Abstract
Background
Experimental studies showed that 25-hydroxy-vitamin D [25(OH)D] deficiency (defined as 25-hydroxy-vitamin D < 15 ng/ml) has been associated with CKD progression. Patients with IgA nephropathy have an exceptionally high rate of severe 25(OH)D deficiency; however, it is not known whether this deficiency is a risk factor for progression of IgA nephropathy. We conducted this study to investigate the relationship between the plasma level of 25(OH)D and certain clinical parameters and renal histologic lesions in the patients with IgA nephropathy, and to evaluate whether the 25(OH)D level could be a good prognostic marker for IgA nephropathy progression.
Methods
A total of 105 patients with biopsy-proven IgA nephropathy were enrolled between 2012 and 2015. The circulating concentration of 25(OH)D was determined using serum samples collected at the time of biopsy. The primary clinical endpoint was the decline of estimated glomerular filtration rate (eGFR; a 30 % or more decline compared to the baseline).
Results
Mean eGFR decreased and proteinuria worsened proportionally as circulating 25(OH)D decreased (P < 0.05). The 25(OH)D deficiency was correlated with a higher tubulointerstitial score by the Oxford classification (P = 0.008). The risk for reaching the primary endpoint was significantly higher in the patients with a 25(OH)D deficiency compared to those with a higher level of 25(OH)D (P = 0.001). As evaluated using the Cox proportional hazards model, 25(OH)D deficiency was found to be an independent risk factor for renal progression [HR 5.99, 95 % confidence intervals (CIs) 1.59–22.54, P = 0.008].
Conclusion
A 25(OH)D deficiency at baseline is significantly correlated with poorer clinical outcomes and more sever renal pathological features, and low levels of 25(OH)D at baseline were strongly associated with increased risk of renal progression in IgAN.
Similar content being viewed by others
Background
IgA nephropathy (IgAN) is the most common form of glomerulonephritis worldwide [1], especially in Asia, and represents one of the main causes of the end-stage renal diseases (ESRD) [2]. Male gender, early-onset, absence of macroscopic hematuria, persistent microscopic hematuria, hypertension, proteinuria, presence of renal dysfunction at the time of diagnosis, and certain histological features of renal lesions have been identified as important risk factors for its progression[3–6].
Recent observations suggest that low vitamin D levels, measured as 25-hydroxyvitamin D [25(OH)D] is significantly associated with a more severe decrease in estimated glomerular filtration rate (GFR) in patients with chronic kidney diseases (CKD) [7, 8]. A series of studies have also suggested a role of vitamin D [9] deficiency (defined as 25-hydroxy-vitamin D < 15 ng/ml) [10] in the short-life expectancy of CKD [11–13]. Bienaim’ et al. also reported that patients with lower serum total 25(OH)D concentrations at 3 months after transplantation exhibited lower kidney allograft function at 1 year after transplantation and had higher risk of the progression of interstitial fibrosis and tubular atrophy. [14] Furthermore, among patients with wide range of renal dysfunctions including ESRD, vitamin D deficiency showed the associations with vascular calcification, vascular endothelial function, cardiovascular events, and cardiovascular mortality. In the Third National Health and Nutrition Examination Survey (NHANES III) cohort, individuals with 25 (OH) vitamin D levels < 15 ng/ml had a higher risk for all-cause mortality despite adjustments for CKD stage and for potential confounders. [15] Therefore, low vitamin D level is considered as a candidate novel risk factor for poor outcome of renal disease. Experimental data have indicated that vitamin D analogues mediate a decrease in albuminuria and slow the progression of kidney injury in several animal models of kidney disease [16, 17]. Moreover, a randomized trial by Liu and colleagues [18] has demonstrated that oral calcitriol results in decrease in proteinuria in IgA nephropathy patients confirming earlier results of Szeto et al. [19]. Experimental studies have revealed that VD insufficiency upregulates the renin-angiotensin system [20, 21] and the NF-κB pathway [22], decreases the nitric oxide synthase transcription in vascular endothelial cells [23–25], increases inflammation and oxidative stress [26, 27], and therefore may be a risk factor for progression of kidney diseases. However, to date there have been few studies exploring the relationship between the 25(OH)D level and the progression of IgAN, we therefore studied this aspect.
Methods
Patients and serum samples
A total of 105 patients with newly diagnosed, biopsy-proven primary IgAN were recruited from The First Affiliated Hospital of Guangxi Medical University between 2012 and 2015. IgAN was diagnosed with mesangial depositions of IgA under immunofluorescence microscope and with electron-dense deposits in the mesangium under electron microscope. Blood samples were collected before initial VD treatment at the time of kidney biopsy.
Basic data and study endpoint
Patient demographics as well as conventional clinical parameters including age, gender, body mass index (BMI), blood pressure (BP), blood chemistry and daily proteinuria were collected at the time of kidney biopsy. Blood chemistry tests included serum creatinine, albumin, uric acid, total cholesterol and IgA. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [28]. Consecutive changes in renal function and the daily proteinuria were recorded during the follow-up period. Primary endpoint was defined as a decline of 30 % or more in eGFR compared with the baseline. Medication history was recorded, including the use of renin-angiotensin system (RAS) blockers such as angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, and immunosuppressors (IS) within 6 months of kidney biopsy and during the follow-up period. Kidney biopsy was performed to those patients with uncontrolled proteinuria or uncontrolled hypertension in spite of the use of RAS blockers. However, according to the clinical judgment of the nephrologists, it would often be performed regardless of the efficacy of RAS blockers. The patients who had a persistant proteinuria greater than 1 g after the treatment of RAS blocker or showed impaired renal function usually received immunosuppressive treatment for 2-4 weeks after the kidney biopsy. Steroid medicines were the mainstay of the treatment. None of the participants had received IS treatment before a renal histological confirmation.
Renal histopathology
All kidney tissue specimens were obtained by percutaneous kidney biopsy, and examined using light microscope, immunofluorescent, and electron microscope. All histological slides were evaluated by an experienced renal pathologist. The four pathologic variables of the Oxford classification, which was used in this study, were scored as follows: mesangial score ≤0.5 (M0) or >0.5 (M1), absence (S0) or presence (S1) of segmental glomerulosclerosis, absence (E0) or presence (E1) of endocapillary hypercellularity, and tubular atrophy/interstitial fibrosis that was graded as T0 (≤25 %), T1 (26–50 %) or T2 (>50 %) [29].
Measurement of the 25-hydroxyvitamin D
Serum 25(OH)D was measured using electrochemiluminescent immunoassay (ECLIA) with a Roche Elecsys 10100/201 system (Roche Diagnosis Elecsys) according to the manufacturer’s protocol. All measurements were performed in a blind manner and in duplicate. Vitamin D deficiency is defined as 25-hydroxy-vitamin D < 15 ng/ml.
Statistical analyses
Descriptive data were analyzed for all variables. For continuous variables, results were presented as mean ± SD. Variables were compared using one-way analysis of variance (ANOVA) and unpaired Student’s t-test for normally distributed variables whereas Kruskal-Wallis Test was used for non-normally distributed variables. Categorical variables were recorded as frequency counts, and intergroup comparisons were analyzed using a chi-squared test. Event free survival curves were derived from the Kaplan-Meier method and differences between the two curves were tested using the logrank test. The model of the Cox proportional hazards was used to identify independent predictors for the development of the primary endpoint. A correlation analysis was conducted in order to avoid multi-collinearity; only one variable in highly correlated variable sets was selected for multivariate analysis. Statistically significant covariables from univariate analysis and clinically important covariables were included in the final multivariate Cox proportional hazard regression analysis, and backward elimination and stepwise selection approaches were conducted. A P < 0.05 is considered statistical significance. Data analysis was performed using the SPSS for Windows, version 12.0 (SPSS, Chicago, IL, USA).
Results
Basic clinical information of the participants
Table 1 shows the clinical characteristics of our study population (n = 105) at the time of kidney biopsy. The median age was 34 years old, and 54.3 % of the participants were men. Average level of the systolic BP, proteinuria, and baseline eGFR were 131 mmHg, 2.04 g/24 h, and 75.46 mL/min/1.73 m2, respectively. One hundred and one patients (96.2 %) had 25 (OH) vitamin D levels < 30 ng/ml, and fifty-one patients (48.6.5 %) had 25 (OH) vitamin D < 15 ng/ml, which are the thresholds for 25 (OH) vitamin D insufficiency and deficiency, respectively. By CDK stage, the proportions of patients with 25(OH)D <15 ng/ml were:55 % (22 of 40) at stage 1,42.9 % (12 of 28) at stage 2,61.9 % (13 of 21) at stage 3,83.3 % (5 of 6) at stage 4, and 90 % (9 of 10) at stage 5(shown as Table 2). A total of 83 (79 %) patients were treated with RAS blockers at the time of the kidney biopsy and 38 (36.2 %) patients received immunosuppressive therapy within 2–4 weeks after the kidney biopsy.
The correlation between clinical parameters and 25(OH)D
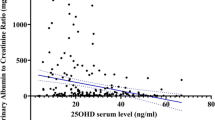
To identify the relationship between the serum level of 25(OH)D and several clinical parameters, we dichotomized the patients, based on their serum concentration of 25(OH)D, into two groups: levels of 25(OH)D < 15 ng/ml vs 15 ng/ml or greater. Compared with those showing a higher 25(OH)D level, those with a 25(OH)D deficiency were significantly associated with a lower eGFR and a higher proteinuria level (Table 2). Age, BMI and serum IgA level were not correlated with the 25(OH)D concentration. Stepwise increases in the uric acid level and BP but a decrease in the serum albumin level were also associated with the 25(OH)D level. These correlations were observed as well when the 25(OH)D level was used as continuous variables (Table 3 and Fig. 1). There was a significant positive correlation between the 25(OH)D level and the eGFR (r = 0.196, P < 0.05). Proteinuria was inversely correlated with the 25(OH)D level (r = -0.553, P < 0.01). We also conducted a correlation analysis between serum phosphorus, calcium, and PTH levels with 25(OH)D level (result shown in Additional file 1).
Relationship between histologic features and the plasma 25(OH)D
We examined the association between the plasma 25(OH)D level at the time of initial diagnosis and the severity of histologic lesions, and found that the plasma 25(OH)D level was associated with the percentage of interstitial fibrosis/tubular atrophy. A lower plasma 25(OH)D level indicated a higher tubulointerstitial score with the aforementioned Oxford classification (P = 0.008; Table 4).
The impact of circulating 25(OH)D on clinical outcome
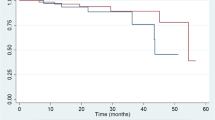
A total of 28 (26.7 %) patients reached the primary end-point (renal progression; eGFR declined by 30 % or more compared with the baseline level) during a median follow-up period of 13 months. The primary endpoint was reached by 24 patients with deficiency (39.3 %) and 4 patients without (9.1 %). Time-to-event analysis showed that those patients with a 25(OH)D deficiency showed a significantly higher risk for renal progression compared with their counterparts (Fig. 2). A Cox proportional hazards regression analysis revealed that 25(OH)D deficiency, is an independent risk factor for renal progression, after adjustment for age, gender, systolic BP, proteinuria, eGFR, subsequent vitamin D therapy, immunosuppressives treatment and ACEI/ARB treatment [HR 5.99, 95 % confidence intervals (CIs) 1.59–22.54, P = 0.008] (Table 5). Moreover, as shown in Fig. 3, the ROC curve was made comparing between combined clinical score (eGFR + proteinuria) vs combined clinical score + vitamin D level. It revealed that vitamin D can add significant predictive information to the renal progression, manifested as a larger area under the ROC curves (AUCs); the combined model (eGFR, proteinuria and vitamin D score), 0.785; combined clinical score, 0.722.
Discussion
Results from this study display several relationships between the plasma 25(OH)D level and several clinical parameters related to the IgAN histological damage and its severity. Our results also suggest that the plasma 25(OH)D level at the time of initial diagnosis may be an independent inverse-predictor of IgAN progression.
Vitamin D deficiency or insufficiency is highly prevalent among patients with CKD. An ethnically homogeneous cohort by Ravani et al. showed that the prevalence of a 25(OH)D deficiency or insufficiency was increased from 25 % in stage 2 to 56 % in stage 5 [8]. Cumulative evidence suggests an association of a low 25(OH)D level with clinical parameters related to a kidney damage. For example, Ravani et al. found that the baseline level of 25(OH)D was directly and significantly correlated with eGFR [8]. Diniz et al. also revealed an inverse correlation between the 25(OH)D level and proteinuria [30]. Furthermore, an inverse relationship between blood pressure and the 25(OH)D level has been documented as well in a number of epidemiological studies [31, 32]. Our observations are consistent with these previous reports.
Our observation of a more severe tubulointerstitial damage in patients with a 25(OH)D deficiency is of interest and suggests that a 25(OH)D deficiency in an early stage of IgAN may point to an advanced renal injury. Interstitial fibrosis together with tubular athrotrophy is a hallmark of chronic renal failure and strongly correlates with deterioration of renal function. Zhang Y. et al. [33] have established vitamin D receptor-null mice that allow a manipulation of unilateral ureteral obstruction for 7 days. Compared with the wild-type mice, the VDR-null mice develop more severe renal damage with marked tubular atrophy and interstitial fibrosis. Gonçalves et al. [34] found recently that a 25(OH)D deficiency might potentiate tubulointerstitial damages (fibrosis, inflammatory infiltration, tubular dilation and atrophy) through those inflammatory pathways that involve TGF-β1. Increased expression of TGF-β1 and decreased expression of VDR and Klotho protein are observed in VD deficient rats. The essential role of TGF-β1 and the protective effect of Klotho protein in pathogenesis of various renal diseases, including IgAN, have been documented in different clinical and experimental models [35–37].
There has been increasing evidence recommending the plasma 25(OH)D level as a good indicator for deterioration of renal function in various kidney diseases. In a prospective study with a cohort of predialysis patients with CKD, Nakano et al. [38] identified the serum 25(OH)D concentration as a significant predictor of renal composite outcome of doubling serum creatinine and ESRD. In the same cohort, Hamano et al. [39] found a nonlinear association between the serum 25(OH)D concentration and annual decline in the renal function. A meta-analysis of clinical observations reveals an inverse association between all-cause mortality and the serum 25(OH)D concentration [12], whereas our study shows that the circulating 25(OH)D level determined at the time of diagnosis predicts the renal progression. This predictability is not only independent of other important risk factors relevant to renal progression in IgAN but is also unaffected by the treatment of vitamin D, RAS blockers or immunosuppresors. Given that tubulointerstitial damage is one of the most important risk factors for renal progression in IgAN, our findings that connect the renal histologic features to the circulating level of 25(OH)D may partly explain why a decreased 25(OH)D level predicts the prognosis of IgAN.
In our study the follow-up period is too short to assess the long-term renal outcomes such as ESRD, and death in this patient population. Nevertheless, it is noteworthy that this is the first study suggesting a prognostic value of the circulating 25(OH)D in IgAN patients, although its prognostic value for the much longer outcome still awaits further study. Moreover, in the last decade observational studies have noted links between vitamin D deficiency and poor cardiovascular outcomes in CKD patients. Our study only demonstrated the reverse relationship of vitamin D and the blood pressure, but did not test the relationship of 25(OH)D with other cardiovascular outcomes such as incident coronary heart disease and heart failure. Given the high-cardiovascular risk of CKD, the issue deserves to be addressed in well powered future studies. Finally, our study did not address the vitamin D treatment effect, thus an adequately powered randomized controlled trial should be designed to determine whether 25 (OH) vitamin D therapy is able to slow the progression of IgAN.
Conclusions
In conclusion, 25(OH)D deficiency is significantly correlated with a poorer renal function and more severe renal pathological features, and is strongly associated with increased risk of renal progression in IgAN patients, making a 25(OH)D deficiency a good prognostic marker to predict the severity and clinical outcome in IgAN patients. Therefore, determining the circulating level of 25(OH)D may be important and informative for the identification of high-risk patients and for the proper management of IgAN.
Abbreviations
- 25(OH)D:
-
25-hydroxy-vitamin D
- ACEI:
-
Angiotensin converting enzyme inhibitor
- ANOVA:
-
One-way analysis of variance
- ARB:
-
Angiotensin II receptor blockade
- BMI:
-
Body mass index
- CIs:
-
Confidence intervals
- CKD:
-
Chronic kidney diseases
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration
- eGFR:
-
Estimated glomerular filtration rate
- ESRD:
-
End-stage renal diseases
- IgAN:
-
IgA nephropathy
- IS:
-
Immunosuppressors
- RAS:
-
Renin-angiotensin system
References
Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347(10):738–48.
Chou YH, Lien YC, Hu FC, Lin WC, Kao CC, Lai CF, Chiang WC, Lin SL, Tsai TJ, Wu KD, et al. Clinical outcomes and predictors for ESRD and mortality in primary GN. Clin J Am Soc Nephrol. 2012;7(9):1401–8.
Radford Jr MG, Donadio Jr JV, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8(2):199–207.
Xie J, Kiryluk K, Wang W, Wang Z, Guo S, Shen P, Ren H, Pan X, Chen X, Zhang W, et al. Predicting progression of IgA nephropathy: new clinical progression risk score. Plos One. 2012;7(6):e38904.
Alamartine E, Sabatier JC, Guerin C, Berliet JM, Berthoux F. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18(1):12–9.
Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L. Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol. 2011;22(4):752–61.
Satirapoj B, Limwannata P, Chaiprasert A, Supasyndh O, Choovichian P. Vitamin D insufficiency and deficiency with stages of chronic kidney disease in an Asian population. Bmc Nephrol. 2013;14:206.
Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75(1):88–95.
Allegretto EA, Shevde N, Zou A, Howell SR, Boehm MF, Hollis BW, Pike JW. Retinoid X receptor acts as a hormone receptor in vivo to induce a key metabolic enzyme for 1,25-dihydroxyvitamin D3. J Biol Chem. 1995;270(41):23906–9.
National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003 42;(4 Suppl 3):S1-201.
Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo Jr CA, Tonelli M, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–13.
Pilz S, Iodice S, Zittermann A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58(3):374–82.
Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Jain A, Schreiber Jr MJ, Simon JF, Srinivas TR, Nally Jr JV. Low 25-hydroxyvitamin D levels and mortality in non-dialysis-dependent CKD. Am J Kidney Dis. 2011;58(4):536–43.
Bienaime F, Girard D, Anglicheau D, Canaud G, Souberbielle JC, Kreis H, Noel LH, Friedlander G, Elie C, Legendre C, et al. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol. 2013;24(5):831–41.
Mehrotra R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, Martins D, Adler SG, Norris KC. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76(9):977–83.
Mizobuchi M, Morrissey J, Finch JL, Martin DR, Liapis H, Akizawa T, Slatopolsky E. Combination therapy with an angiotensin-converting enzyme inhibitor and a vitamin D analog suppresses the progression of renal insufficiency in uremic rats. J Am Soc Nephrol. 2007;18(6):1796–806.
Deb DK, Sun T, Wong KE, Zhang Z, Ning G, Zhang Y, Kong J, Shi H, Chang A, Li YC. Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int. 2010;77(11):1000–9.
Liu LJ, Lv JC, Shi SF, Chen YQ, Zhang H, Wang HY. Oral calcitriol for reduction of proteinuria in patients with IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2012;59(1):67–74.
Szeto CC, Chow KM, Kwan BC, Chung KY, Leung CB, Li PK. Oral calcitriol for the treatment of persistent proteinuria in immunoglobulin a nephropathy: an uncontrolled trial. Am J Kidney Dis. 2008;51(5):724–31.
Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55(5):1283–8.
Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, Marz W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin chim acta. 2010;411(17-18):1354–60.
Li YC. Renoprotective effects of vitamin D analogs. Kidney Int. 2010;78(2):134–9.
Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circulation research. 2014;114(2):379–93.
Ott C, Raff U, Schneider MP, Titze SI, Schmieder RE. 25-hydroxyvitamin D insufficiency is associated with impaired renal endothelial function and both are improved with rosuvastatin treatment. Clin Res Cardiol. 2013;102(4):299–304.
Abu El Maaty MA, Hassanein SI, Hanafi RS, Gad MZ. Insights on vitamin D’s role in cardiovascular disease: investigating the association of 25-hydroxyvitamin D with the dimethylated arginines. J Nutr Sci Vitaminol. 2013;59(3):172–7.
Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188(5):2127–35.
Luchi WM, Shimizu MH, Canale D, Gois PH, de Braganca AC, Volpini RA, Girardi AC, Seguro AC. Vitamin D deficiency is a potential risk factor for contrast-induced nephropathy. Am J Physiol Regul Integr Comp Physiol. 2015;309(3):R215–22.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro 3rd AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76(5):546–56.
Diniz HF, Romao MF, Elias RM, Romao Junior JE. Vitamin D deficiency and insufficiency in patients with chronic kidney disease. Jornal brasileiro de nefrologia : ‘orgao oficial de Sociedades Brasileira e Latino-Americana de Nefrologia. 2012;34(1):58–63.
Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the third national health and nutrition examination survey. Am J Hypertens. 2007;20(7):713–9.
Hintzpeter B, Mensink GB, Thierfelder W, Muller MJ, Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62(9):1079–89.
Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol. 2010;21(6):966–73.
Goncalves JG, de Braganca AC, Canale D, Shimizu MH, Sanches TR, Moyses RM, Andrade L, Seguro AC, Volpini RA. Vitamin D deficiency aggravates chronic kidney disease progression after ischemic acute kidney injury. Plos One. 2014;9(9):e107228.
Morinaga J, Kadomatsu T, Miyata K, Endo M, Terada K, Tian Z, Sugizaki T, Tanigawa H, Zhao J, Zhu S, et al. Angiopoietin-like protein 2 increases renal fibrosis by accelerating transforming growth factor-beta signaling in chronic kidney disease. Kidney Int. 2016;89(2):327–41.
Munoz-Felix JM, Gonzalez-Nunez M, Martinez-Salgado C, Lopez-Novoa JM. TGF-beta/BMP proteins as therapeutic targets in renal fibrosis. Where have we arrived after 25 years of trials and tribulations? Pharmacol Ther. 2015;156:44–58.
Yu R, Mao J, Yang Y, Zhang Y, Tian Y, Zhu J. Protective effects of calcitriol on diabetic nephropathy are mediated by down regulation of TGF-beta1 and CIP4 in diabetic nephropathy rat. Int J Clin Exp Pathol. 2015;8(4):3503–12.
Nakano C, Hamano T, Fujii N, Matsui I, Tomida K, Mikami S, Inoue K, Obi Y, Okada N, Tsubakihara Y, et al. Combined use of vitamin D status and FGF23 for risk stratification of renal outcome. Clin J Am Soc Nephrol. 2012;7(5):810–9.
Hamano T, Nakano C, Obi Y, Fujii N, Matsui I, Tomida K, Mikami S, Inoue K, Shimomura A, Kusunoki Y, et al. Fibroblast growth factor 23 and 25-hydroxyvitamin D levels are associated with estimated glomerular filtration rate decline. Kidney Int Suppl. 2013;3(5):469–75.
Acknowledgements
We would like to thank Dr. Fred Bogott at the Medical Center of Austin of Minnesota, Mayo Clinic, and Dr. Joshua Liao of Hormel Institute, University of Minnesota, for their excellent English editing of this manuscript.
Funding
This work was supported by a grant from Natural Science Foundation of Guangxi (Contract Number: 2010GXNSFA013178) and a grant of Guangxi Science and Technology Ministry (Contract Number: Guigongke1598011-6) to Yun-Hua Liao.
Availability of data and materials
The dataset supporting the conclusions of this article is available from the corresponding author on reasonable request, and is also available in the IgAN medidata repository, http://igan.medidata.cn/login.jsp (the user name and password will be provided by the corresponding author on reasonable request.)
Authors’ contributions
LXH and HXP are the first authors who were involved in the study design, sample collection, analysis and interpretation of the data and in the writing of the report. WCY, LYZ, WY, HYM, PX, QJ, NFW acquired the data and participated in the interpretation of the data. YZH, PL revised the article critically for important intellectual content. LYH is the corresponding author. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable (There are no details on individuals reported within the manuscript.)
Ethics approval and consent to participate
This study was approved and abided by the Institutional Ethical Committee of the First Affiliated Hospital of Guangxi Medical University. All clinical investigations were conducted in accordance with the guidelines of the 2013 version of the Declaration of Helsinki. Written informed consents were obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1:
A correlation analysis between serum phosphorus, calcium, and PTH levels with vit D levels. 25(OH) positively related with serum calcium and negatively related with serum phosphorus and PTH. (DOC 82 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, XH., Huang, XP., Pan, L. et al. Vitamin D deficiency may predict a poorer outcome of IgA nephropathy. BMC Nephrol 17, 164 (2016). https://doi.org/10.1186/s12882-016-0378-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-016-0378-4