Abstract
Background
Investigating the effect of metabolic disorders on chronic kidney disease (CKD) in the presence or the absence of obesity is of great importance. This study aimed to examine the independent and joint relationships of obesity and metabolic syndrome (MetS) with CKD.
Methods
The present study was performed on 9,762 participants from the baseline phase of the Ravansar non- communicable diseases (RaNCD) study. Thereafter, the CKD was estimated by glomerular filtration rate (eGFR) using the Modification of Diet in Renal Disease (MDRD) equation. All the included participants were categorized into the following four phenotypes: metabolically healthy non-overweight/obesity (MHNO), metabolically unhealthy non-overweight/obesity (MUNO), metabolically healthy overweight/obesity (MHO), and metabolically unhealthy overweight/obesity (MUO). Finally, Logistic regression analysis was used to estimate the odds ratio (ORs).
Results
The mean age of the included participants was 47.33 ± 8.27 years old, %48.16 (4,701) of whom were men. As well, 1,058(10.84%) participants had CKD (eGFR less than 60 ml/min/1.73m2). The overweight/obesity was not significantly associated with odds of CKD. The odds of CKD in male subjects with MetS was 1.48 times higher than non-MetS ones (95% CI: 1.10, 2.01). After adjusting the confounders, the odds of CKD were 1.54 times (95% CI: 1.12, 2.11) higher in the MUNO and 2.22 times (95% CI: 1.44, 3.41) higher in the MUO compared to MHNO phenotype in male subjects. The odds of CKD in the MUNO and MUO was 1.31 times (95% CI: 1.10, 1.60) and 1.23 times (95% CI: 1.01, 1.54) higher than MHNO phenotype in female subjects, respectively.
Conclusion
The odds of CKD were higher in MUNO and MUO phenotypes. Therefore, lifestyle modification is recommended to control normal weight and healthy metabolism.
Similar content being viewed by others
Introduction
The term chronic kidney disease (CKD) is used for a wide range of kidney diseases characterized by the gradual loss of functional nephrons [1]. The Global Burden of Disease (GBD) study in 2017 has reported that approximately 700 million people have CKD worldwide [2]. In a population-based study conducted on 30,041 Iranian (2021), the prevalence of CKD stage III + was estimated to be 7.1 and 5.5 based on Diet in Renal Disease (MDRD) and CKD Epidemiology Collaboration (CKD-EPI), respectively [3]. It is noteworthy that the CKD may eventually progress to end-stage renal disease (ESRD), in which patients survive if kidney substitutes such as dialysis or transplants are applied. Moreover, CKD can play important roles in morbidity and mortality in populations [4, 5]. The CKD-EPI and MDRD are the two widely-used, accurate methods for estimating GFR, which are applied in large and diverse populations [6, 7]. Therefore, the estimation of glomerular filtration rate (eGFR) based on endogenous filtration markers like serum creatinine, is often applied as a measure of general kidney function in both clinical and population-based studies [8,9,10].
Studies have previously reported different risk factors for CKD, including age, hypertension, low HDL serum, type 2 diabetes mellitus (T2DM), and central obesity (waist and hip circumferences) [11,12,13]. Among the above-mentioned risk factors, obesity is the most important factor. Accordingly, obesity is the cause of many non- communicable diseases (NCDs), which is also effective on the development of kidney disease [14, 15]. Although the mechanisms by which obesity causes the occurrence and exacerbation of CKD are still unknown, aligning obesity with metabolic risk factors and CVDs may partly help to identify the related mechanisms, and this requires performing extensive studies in diverse populations. Therefore, a direct cause of kidney disease should be clarified; either it is obesity or metabolic disorders caused by obesity [16, 17]. Scientific report suggested that obesity, due to its anti-inflammatory and oxidative stress effects, can lead to the development of dyslipidaemia, insulin resistance (IR), and other metabolic disorders, which in general metabolic unhealthy obesity (MUO) is considered [18, 19]. A retrospective cohort study performed on Japanese population (2015), indicated that the metabolically unhealthy obese (MUO) phenotype, unlike the metabolically healthy obese (MHO) phenotype, is associated with the risk of CKD [14]. Nevertheless, a study conducted on 42,128 adults in Pennsylvania found that metabolically healthy obesity (MHO) is associated with the increased risk of kidney disease, regardless of either the presence or the absence of metabolic unhealthy [20]. These contradictions may possibly be due to differences in populations, ethnicities, or sample sizes, which require further investigations. Therefore, the current study aimed to assess the association between metabolic obesity phenotypes and CKD among Iranian Kurdish adults.
Methods
Study design and population
This cross-sectional study was conducted using the baseline phase data obtained from the Ravansar non- communicable diseases (RaNCD) cohort study in 2021. Ravansar is a city with 50,000 populations, located in Kermanshah province, western Iran. Of note, the RaNCD cohort study is a 15-year prospective epidemiological study, the baseline phase of which was started in 2014. The sample size of the study was estimated as 10,047 people, which was established to study a prospective national group (PERSIAN)Footnote 1 in people aged between 35 and 65 years old in Ravansar. A detailed description of the design of RaNCD study has been published previously [21].
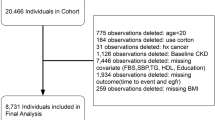
Of the total RaNCD participants, 285 cases were excluded from the present study due to the following reasons: participants with cancer (n = 81), pregnant women (n = 138), and subjects with incomplete information (n = 66). Finally, 9762 participants were included in the present study (Fig. 1).
Data collection and measurements
Demographic information of the participants was collected by the trained experts of RaNCD cohort centre using a digital questionnaire. Moreover, blood pressure, height, weight, and BMI were measured in terms of the cohort profile [21].
Socio-economic status (SES): The socio-economic status (SES) was created by 18 items using principal component analysis (PCA) method. Next, the SES was categorized in three groups ordered from the lowest to the highest one.
Smoking and alcohol consumption: Current smokers were people who reported they had smoked at least 100 cigarettes. Alcohol consumption is defined as drinking approximately 200 ml of beer OR 45 ml of alcohol, once per week for at least six months.
Physical activity: To assess physical activity, at first, a standard 22-item questionnaire of Ravansar cohort was categorized into the following three levels: low (24–36.5 MET/hour per day), medium (36.6–44.4 MET/hour per day), and high (≥ 44.5 MET/hour per day) according to Met/hour per day.
Nutritional information: The nutritional information and energy intake level were collected using Food Frequency Questionnaire (FFQ) with 118 items.
Biochemical measurements: After 12 h of fasting, biochemical markers, including creatinine (Cr), blood urea nitrogen (BUN), high-density lipoprotein Cholesterol (HDL-C), low-density lipoprotein Cholesterol (LDL-C), triglyceride (TG), Total cholesterol (T-C), fasting blood sugar (FBS), γ-glutamyltransferase (GGT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured.
Muscle strength: Muscle strength (Handgrip strength) was measured using a digital dynamometer (Model: Saehan, SH5003, Saehan Co, South Korea). Anthropometric indices, including weight, BMI, body fat mass (BFM), Fat free mass (FFM), and waist-to-hip ratio (WHR) were measured using bioelectric impedance InBody 770 model (Inbody Company, Seoul, Korea).
Obesity phenotypes
At this stage, general obesity was measured by BMI (kg/m2) and then classified into normal weight (BMI: 18.5–24.9), overweight (BMI: 25–29.9), and obesity (BMI: ≥ 30). Metabolic health was defined in terms of the International Diabetes Federation (IDF) criteria for MetS [22]. Subsequently, four phenotypes (BMI-MetS categories) were categorized based on BMI (normal weight, overweight/obese) and MetS (yes/no) as follows: (1) MHNO; MHO; MUNO; MUO.
Definitions
In the present study, the definitions were provided in terms of the RaNCD cohort study protocol, so that the participants with a history of hospitalization and/or treatment for one or more types of heart diseases such as stroke, Myocardial Infarction (MI), and coronary artery disease, and/or those who were consuming medications for these conditions, were considered as CVDs [23]. Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg and/or those who were using antihypertensive drugs [24]. Dyslipidaemia was defined as a disorder in lipid profile and/or a history of consuming medication for lipid disorders [25]. In addition, Type 2 diabetes mellitus (T2DM) was defined as Fasting Blood Sugar (FBS) > = 126 mg/dl and/or history of using medication for T2DM treatment [26]. CKD was estimated by eGFR using the Modification of Diet in Renal Disease (MDRD) equation [10] as follows:
The decreased kidney function was defined as eGFR less than 60 mL/min/1.73m2 in terms of the Kidney Disease Improving Global Outcomes criteria for CKD.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD), and categorical variables were presented as frequency (%). To compare demographic and biochemical characteristics among the four groups of phenotype obesity, one-way analysis of variance (ANOVA) was performed for continuous variables, and the Chi square test was done for categorical variables. The demographic, biochemical characteristics, and comorbidity were compared by t-test between the two groups of eGFR (eGFR less than 60 and equal to or more than 60 ml/min/1.73m2) for continuous variables. Additionally, the Chi square test was used for categorical variables. Moreover, logistic regression analysis was performed to determine the association between phenotype obesity and CKD. Regression models were also adjusted for potentially confounding variables, including age, SES, physical activity, smoking, alcohol use, and CVD. The estimations were presented with 95% confidence interval and P < 0.05. All these analyses were done using STATA software version 14.2 (Stata Corp, College Station, Tex).
Results
Table 1 presents the baseline demographic, clinical, biochemical, and nutritional characteristics of the included participants according to eGFR groups. A total of 9,762 participants with a mean age of 47.33 ± 8.27 years old were studied in this research. Correspondingly, %48.16 (4701) of the participants were men, and %11.72 (1138) of them were current smokers. The prevalence rates of hypertension and CVD were significantly lower in the participants with eGFR equal to or more than 60 compared to the less than 60 ml/min/1.73 m2 (P < 0.001). The means of intake levels of vitamin D and B12 were significantly higher in the participants with eGFR equal to or more than 60 compared to less than 60 ml/min/1.73 m2. Table 2 presents the baseline demographic, clinical, biochemical, and nutritional characteristics of the participants based on obesity phenotype categories. The prevalence rates of kidney stones (P = 0.007) and CVDs (P < 0.001) in the MUNO and MUO groups were significantly higher than those of the MHNO and MHO groups. The mean eGFR was found to be significantly different in these four groups, which was higher in the MHNO group compared to the other three groups (P < 0.001). Notably, the means of creatinine, BUN, liver enzymes, sodium, energy intake, and percentage of energy intake from protein and lipid in the four groups of obesity phenotypes were statistically significant (P < 0.05).
Table 3 shows the associations of obesity, MetS, and obesity phenotypes with CKD. After adjusting potential confounders, overweight/obesity was observed to be associated with higher risk of CKD in male subjects (OR = 1.10; 95% CI: 0.78, 1.43); however, this association was not statistically significant. The odds of CKD in male participants with MetS was 88% higher than the odds in non-MetS ones (OR: 1.88; 95% CI: 1.43, 2.45). Accordingly, this association remained significant after adjusting some factors, including age, SES, physical activity, smoking, alcohol use, sodium intake, CVD, GGT, ALT, and AST. The odds of CKD in the MUNO and MUO were 1.70 and 2.20 times higher than those of MHNO phenotype in male cases, respectively. After adjusting the confounders, the odds of CKD in the MUNO and MUO were 1.54 times (95% CI: 1.12, 2.11) and 2.22 times (95% CI: 1.44, 3.41) higher than those of MHNO phenotype in male subjects, respectively. After adjusting the confounders, the odds of CKD in the MUNO and MUO were 1.31 times (95% CI: 1.10, 1.60) and 1.23 times (95% CI: 1.01, 1.54) higher than those of MHNO phenotype in female cases, respectively.
Figure 2 shows the prevalence of MetS components in the four groups defined based on obesity phenotype. Correspondingly, the WC was high in the healthy and unhealthy groups. Moreover, the prevalence rates of all the components of MetS were higher in the metabolically unhealthy groups (MUNO and MUO) compared to the metabolically healthy groups (MHNO and MHO).
Discussion
In this population-based study on Kurdish adults, the finding showed that MetS was independently associated with higher risk of CKD in male cases. Accordingly, this association remained significant after adjusting potentially confounding variables. In contrast, overweight/obesity had no significant association with higher risk of CKD.
In this regard, previous research has identified overweight/Obesity and MetS as risk factors for CKD, and reported that after controlling the effect of gender, a significant association existed between overweight/Obesity and MetS and an increased risk of CDK [20, 27, 28]. Moreover, in a study by Jay et al., it was shown that the components of the MetS, including disorders of FBS, lipid profile, blood pressure, and central obesity are correlated with low eGFR [27]. However, in this study, we examined the risk of CKD by gender using subgroup analysis, and it was indicated that MetS are associated with odds of CKD, which was not significant in female cases. One of the reasons for the difference between male and female subjects may be the hormones’ effects, which were not measured in this study.
The mechanism of the association between overweight/Obesity and MetS and kidney function is not exactly known yet. Accordingly, one possible mechanism is the number of nephrons, which is determined at birth time, but as weight increases, the GFR of a single nephron must increase as well, in order to keep pace with metabolic needs. According to this hypothesis, individuals born with the lowest number of nephrons will have the highest risk of glomerular hypertrophy if they become obese. This may be due to the reason that obesity requires an additional burden on the nephron, which promotes kidney dysfunction [29, 30]. In addition, MetS, through insulin resistance, leads to the development of a pre-inflammatory condition in the body. As well, the plasma concentration of some pro-inflammatory adipokines increases in MetS patients, while that of other anti-inflammatory adipokines decreases, contributing to the development and progression of kidney diseases [31, 32].
According to the performed analysis, the joint effect of both obesity and MetS as obesity phenotypes was observed, and the odds of CKD in the MUNO and MUO phenotype were found to be significantly higher than those of MHNO phenotype in both male and female cases. Therefore, we conclude that in populations with Kurdish ethnicity, MetS with overweight/Obesity (MetS-obesity) can independently and simultaneously be considered as a risk factor for CKD. In consistent with the findings of our study, a study by Chou et al. showed that Korean female with the MANW phenotype (metabolically abnormal normal weight) are at greater risk for early renal function decline compared to the MHNW (metabolically-healthy normal weight) phenotype [27]. A prospective cohort study on 41,194 Korean adults reported that the risk of CDK was significantly higher in individuals with the MHO and MUNO phenotypes [33]. Moreover, another cohort study of 6,852 Chinese adults with 5-year follow-up reported that both MHO and MUNO phenotypes were associated with the increased risk of CKD [34]. As well, in a prospective study conducted on 3,136 Japanese adults, it was shown that MHO and MUNO phenotypes were not associated with higher risk of CKD [14]. The reasons for this discrepancy may possibly be the low number of new cases, participants underweight in the reference group (MHNO), and the incomplete adjustment of important confounders in the analysis.
The mechanisms of this process have not been identified yet. However, it is assumed that complex pathophysiological factors such as adipocytokines, insulin resistance, renin–angiotensin–aldosterone activation, endothelial dysfunction, and oxidative stress, play a role in this association [35, 36]. The importance of diet and lifestyle (smoking, alcohol use, and physical activity) in kidney function, obesity, and MetS is undeniable, so their effects were adjusted in the analysis.
The most important strengths of the present study are its large sample size and controlling a great number of potential confounders. To the best of our knowledge, this is the first study conducted on Iranian adults with Kurdish ethnicity. Due to the cross-sectional nature of the study, it was not possible to investigate the causal association, which was one of the limitations of the study. Another limitation was that hormones were not measured and their effects on the obesity phenotype and kidneys function were not controlled. However, to prove the generalizability of the results, it is necessary to conduct further research in different regions and with different dietary patterns.
Conclusion
The present study demonstrated that MetS are independently associated with higher odds of CKD in male cases. In contrast, overweight/obesity was found to be independently, but not significantly, associated with higher risk of CKD. According to the analysis, the joint effect of metabolic obesity phenotype was observed, and the odds of CKD in the MUNO and MUO phenotypes were significantly higher than those of MHNO phenotype in male and female cases. However, we conclude that in populations with Kurdish ethnicity, MetS with overweight/Obesity (MetS-obesity) can independently and simultaneously be considered as a risk factor for CKD. Therefore, lifestyle modification is recommended to control normal weight and healthy metabolism.
Availability of data and materials
The data sets generated during this study are available from the correspondence author on reasonable request via email.
Notes
Prospective Epidemiological Research Studies in Iran.
Abbreviations
- CKD:
-
Chronic kidney disease
- MetS:
-
Metabolic syndrome
- RaNCD:
-
Ravansar Non-Communicable Diseases
- GFR:
-
Glomerular filtration rate
- MDRD:
-
Modification of Diet in Renal Disease
- MHNO:
-
Metabolically healthy non-overweight/obesity
- MUNO:
-
Metabolically unhealthy non-overweight/obesity
- MHO:
-
Metabolically healthy overweight/obesity
- MUO:
-
Metabolically unhealthy overweight/obesity
- CI:
-
Confidence interval
- SD:
-
Standard deviation
- CKD-EPI:
-
CKD Epidemiology Collaboration
- ESRD:
-
Eventually progress to end-stage renal disease
- T2DM:
-
Type 2 diabetes mellitus
- NCDs:
-
Non-communicable diseases
- SES:
-
Socio-economic status
- TG:
-
Triglyceride
- LDL-C:
-
Low-density lipoprotein Cholesterol
- HDL-C:
-
High-density lipoprotein Cholesterol
- T-C:
-
Total cholesterol; FBS: Fasting blood sugar
- GBD:
-
Global Burden of Disease
- CVDs:
-
Cardiovascular disease
- IR:
-
Incidence rate
- PERSIAN:
-
Prospective Epidemiological Research Studies in Iran
- FFQ:
-
Food frequency questionnaire
- BP:
-
Blood pressure
- DBP:
-
Diastolic Blood Pressure
- SBP:
-
Systolic Blood Pressure
- PCA:
-
Principal component analysis
- GGT:
-
γ-Glutamyltransferase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- BFM:
-
Body fat mass
- FFM:
-
Fat free mass
- WHR:
-
Waist-to-hip ratio
References
Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. The Lancet. 2017;389(10075):1238–52.
Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2020;395(10225):709–33.
Alvand S, Abolnezhadian F, Alatab S, Mohammadi Z, Hayati F, Noori M, et al. Prevalence of impaired renal function and determinants in the southwest of Iran. BMC Nephrol. 2021;22(276):1–10.
Saeed F, Arrigain S, Schold JD, Nally JV Jr, Navaneethan SD. What are the Risk Factors for One-Year Mortality in Older Patients with Chronic Kidney Disease? An Analysis of the Cleveland Clinic CKD Registry. Nephron. 2018;141(2):98–104.
van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–52.
Eknoyan G, Lameire N, Eckardt K, Kasiske B, Wheeler D, Levin A, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(1):5–14.
Levey AS. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann Intern Med. 1999;130(6):461.
Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am J Kidney Dis. 2014;63(5):713–35.
Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009;150(9):604.
Zabell JR, Larson G, Koffel J, Li D, Anderson JK, Weight CJ. Use of the Modification of Diet in Renal Disease Equation for Estimating Glomerular Filtration Rate in the Urologic Literature. J Endourol. 2016;30(8):930–3.
Khajehdehi P, Malekmakan L, Pakfetrat M, Roozbeh J, Sayadi M. Prevalence of Chronic Kidney Disease and Its Contributing Risk Factors in Southern Iran A Cross-sectional Adult Population-based Study. Iran J Kidney Dis. 2014;8(2):109–15.
Najafi I, Attari F, Islami F, Shakeri R, Malekzadeh F, Salahi R, et al. Renal function and risk factors of moderate to severe chronic kidney disease in Golestan Province, northeast of Iran. PLoS One. 2010;5(12):e14216-e.
Sepanlou SG, Barahimi H, Najafi I, Kamangar F, Poustchi H, Shakeri R, et al. Prevalence and determinants of chronic kidney disease in northeast of Iran: Results of the Golestan cohort study. PLoS ONE. 2017;12(5):e0176540.
Hashimoto Y, Tanaka M, Okada H, Senmaru T, Hamaguchi M, Asano M, et al. Metabolically healthy obesity and risk of incident CKD. Clin J Am Soc Nephrol. 2015;10(4):578–83.
Noori N, Hosseinpanah F, Nasiri AA, Azizi F. Comparison of Overall Obesity and Abdominal Adiposity in Predicting Chronic Kidney Disease Incidence Among Adults. J Ren Nutr. 2009;19(3):228–37.
Chen S, Zhou S, Wu B, Zhao Y, Liu X, Liang Y, et al. Association between metabolically unhealthy overweight/obesity and chronic kidney disease: The role of inflammation. Diabetes Metab. 2014;40(6):423–30.
Hanks LJ, Tanner RM, Muntner P, Kramer H, McClellan WM, Warnock DG, et al. Metabolic subtypes and risk of mortality in normal weight, overweight, and obese individuals with CKD. Clin J Am Soc Nephrol. 2013;8(12):2064–71.
Cӑtoi AF, Pârvu AE, Andreicuț AD, Mironiuc A, Crӑciun A, Cӑtoi C, et al. Metabolically Healthy versus Unhealthy Morbidly Obese: Chronic Inflammation, Nitro-Oxidative Stress, and Insulin Resistance. Nutrients. 2018;10(9):1199.
Kim Y, Chang Y, Cho YK, Ahn J, Shin H, Ryu S. Metabolically healthy versus unhealthy obesity and risk of fibrosis progression in non-alcoholic fatty liver disease. Liver Int. 2019;39(10):1884–94.
Chang AR, Surapaneni A, Kirchner HL, Young A, Kramer HJ, Carey DJ, et al. Metabolically Healthy Obesity and Risk of Kidney Function Decline. Obesity (Silver Spring). 2018;26(4):762–8.
Pasdar Y, Najafi F, Moradinazar M, Shakiba E, Karim H, Hamzeh B, et al. Cohort Profile: Ravansar Non-Communicable Disease cohort study: the first cohort study in a Kurdish population. Int J Epidemiol. 2019;48(3):682–3f.
Alberti KGMM, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. The Lancet. 2005;366(9491):1059–62.
Moradinazar M, Samadi M, Hamzeh B, Najafi F, Karimi S, Faraji F, et al. Association of Dietary Inflammatory Index with cardiovascular disease in Kurdish adults: results of a prospective study on Ravansar non-communicable diseases. BMC Cardiovasc Disord. 2020;20(1):1–8.
Rajati F, Hamzeh B, Pasdar Y, Safari R, Moradinazar M, Shakiba E, et al. Prevalence, awareness, treatment, and control of hypertension and their determinants: Results from the first cohort of non-communicable diseases in a Kurdish settlement. Sci Rep. 2019;9(1):12409.
Rezaei M, Fakhri N, Pasdar Y, Moradinazar M, Najafi F. Modeling the risk factors for dyslipidemia and blood lipid indices: Ravansar cohort study. Lipids Health Dis. 2020;19(1):176.
Safari-Faramani R, Rajati F, Tavakol K, Hamzeh B, Pasdar Y, Moradinazar M, et al. Prevalence, Awareness, Treatment, Control, and the Associated Factors of Diabetes in an Iranian Kurdish Population. J Diabetes Res. 2019;2019:5869206.
Choi JI, Cho YH, Lee SY, Jeong DW, Lee JG, Yi YH, et al. The Association between Obesity Phenotypes and Early Renal Function Decline in Adults without Hypertension, Dyslipidemia, and Diabetes. Korean J Fam Med. 2019;40(3):176–81.
Wang Y, Sun B, Sheng L-T, Pan X-F, Zhou Y, Zhu J, et al. Association between weight status, metabolic syndrome, and chronic kidney disease among middle-aged and elderly Chinese. Nutr Metab Cardiovasc Dis. 2020;30(11):2017–26.
Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and Risk for Chronic Renal Failure. J Am Soc Nephrol. 2006;17(6):1695–702.
Wickman C, Kramer H. Obesity and Kidney Disease: Potential Mechanisms. Semin Nephrol. 2013;33(1):14–22.
Prasad GVR. Metabolic syndrome and chronic kidney disease: Current status and future directions. World J Nephrol. 2014;3(4):210–9.
Wisse BE. The Inflammatory Syndrome: The Role of Adipose Tissue Cytokines in Metabolic Disorders Linked to Obesity. J Am Soc Nephrol. 2004;15(11):2792–800.
Jung CH, Lee MJ, Kang YM, Hwang JY, Kim EH, Park J-Y, et al. The risk of chronic kidney disease in a metabolically healthy obese population. Kidney Int. 2015;88(4):843–50.
Cao X, Zhou J, Yuan H, Wu L, Chen Z. Chronic kidney disease among overweight and obesity with and without metabolic syndrome in an urban Chinese cohort. BMC Nephrol. 2015;16(1):1–9.
Nashar K, Egan BM. Relationship between chronic kidney disease and metabolic syndrome: current perspectives. Diabetes Metab Syndr Obes. 2014;7:421–35.
Slee AD. Exploring metabolic dysfunction in chronic kidney disease. Nutr Metab (Lond). 2012;9(1):36.
Acknowledgements
The authors thank the PERSIAN cohort Study collaborators and of Kermanshah University of Medical Sciences.The Iranian Ministry of Health and Medical Education has also contributed to the funding used in the PERSIAN Cohort through Grant no 700/534.
Funding
This research was supported by Kermanshah University of Medical Sciences (grant number: 92472).
Author information
Authors and Affiliations
Contributions
YP and SAJ designed the study. MD and FN conducted data analyses and interpreted the results. SAJ and BM drafted the manuscript, and all authors revised it critically for important intellectual content and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Kermanshah University of Medical Sciences (KUMS.REC.1394.318). All methods were carried out in accordance with relevant guidelines and regulations. All the participants were provided oral and written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jam, S.A., Moloudpour, B., Najafi, F. et al. Metabolic obesity phenotypes and chronic kidney disease: a cross-sectional study from the RaNCD cohort study. BMC Nephrol 23, 233 (2022). https://doi.org/10.1186/s12882-022-02858-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02858-9