/1413-81232018236.09022018.
Acknowledgements
The authors thank the staff and participants of the ELSA-Brasil for their important contributions. The study was supported by the Brazilian Ministries of Health (DECIT) and of Science and Technology (FINEP/CNPq).
Funding
The ELSA-Brasil baseline study was supported by the Brazilian Ministry of Health (Science and Technology Department) and the Brazilian Ministry of Science and Technology (Financiadora de Estudos e Projetos and CNPq National Research Council), grants 01 06 0010.00 RS, 01 06 0212.00BA, 01 06 0300.00 ES, 01 06 0278.00 MG, 01 06 0115.00SP, 01 06 0071.00 RJ.
Author information
Authors and Affiliations
Contributions
All authors were responsible for the study design, analysis and interpretation of data, the writing of the manuscript, and the decision to submit for publication. All authors had full access to the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Research Ethics Committees of the participating institutions and by the National Committee for Research Ethics (CONEP 976/2006) of the Ministry of Health. This study was carried out in accordance with the Declaration of Helsinki’s ethical principles. All patients provided informed consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
dos Santos, A.S., de Menezes, S.T., Silva, I.R. et al. Kidney function decline associated with proton pump inhibitors: results from the ELSA-Brasil cohort. BMC Nephrol 24, 285 (2023). https://doi.org/10.1186/s12882-023-03300-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-023-03300-4