Abstract
Aims
This cross-sectional study aimed to investigate the association between plasma homocysteine (Hcy) and chronic kidney disease (CKD) in US patients with type 2 diabetes mellitus (T2DM).
Methods
We used data from the 2003–2006 National Health and Nutritional Examination Surveys (NHANES). CKD was defined as an estimated glomerular filtration rate < 60 ml/min/1.73 m2 and/or urinary albumin-creatine ratio ≥ 3 mg/mmol.
Results
This study included 1018 patients with T2DM. The mean Hcy value was 10.2 ± 4.6 μmol/L. Among the patients, 417 (40.96%) had hyperhomocysteinemia (HHcy) and 480 (47.15%) had CKD. The Hcy level was higher in patients with CKD than in those without CKD. Compared to patients with normal Hcy, those with HHcy were older and had worse renal function. After full multivariate adjustment, HHcy was positively associated with the risk of CKD in US patients with T2DM (OR, 1.17; 95% CI, 1.11–1.22; P < 0.001), which for women was 1.15 (95% CI, 1.08 ~ 1.23; P < 0.001) and for men was 1.18 (95% CI, 1.1 ~ 1.27; P < 0.001).
Conclusions
HHcy was independently associated with CKD in patients with T2DM. Further prospective studies are warranted to investigate the effect of Hcy on CKD in patients with T2DM.
Similar content being viewed by others
Introduction
Type 2 diabetes mellitus (T2DM) is the most common type of diabetes and accounts for over 90% of all diabetes cases worldwide. An estimated 537 million adults aged 20–79 years worldwide (10.5% of all adults in this age group) have diabetes according to the latest International Diabetes Federation (IDF) Diabetes Atlas [1]. Up to 40% of patients with diabetes have comorbidity of chronic kidney disease (CKD), which has the risk of progression to end-stage renal disease (ESKD) and cause high morbidity, mortality, and poor quality of life [2]. The patients with ESKD must receive maintenance dialysis or kidney transplantation to survive, leading to a heavy burden on the health system globally [3]. In addition, CKD patients with diabetes would be at a higher risk of cardiovascular (CV) disease, including myocardial infarction, ischaemic stroke, and all-cause mortality than kidney disease or diabetes itself.
Homocysteine (Hcy) may play a role in the development of cardiovascular (CV) diseases. The role of Hcy in the development of the vascular complications associated with DM was not clearly defined [4]. The 2006 US Stroke guidelines clearly indicated that plasma Hcy > 10 μmol/L was defined as hyperhomocysteinemia (HHcy) [5]. HHcy could reflect the abnormal state of body metabolism for the sake of its damaging effect on cells, tissues and organs [6]. It was an important risk factor for the occurrence of many chronic diseases and regarded as one of the important indicators for many physical illnesses, such as hypertension, hyperlipidemia and hyperglycaemia [7]. Hcy appeared to cause endothelial cell damage and influence the activity and production of coagulation factors [8]. Furthermore, several studies have found that Hcy was associated with eGFR and CKD in the general population [9]. However, few studies have reported the association between Hcy and CKD in patients with diabetes.
The aim of this study was to assess the association between Hcy and CKD in T2DM patients. The various confounding factors which had the possibility to affect the progression to CKD would be adjusted in the cross-sectional study.
Materials and methods
Data source and study population
A NHANES database was used for this study. NHANES is a nationwide population-based survey of the US that has been conducted to assess the health and nutritional status of the US population since 1999. In our research, NHANES data from 2003 to 2004 and 2005–2006 have been chosen because of the integrity of these two time periods for Hcy measurements. Of a total of 16,625 patients, 1112 type 2 diabetes patients were included in this study. A total of 74 patients younger than 18 and older than 80 were excluded. Twenty patients were excluded because 16 had missing data on Hcy and 4 had missing data on eGFR. Finally, the data from 1018 participants were employed for this study (Fig. 1). The research method was cross-sectional study. NHANES was approved by the US National Center for Health Statistics Research Ethics Review Board, and all participants provided informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement were followed when reporting this cross-sectional study [10].
Data collection
We evaluated both clinical characteristics (sex, age, and race) and biochemical parameters (levels of triglycerides [TG], cholesterol [CHO], albumin [ALB], blood urea nitrogen [BUN], serum creatinine [Scr], uric acid [UA], HbA1c, urinary albumin-creatinine ratio [ACR] and plasma homocysteine [Hcy]) collected from the NHANES Laboratory Data. The TG, CHO, ALB, BUN, Scr, and UA analyses were performed using a Beckman Synchron LX20 (Beckman Coulter, Inc).HbA1c measurements were performed by the Diabetes Laboratory at the University of Minnesota using a Tosoh A1c 2.2 Plus Glycohemoglobin Analyser (Tosoh Medics, Inc., San Francisco, CA). Hcy in plasma was measured by the Abbott Homocysteine (Hcy) assay, which is a fully automated fluorescence polarization immunoassay (FPIA) from Abbott Diagnostics.
The albumin in the urine was measured via a fluorescent immunoassay. The urinary creatinine analysis uses a Jaffé rate reaction, in which creatinine reacts with picrate in an alkaline solution to form a red creatinine-picrate complex.
Measurement of chronic kidney disease
We used ACR and eGFR values as parameters to determine CKD [11]. To calculate the eGFR, the following CKD-EPI formula was applied [12]:
eGFR CKD-EPI (mL/min/1.73 m2).
GFR = a × (Scr/b) c × (0.993)age.
In this formula, Scr denotes the serum creatinine level (μmol/L);a is 166 for black females and 163 for males;a is 144 for non-black females and 141 for males; b is 0.7 for females and 0.9 for males, and c is − 0.329 for females with serum creatinine ≤62 μmol/L and − 0.411 for males with serum creatinine ≤80 μmol/L. c is − 1.209 for females with serum creatinine≥62 μmol/L and for males with serum creatinine ≥80 μmol/L. The ACR was calculated as the urinary albumin/creatinine ratio. Participants with an ACR above 3 mg/mmoL or an ALFF below 60 mL/min/1.73 m2 were classified as CKD patients according to the Kidney Disease Outcome Quality Initiative clinical practice guidelines for CKD.
Statistical analysis
Data were presented as the mean ± standard deviation (SD) for normally distributed variables or the median (interquartile range) for nonnormally distributed variables and as the frequency or percentage for categorical variables. For the baseline characteristics analysis, the significant differences between two groups were tested based on t test, nonparametric tests for continuous variables and chi-square or Fisher tests for categorical variables. Multiple logistic regression analysis was performed to evaluate the associations between Hcy and CKD, with the results expressed as odds ratios (ORs) and 95% confidence intervals (CIs). The covariates entered into the model were based on univariate analyses and a literature review [13]. All analyses were performed using R Statistical Software (http://www.R-project.org, The R Foundation) and the Free Statistics analysis platform. P values< 0.05 (two-tailed) were considered statistically significant.
Results
Clinical and biochemical characteristics of the study subjects
The baseline clinical characteristics of the included patients were summarized in Table 1. The mean age of the included patients was 59.9 ± 13.1 years, and 51.2% were male. Of all patients, 480 (47.1%) had CKD. Patients with CKD were older (P < 0.001). Levels of Hcy (P < 0.001), ACR (P < 0.001), Scr (P < 0.001), BUN (P < 0.001) and UA (P < 0.001) were higher in patients with CKD than in those without CKD. No significant differences were found in the proportion of race, HbA1c, TG and CHO between the two groups. Table 2 compared the characteristics of the normal plasma homocysteine and hyperhomocysteinemia groups in which the plasma homocysteine concentration exceeded 10 μmol/L. Compared with patients with normal Hcy, those with HHcy were older (P < 0.001). They also had higher levels of ACR and worse renal function (P < 0.001) but had lower HbA1c (P < 0.001) and CHO (P = 0.002).
Association of plasma homocysteine with CKD
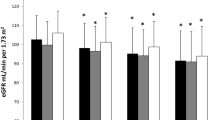
Table 3 showed the results of the univariate logistic regression analysis for the associations between Hcy and CKD. In the multivariate model that included age, race, HbA1c, ALB, CHO, TG and UA, the prevalence of CKD increased with increasing Hcy in all patients (OR, 1.17; 95% CI, 1.11–1.22; P < 0.001). For male and female patients, the ORs for CKD all tended to increase with increasing Hcy levels (male OR, 1.18; 95% CI, 1.1 ~ 1.27; P < 0.001; female OR, 1.15; 95% CI, 1.08 ~ 1.23; P < 0.001). Fig. 2 showed the homocysteine plasma levels at different stages of renal impairment in type 2 diabetes. Significant differences were observed among the five groups (P < 0.001), and the mean value of Hcy in the CKD5 stage was the highest.
Plasma homocysteine levels at different stages of renal impairment in type 2 diabetes. CKD1 = eGFR ≥90 mL/min/1.73 m2; CKD 2 = eGFR Between 60 and 89 mL/min/1.73 m2; CKD 3 = eGFR between 30 and 59 mL/min/1.73 m2; CKD 4 = eGFR between 15 and 29 mL/min/1.73 m2; CKD 5 = eGFR<15 mL/min/1.73 m2. In CKD stage 1, the mean value of Hcy was 8.2 μmol/L. In CKD stage 1, the mean value of Hcy was 8.2 μmol/L. In CKD stage 2, the mean value of Hcy was 9.8 μmol/L. In CKD stage 3, the mean value of Hcy was 13.5 μmol/L. In CKD stage 4, the mean value of Hcy was 16.4 μmol/L. In CKD stage 5, the mean value of Hcy was 17.2 μmol/L
Discussion
In this cross-sectional study of 1018 patients with T2DM, we investigated the association between Hcy and the prevalence of CKD. We found that the prevalence of CKD in T2DM patients increased with higher Hcy value, independent of age, race, gender, and other clinical variables. The results also showed that gender, age, UA, TG, and HbA1c were associated with CKD. However, the associations among HbA1c, TG, and CKD were not statistically significant. Previously, diabetes, hypertension, hyperuricaemia, obesity, and hyperlipidaemia were identified as risk factors for CKD. This research have investigated the association between Hcy and CKD in US patients with T2DM.
In the last 10 years, Hcy has been considered as a marker of cardiovascular disease and a independent risk factor for many other diseases. HHcy is a trigger for many diseases, such as atherosclerosis, congestive heart failure, c, Alzheimer’s disease and hearing loss [14]. Several previous reports have assessed the association between Hcy and CKD. Li reported that Hcy is associated with tubular interstitial lesions in the early stages of IgA nephropathy [15]. Wang found that HHcy was more prevalent in patients with IgA nephropathy than in patients with other primary glomerular diseases, especially in the early stages of CKD, and may be a predictor of accelerated decline in renal function and future incidence of CKD [16]. Spence has shown that Hcy plays a significant role in the effect of renal dysfunction on atherosclerosis [17].
Diabetes mellitus is the most common cause of CKD. The hemodynamic changes and abnormal glucose metabolism caused by hyperglycemia are the basis of renal damage. However, The exact mechanisms underlying the association between HHcy and renal disease progression are not fully understood. Possible reasons are that HHcy inhibits vasodilation of renal arteries and promoting acceleration of progression of renal damage and glomerulosclerosis [18,19,20]. Increasing evidence indicated that there was a graded association between Hcy quartiles and eGFR decline. Compared with participants with the lowest quartile of Hcy levels, those with the highest quartile had significantly increased risk for rapid eGFR decline [21]. Figure. 2 indicated that renal function gradually deteriorated with increasing Hcy levels. An Israeli study found that subjects with homocysteine> 15 μmol/L were more likely to have an eGFR< 60 ml/min and proteinuria. At a GFR < 60 ml/min, homocysteine was progressively elevated in stages 3 and 4 of CKD [9, 22]. This is consistent with our findings. It was found that the longer the duration of T2DM, the higher the levels of Hcy in the plasma. A meta-analysis showed that Hcy levels were significantly higher in type 2 diabetic nephropathy (T2DN) patients with macro-albuminuria compared with T2DM patients without albuminuria and T2DN patients with micro-albuminuria, while Hcy status was significantly higher in T2DN patients with micro-albuminuria compared to T2DM patients without albuminuria [23].
This study had several limitations. Firstly, this was a retrospective cross-sectional study that did not confirm the causal relationship between Hcy and CKD in patients with T2DM. Secondly, the covariates in this study did not include medications that might affect the results of Hcy detection. Finally, there was a wide variation in the prevalence of MTHFR gene polymorphism across different populations around the world. MTHFR gene have been reported to be involved in the biological metabolism of folate, vitamin B12 and Hcy, maintaining DNA methylation patterns [24]. There was no genetic testing in this study, and the influence of MTHFR polymorphisms on the study results was unknown.
Nevertheless, the strength of this study was the relatively large sample size in patients with T2DM. This study provided useful and convincing epidemiological evidence for the association between Hcy and CKD in patients with T2DM. In conclusion, Hcy was found to be independently associated with CKD in patients with T2DM. In the future, a prospective cohort study is warranted to further investigate the impact of Hcy on CKD in patients with T2DM.
Availability of data and materials
Data can be downloaded from the ‘NHANES’database (https://www.cdc.gov/nchs/nhanes/index.htm).
References
Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. https://doi.org/10.1016/j.diabres.2021.109119.
Shubrook JH, Neumiller JJ, Wright E. Management of chronic kidney disease in type 2 diabetes: screening, diagnosis and treatment goals, and recommendations. Postgrad Med. 2021:1–12. https://doi.org/10.1080/00325481.2021.2009726.
Skolnik NS, Style AJ. Importance of early screening and diagnosis of chronic kidney disease in patients with type 2 diabetes. Diabetes Ther. 2021;12:1613–30. https://doi.org/10.1007/s13300-021-01050-w.
Muzurović E, Kraljević I, Solak M, Dragnić S, Mikhailidis DP. Homocysteine and diabetes: role in macrovascular and microvascular complications. J Diabetes Complicat. 2021;35:107834. https://doi.org/10.1016/j.jdiacomp.2020.107834.
Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association stroke council: cosponsored by the atherosclerotic peripheral vascular disease interdisciplinary working group; cardiovascular nursing council; clinical cardiology council; nutrition, physical activity, and metabolism council; and the quality of care and outcomes research interdisciplinary working group: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–633. https://doi.org/10.1161/01.str.0000223048.70103.f1.
Jiang Q, Wang L, Si X, et al. Current progress on the mechanisms of hyperhomocysteinemia-induced vascular injury and use of natural polyphenol compounds. Eur J Pharmacol. 2021;905:174168. https://doi.org/10.1016/j.ejphar.2021.174168.
Martí-Carvajal AJ, Solà I, Lathyris D, Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:CD006612. https://doi.org/10.1002/14651858.CD006612.pub5.
Badri S, Vahdat S, Seirafian S, Pourfarzam M, Gholipur-Shahraki T, Ataei S. Homocysteine-lowering interventions in chronic kidney disease. J Res Pharm Pract. 2021;10:114–24. https://doi.org/10.4103/jrpp.jrpp_75_21.
Cohen E, Margalit I, Shochat T, Goldberg E, Krause I. The relationship between the concentration of plasma homocysteine and chronic kidney disease: a cross sectional study of a large cohort. J Nephrol. 2019;32:783–9. https://doi.org/10.1007/s40620-019-00618-x.
Elm E, Altman DG, Egger M, Pocock SJ, Gatzsche PC, Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806. https://doi.org/10.1136/bmj.39335.541782.AD.
Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. 2005;67:2089–100. https://doi.org/10.1111/j.1523-1755.2005.00365.x.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. https://doi.org/10.7326/0003-4819-150-9-200905050-00006.
Smith AD, Refsum H. Homocysteine - from disease biomarker to disease prevention. J Intern Med. 2021;290:826–54. https://doi.org/10.1111/joim.13279.
Kim J, Kim H, Roh H, Kwon Y. Causes of hyperhomocysteinemia and its pathological significance. Arch Pharm Res. 2018;41:372–83. https://doi.org/10.1007/s12272-018-1016-4.
Li Z, Han Q, Ye H, et al. Serum homocysteine is associated with tubular interstitial lesions at the early stage of IgA nephropathy. BMC Nephrol. 2022;23:78. https://doi.org/10.1186/s12882-021-02632-3.
Wang YN, Xia H, Song ZR, Zhou XJ, Zhang H. Plasma homocysteine as a potential marker of early renal function decline in IgA nephropathy. Front Med. 2022;9:812552. https://doi.org/10.3389/fmed.2022.812552.
Spence JD, Urquhart BL, Bang H. Effect of renal impairment on atherosclerosis: only partially mediated by homocysteine. Nephrol Dial Transplant. 2016;31:937–44. https://doi.org/10.1093/ndt/gfv380.
Ostrakhovitch EA, Tabibzadeh S. Homocysteine in chronic kidney disease. Adv Clin Chem. 2015;72:77–106. https://doi.org/10.1016/bs.acc.2015.07.002.
Emsley AM, Jeremy JY, Gomes GN, Angelini GD, Plane F. Investigation of the inhibitory effects of homocysteine and copper on nitric oxide-mediated relaxation of rat isolated aorta. Br J Pharmacol. 1999;126:1034–40. https://doi.org/10.1038/sj.bjp.0702374.
Zhong MF, Zhao YH, Xu H, Tan X, Wang YK, Wang WZ. The cardiovascular effect of systemic homocysteine is associated with oxidative stress in the rostral ventrolateral medulla. Neural Plast. 2017;2017:3256325. https://doi.org/10.1155/2017/3256325.
Xiao W, Ye P, Wang F, Cao R, Bai Y, Wang X. Plasma homocysteine is a predictive factor for accelerated renal function decline and chronic kidney disease in a community-dwelling population. Kidney Blood Press Res. 2021;46:541–9. https://doi.org/10.1159/000514360.
Perna AF, Ingrosso D. Homocysteine and chronic kidney disease: an ongoing narrative. J Nephrol. 2019;32:673–5. https://doi.org/10.1007/s40620-019-00622-1.
Mao S, Xiang W, Huang S, Zhang A. Association between homocysteine status and the risk of nephropathy in type 2 diabetes mellitus. Clin Chim Acta. 2014;431:206–10. https://doi.org/10.1016/j.cca.2014.02.007.
Cai C, Xiao R, Van Halm-Lutterodt N, et al. Association of MTHFR, SLC19A1 genetic polymorphism, serum folate, vitamin B12 and Hcy status with cognitive functions in Chinese adults. Nutrients. 2016;8(10):665. https://doi.org/10.3390/nu8100665.
Acknowledgements
We gratefully thank Dr. Jing Hu of Beijing Institute of Traditional Chinese Medicine for her contribution to the study design and comments regarding the manuscript.
Authors’ contributions
Zilong Shen contributed to the conception and design, wrote the manuscript, contributed to the research data discussion, reviewed the data and wrote the initial draft. Zhengmei Zhang contributed to the research data and editing. Wenjing Zhao contributed to the conception, design and critical review of the manuscript. The author(s) read and approved the final manuscript.
Funding
Natural Science Foundation of Beijing (7222271). Research and Cultivation Program of Beijing Municipal Hospitals (PZ20231016). The funding source had no involvement in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was reviewed and approved by the National Center for Health Statistics Research Ethics Review Board. Written informed consent was obtained from all participants for their participation in this study. The study protocol was performed in accordance with performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shen, Z., Zhang, Z. & Zhao, W. Relationship between plasma homocysteine and chronic kidney disease in US patients with type 2 diabetes mellitus: a cross-sectional study. BMC Nephrol 23, 419 (2022). https://doi.org/10.1186/s12882-022-03045-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-03045-6