Abstract
Background
Galloway-Mowat syndrome (GAMOS) is a rare autosomal recessive disorder characterized by early-onset nephrotic syndrome and microcephaly with brain anomalies. WDR73 pathogenic variants were described as the first genetic cause of GAMOS and, very recently, four novel causative genes, OSGEP, LAGE3, TP53RK, and TPRKB, have been identified.
Case presentation
We present the clinical and genetic characteristics of two unrelated infants with clinical suspicion of GAMOS who were born from consanguineous parents. Both patients showed a similar clinical presentation, with early-onset nephrotic syndrome, microcephaly, brain atrophy, developmental delay, axial hypotonia, and early fatality. We identified two novel likely disease-causing variants in the OSGEP gene. These two cases, in conjunction with the findings of a literature review, indicate that OSGEP pathogenic variants are associated with an earlier onset of nephrotic syndrome and shorter life expectancy than WDR73 pathogenic variants.
Conclusions
Our findings expand the spectrum of pathogenic variants in the OSGEP gene and, taken in conjunction with the results of the literature review, suggest that the OSGEP gene should be considered the main known monogenic cause of GAMOS. Early genetic diagnosis of GAMOS is of paramount importance for genetic counseling and family planning.
Similar content being viewed by others
Background
Galloway-Mowat syndrome (GAMOS) (OMIM #251300) is a rare autosomal recessive syndrome first described in 1968. It is a clinically heterogeneous condition characterized by early-onset nephrotic syndrome associated with microcephaly, gyral abnormalities of the brain, and delayed psychomotor development. Most patients also present dysmorphic facial features, including hypertelorism, ear abnormalities, and micrognathia. Most affected individuals die in early childhood [1, 2]. The estimated prevalence is < 1/1,000,000 but it is likely that many cases remain misdiagnosed or undiagnosed.
Truncating variants in WDR73 gene were described as the first monogenic cause of GAMOS in two families [3]. The protein encoded by this gene is implicated in the regulation of the microtubule network during cell cycle progression, proliferation, and survival [3,4,5]. Homozygous missense variants in the WDR73 gene were later reported [6, 7]. Recently, pathogenic variants in the OSGEP, LAGE3, TP53RK, and TPRKB genes have been identified as novel monogenic causes of GAMOS. These genes encode four subunits of the evolutionary highly conserved KEOPS (kinase, endopeptidase, and other proteins of small size) complex. This complex plays an important role in brain and renal development. To date, the genetic cause of GAMOS has been reported in 54 families: OSGEP in 26 (48.15%), WDR73 in 19 (35.18%), TP53RK in 4 (7.40%), LAGE3 in 3 (5.55%), and TPRKB in 2 (3.70%) [2,3,4,5,6,7,8,9,10,11].
Here, we report two unrelated patients with GAMOS carrying homozygous pathogenic variants in the newly identified OSGEP gene.
Case presentation
Patient 1 was a first-child male born to healthy consanguineous parents from Spain with no previous family history of kidney disease (Table 1, Fig. 1A). He was born at 39.4 weeks of gestation. Birth weight was 3400 g, height was 51 cm, and head circumference was 34 cm, with no dysmorphic features. Forty-five days after birth, the patient was diagnosed as having congenital nephrotic syndrome with severe proteinuria, hypertension, and hypothyroidism. He also presented edema, hyperkalemia, hyponatremia, and hypomagnesemia. Renal ultrasound showed poor corticomedullary differentiation in the right kidney. Renal biopsy showed diffuse mesangial sclerosis, tubular atrophy, and primitive glomeruli (Fig. 1C). The patient progressed to end-stage renal disease and required peritoneal dialysis. He also presented left eye evisceration, dry right eye, and gastroesophageal reflux. At 5 months of age, the neurological examination revealed microcephaly with a head circumference of 38.5 cm (percentile < 1, − 4.72 SD), severe psychomotor delay for his age, and axial hypotonia. Cranial magnetic resonance imaging (CMRI) revealed brain atrophy and absence of normal myelination of the brainstem, cerebellar white matter, bilateral hemispheric white matter, internal capsules, and corpus callosum as well as abnormal intensity signal in the dentate nucleus and thalamus (Fig. 1D). In view of the congenital nephrotic syndrome and microcephaly with brain anomalies, a clinical diagnosis of GAMOS was suspected. The patient presented progressive neurological deterioration and died at 8 months of age.
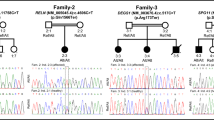
a Pedigree of patient 1 with a likely pathogenic OSGEP variant, c.81C > G p.(Asn27Lys), in homozygosity while his consanguineous parents are healthy heterozygous carriers. b The identified missense variant c.81C > G p.(Asn27Lys) affects a totally conserved amino acid N27 in OSGEP orthologs. c Silver-stained renal biopsy from patient 1 showed glomerular collapse with mesangial matrix increase, atrophic tubules, and interstitial fibrosis on light microscopy. d CMRI performed in patient 1 at 8 months of age: sagittal 3 Dimensional Imaging T1 sequence (a), axial reconstructions (b–e), and axial Turbo Spin Echo (TSE) T2 (f–j). k–o: Sequence TSE T2 of normal control individual. MRI revealed craniofacial disproportion in relation to microcephaly (a); supratentorial cortico-subcortical atrophy with increased extra-axial space, prominence of the frontal horns, and thinning of the corpus callosum (red arrow in a); bilateral subdural frontoparietal hygromas (red asterisks in h–j); and atrophy of the basal ganglia (h). A decrease in the number and depth of the grooves was observed (h–j) and there was an absence of normal myelination of the brainstem (red arrow in f), cerebellar peduncles (blue arrow in f), internal capsules (red arrow in h), and white bihemispheric substance (arrows in i and j). Hypointense T2 signal of the thalamus was evident (blue arrow in h). Enucleation of the left eye is denoted by the yellow arrow in f. Finally, there was an increase in the thickness of the cranial and facial subcutaneous cellular tissue (green arrows in a and b)
Patient 2 was a female infant with normal karyotype (46, XX) born to healthy consanguineous parents from Pakistan. The patient had two healthy sisters and there was no family history of kidney disease (Table 1, Fig. 2a). She was born at 40.3 weeks of gestation. Birth weight was 2940 g, height was 49 cm, and head circumference was 32 cm with signs of microcephaly. Screening for metabolic disorders and cerebral ultrasound were normal. The patient presented dysmorphic features (wide nasal bridge, aquiline nose and retrognathia, low set ears, and arachnodactyly) with axial hypotonia and poor eye contact. Seventy-five days after birth, she presented with nephrotic range proteinuria, hypoproteinemia with severe hypoalbuminemia, hypertriglyceridemia and hypercholesterolemia. Serum creatinine and urea were normal. Abdominal ultrasound showed normal-sized kidneys and correct corticomedullary differentiation with cortical hyperechogenicity, bilateral pleural effusion, and a discrete amount of fluid in the abdominal cavity. Renal biopsy showed one glomerulus with increased mesangial matrix and two normal glomeruli; fibrosis and tubular atrophy were absent. CMRI revealed severe brain atrophy with normal cerebellum and brainstem. Electroencephalogram showed normal brain activity with low-amplitude brain waves and occasional frontal left epileptiform activity. The patient evolved with failure to thrive, anemia, and electrolyte disorders and finally died from cardiorespiratory arrest in a sepsis context at 7 months of age.
Genetic study
Variant analyses of patients 1 and 2 were performed by targeted massive parallel sequencing using an updated version of our kidney disease gene panel that includes more than 200 genes causative of or associated with inherited kidney diseases (including WDR73, TPRKB, TP53RK, LARGE3, and OSGEP genes) [12].
Briefly, libraries were prepared according to the manufacturer’s standard protocol, NimbleGen SeqCap EZ Library SR version 4.3. Patients’ DNAs were fragmented and hybridized to the custom NimbleGen SeqCap EZ Choice gene panel and sequenced on a NextSeq 500 instrument (Illumina). Sequence data analysis was performed using an open-source in-house bioinformatic pipeline, as previously reported [12,13,14]. The mean depth of coverage per exon of OSGEP ranged from 153 to 433, with 100% of the bases covered at least 100X. Prediction of pathogenicity was evaluated using different bioinformatic algorithms (DANN, GERP, dbNSFP.FATHMM, LRT, MetaLR, MetaSVM, MutationAssessor, PROVEAN, SIFT, and MutationTaster). Clinical interpretation of variants was based on American College of Medical Genetics (ACMG) recommendations [15]. All candidate pathogenic variants were validated by conventional polymerase chain reaction amplification and Sanger sequencing. Familial segregation analysis was assessed. Analysis of copy number variations (CNVs) was performed using CoNVaDING (copy number variation detection in next-generation sequencing gene panels) software [16].
Patient 1 carried a homozygous missense variant c.81C > G p.(Asn27Lys) in exon 1 of the OSGEP gene (NM_017807), not previously described in the literature. This variant was predicted to be pathogenic by seven prediction tools (DANN, GERP, LRT, MutationAssessor, MutationTaster, SIFT, and PROVEAN) and benign by three (dbNSFP.FATHMM, MetaLR, and MetaSVM). This variant altered an evolutionarily highly conserved residue and was absent from the population databases Genome Aggregation Database (gnomAD) and 1000 Genomes (Fig. 1B). Segregation analysis showed that both parents were heterozygous carriers of this OSGEP variant (Fig. 1A). We concluded that this variant was likely pathogenic (Table 2).
Patient 2 carried a homozygous missense variant c.157A > T p.(Ile53Phe) localized in exon 2 of the OSGEP gene. This variant has not been previously reported in literature. The variant c.157A > T p.(Ile53Phe) was predicted to be pathogenic by five prediction tools (DANN, LRT, MutationAssessor, MutationTaster, and PROVEAN) and benign by five (GERP, dbNSFP.FATHMM, MetaLR, SIFT, and MetaSVM). This variant was conserved in OSGEP orthologs to C. elegans and is extremely rare in the general population (Fig. 2b), with a minor allele frequency in South Asians is 0.00009799 (3 of 30,782 sequenced alleles, no homozygous individuals) in the gnomAD database. The global allele frequency was lower than the 0.0001 threshold for recessive gene OSGEP. The parents were confirmed to be heterozygous carriers (Fig. 2a). We classified this variant as likely pathogenic (Table 2).
Discussion and conclusions
We report two patients who presented with nephrotic syndrome with onset at < 3 months old, primary microcephaly, and developmental delay, which are hallmarks of GAMOS. Both patients carried homozygous likely disease-causing variants in the OSGEP gene. This gene was recently identified as causative of GAMOS in a large cohort of 907 individuals with nephrotic syndrome [2]. Pathogenic variants in one of the four genes TP53RK, TPRKB, LAGE3, and OSGEP, encoding KEOPS complex subunits, were found in 37 out of 91 patients with GAMOS. Independently, a homozygous pathogenic variant in the OSGEP gene was reported in two siblings with a similar renal-neurological phenotype, also by whole exome sequencing [8].
The OSGEP gene encodes the O-sialoglycoprotein endopeptidase enzyme, which regulates the second biosynthetic step in the formation of N-6-threonylcarbamoyladenosine in the cytosol, essential for mRNA translational initiation and efficiency. The highly conserved KEOPS complex is implicated in several cell processes, such as control of telomere length, telomere-associated DNA damage response signaling, and genome maintenance. Zebrafish larvae knockout of the osgep gene resulted in primary microcephaly, with increased apoptosis in the brain compared with controls and early lethality. Knockout mouse embryos also showed microcephaly compared with wild-type embryos. Neither mutant fish nor mice showed any renal phenotype, possibly due to embryonic early lethality [2].
Great strides have been made in the understanding of GAMOS disease over the past 4 years, with the identification of its genetic bases in some patients. However, the genetic etiology of more than three-quarters of patients with a clinical diagnosis of GAMOS remains elusive, suggesting that additional causative genes remain to be identified. Currently, the principal known causative genes of GAMOS are OSGEP and WDR73.
A review of the literature based on 31 patients (26 families) bearing OSGEP pathogenic variants and 23 patients (13 families) with WDR73 pathogenic variants indicates that OSGEP causes earlier onset of nephrotic syndrome than WDR73 [2,3,4, 6,7,8,9,10]. Eighty percent (25/31) of patients with OSGEP pathogenic variants developed nephrotic syndrome with a mean age at onset of 10.36 months (ranging onset from birth to 13 years). In comparison, 35% (8/23) of patients with WDR73 pathogenic variants presented nephrotic syndrome at a mean age of 7.7 years (ranging from 0.5 to 16 years). Our two patients carrying OSGEP pathogenic variants presented with nephrotic syndrome before 3 months of age.
Renal manifestations described in GAMOS patients vary from isolated proteinuria to steroid-resistant nephrotic syndrome, and some patients even have no renal alterations during follow-up period [2,3,4,5,6,7,8]. Intrafamilial clinical variability has also been described in GAMOS. For instance, two siblings carrying a WDR73 pathogenic variant manifested contrasting renal phenotype [3]. One of the affected siblings presented with nephrotic syndrome at the age of 5 years, rapidly developed chronic renal insufficiency, and died after a month, while the other had no renal symptoms at the age of 7 years [3]. A homozygous OSGEP pathogenic variant, c.974A > G p.(Arg325Gln), has also been associated with renal tubular anomalies [10]. It was detected in a girl with magnesium-wasting tubulopathy and partial Fanconi syndrome with a normal glomerular filtration rate who never developed nephrotic syndrome [10]. Interestingly, this variant was previously identified in two siblings with severe hypomagnesemia, hypercalciuria, and proteinuria but normal albumin levels [8]. The authors raised the question of whether these patients should be considered to be affected by a different clinical entity [8, 10].
The review of the literature also indicates that patients with OSGEP pathogenic variants have a shorter life expectancy than those with WDR73 pathogenic variants. Seventy-one percent (22/31) of patients with OSGEP pathogenic variants died at a mean age of 1.5 years (ranging from 6 weeks to 8 years). In line with these reported cases, our patients died at 8 and 7 months of age. However, seven patients with OSGEP pathogenic variants were alive at 13 (2), 10.5 (1), 7 (1), 3.5 (1), and 2 (1) years and at 7 (1) months [2, 10]. It should be noted that four of them carried the above-mentioned OSGEP variant, c.974A > G p.(Arg325Gln), associated with renal tubular anomalies [10]. Twenty-two percent (5/23) of patients carrying pathogenic variants in the WDR73 gene died at a mean age of 8.1 years (ranging from 2.5 to 17 years).
Nearly all OSGEP variants reported as causative of GAMOS are missense, except for two splicing variants [2, 8]. These variants are located throughout the OSGEP gene. By contrast, different types of variant in WDR73 causative of GAMOS have been reported in the literature, including nonsense (3), frameshift (3), and missense (4). No correlation seems to exist between the type or position of the variant and particular clinical features. Identification of the causative pathogenic variant in patients 1 and 2 confirmed the initial clinical suspicion of GAMOS and allowed precise genetic counseling to their parents. In particular, it allowed prenatal diagnosis of a baby girl without GAMOS for the parents of patient 1.
In conclusion, we report two patients with GAMOS caused by OSGEP pathogenic variants. These two cases, in conjunction with the reported cases in the literature, add evidence that OSGEP pathogenic variants are the most prevalent cause of GAMOS and are associated with a more severe phenotype than WDR73 pathogenic variants. For these reasons, OSGEP variant analysis should be considered as the first step in genetic diagnosis of patients with clinical suspicion of GAMOS; this is especially true for those labs that do not perform massive parallel sequencing and for those cases with early and severe onset of the disease. Genetic diagnosis of GAMOS is of paramount importance for genetic counseling and family planning and allows prenatal or preimplantation genetic diagnosis for future pregnancies.
Abbreviations
- CMRI:
-
Cranial magnetic resonance imaging
- DMS:
-
Diffuse mesangial sclerosis
- GAMOS:
-
Galloway-Mowat syndrome
- gnomAD:
-
Genome Aggregation Database
- KEOPS:
-
Kinase, endopeptidase and other proteins of small size
- TSE:
-
Turbo Spin Echo
References
Galloway WH, Mowat AP. Congenital microcephaly with hiatus hernia and nephrotic syndrome in two sibs. J Med Genet. 1968;5:319–21.
Braun DA, Rao J, Mollet G, Schapiro D, Daugeron MC, Tan W, et al. Mutations in KEOPS-complex genes cause nephritic syndrome with primary microcephaly. Nat Genet. 2017;49:1529–38.
Colin E, Huynh Cong E, Mollet G, Guichet A, Gribouval O, Arrondel C, et al. Loss-of-function mutations in WDR73 are responsible for microcephaly and steroid-resistant nephrotic syndrome: Galloway-mowat syndrome. Am J Hum Genet. 2014;95:637–48.
Ben-Omran T, Fahiminiya S, Sorfazlian N, Almuriekhi M, Nawaz Z, Nadaf J, et al. Nonsense mutation in the WDR73 gene is associated with Galloway-Mowat syndrome. J Med Genet. 2015;52:381–90.
Jinks RN, Puffenberger EG, Baple E, Harding B, Crino P, Fogo AB, et al. Recessive nephrocerebellar syndrome on the Galloway-Mowat syndrome spectrum is caused by homozygous protein-truncating mutations of WDR73. Brain. 2015;138:2173–90.
Vodopiutz J, Seidl R, Prayer D, Khan MI, Mayr JA, Streubel B, et al. WDR73 mutations cause infantile neurodegeneration and variable glomerular kidney disease. Hum Mutat. 2015;36:1021–8.
Rosti RO, Dikoglu E, Zaki MS, Abdel-Salam G, Makhseed N, Sese JC, et al. Extending the mutation spectrum for Galloway-Mowat syndrome to include homozygous missense mutations in the WDR73 gene. Am J Med Genet Part A. 2016;170:992–8.
Edvardson S, Prunetti L, Arraf A, Haas D, Bacusmo JM, Hu JF, et al. TRNA N6-adenosine threonylcarbamoyltransferase defect due to KAE1/TCS3 (OSGEP) mutation manifest by neurodegeneration and renal tubulopathy. Eur J Hum Genet. 2017;25:545–51.
Al-Rakan MA, Abothnain MD, Alrifai MT, Alfadhel M. Extending the ophthalmological phenotype of Galloway-Mowat syndrome with distinct retinal dysfunction: a report and review of ocular findings. BMC Ophthalmol. 2018;18:4–7.
Wang PZT, Prasad C, Rodriguez Cuellar CI, Filler G. Nephrological and urological complications of homozygous c . 974G > A (p . Arg325Gln) OSGEP mutations. 2018;:10–3.
Hyun HS, Kim SH, Park E, Cho MH, Kang HG, Lee HS, et al. A familial case of Galloway-Mowat syndrome due to a novel TP53RK mutation: a case report. BMC Med Genet. 2018;19:2–7.
Bullich G, Domingo-Gallego A, Vargas I, Ruiz P, Lorente-Grandoso L, Furlano M, et al. A kidney-disease gene panel allows a comprehensive genetic diagnosis of cystic and glomerular inherited kidney diseases. Kidney Int. 2018;94:363–71.
Trujillano D, Bullich G, Ossowski S, Ballarín J, Torra R, Estivill X, et al. Diagnosis of autosomal dominant polycystic kidney disease using efficient PKD1 and PKD2 targeted next-generation sequencing. Mol Genet Genomic Med. 2014;2:412–21. https://doi.org/10.1002/mgg3.82.
Bullich G, Trujillano D, Santín S, Ossowski S, Mendizábal S, Fraga G, et al. Targeted next-generation sequencing in steroid-resistant nephrotic syndrome: mutations in multiple glomerular genes may influence disease severity. Eur J Hum Genet. 2015;23:1192–9.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405.
Johansson LF, van Dijk F, de Boer EN, van Dijk-Bos KK, Jongbloed JDH, van der Hout AH, et al. CoNVaDING: single exon variation detection in targeted NGS data. Hum Mutat. 2016;37:457–64.
Acknowledgements
We thank the patients’ families for taking part in this study and the referring physicians who participated in this study, especially Dr. Augusto Luque, Dra. Alejandra Aguado del Hoyo, and Dr. Francisco Diaz-Crespo (Hospital General Universitario Gregorio Marañón). We furthermore thank Patricia Ruiz and Laura Lorente for technical support and dedication and the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR 2017/SGR-00676).
Funding
The study was funded by the Instituto de Salud Carlos III (ISCIII)/Fondo Europeo de Desarrollo Regional (FEDER) funds (PI16/01998, PI18/00362). The funding body contributed to the design of study, collection, analysis and interpretation of data, as well as the manuscript writing. AD is funded by Red de Investigación Renal (REDINREN ISCIII) RD16/0009/0019.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
RT and EA designed and organized the study. DB, ABM, and EMM cared for the patients, acquired the clinical data, and prepared the samples from the family members. ADG, MF, MP, RT, and EA wrote the manuscript that was edited by all other authors. ADG, MP, and EA performed Sanger sequencing of the OSGEP gene. ADG, MP, and EA performed the NGS analysis, interpreted the NGS data, and drafted the genetic diagnostic report. RT and EA obtained funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Fundació Puigvert Institutional Review Board. The parents of the patients provided written informed consent to participate in this study.
Consent for publication
The parents of the patients provided written informed consent to publish this case report, including case description, medical data, and images, maintaining anonymity.
Competing interests
RT is the Editorial Board Member of BMC Nephrology. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Domingo-Gallego, A., Furlano, M., Pybus, M. et al. Novel homozygous OSGEP gene pathogenic variants in two unrelated patients with Galloway-Mowat syndrome: case report and review of the literature. BMC Nephrol 20, 126 (2019). https://doi.org/10.1186/s12882-019-1317-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-019-1317-y