Abstract
Background
The calcium-sensing receptor (CaSR) is a calcium (Ca2+) sensitive G protein-coupled receptor implicated in various biological processes. In particular, it regulates Ca2+/Mg2+- homeostasis and senses interstitial Ca2+ levels and thereby controls downstream signalling cascades. Due to its expression in the gut epithelium, the enteric nervous system and smooth muscles and its key function in regulation and coordination of muscular contraction and secretion, it represents an excellent candidate gene to be investigated in the pathophysiology of irritable bowel syndrome (IBS). Disturbed CaSR structure and function may impact gastrointestinal regulation of muscular contraction, neuronal excitation and secretion and consequently contribute to symptoms seen in IBS, such as disordered defecation as well as disturbed gut motility and visceral sensitivity.
Methods
We have therefore genotyped the functional CASR SNP rs1801725 in three case control samples from the UK, Belgium and the USA.
Results
Genotype frequencies showed no association in the three genotyped case–control samples, neither with IBS nor with IBS subtypes.
Conclusions
Although we could not associate the SNP to any of the established bowel symptom based IBS subtypes we cannot rule out association to altered Ca2+ levels and disturbed secretion and gut motility which were unfortunately not assessed in the patients genotyped. This underlines the necessity of a more detailed phenotyping of IBS patients and control individuals in future studies.
Similar content being viewed by others
Background
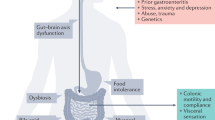
The calcium-sensing receptor (CaSR) is a multifunctional G protein-coupled receptor implicated in various biological processes [1, 2]. Besides its expression in tissues maintaining systemic calcium/magnesium (Ca2+/Mg2+) homeostasis such as parathyroid, thyroid glands and the kidney, it can also be found in the gastrointestinal (GI) tract. The receptors have been detected in the gut epithelium from the stomach to the small and large intestine [3, 4], as well as in the plexus submucosus and plexus myentericus of the enteric nervous system (ENS) [5] and in smooth muscle cells [6]. To date, the CaSR has been shown to be involved in the regulation of colonic secretion [7, 8] and modulation of gut motility in response to changes of interstitial Ca2+ levels [9–11]. Interestingly, studies have shown that constipation in hyperparathyroidism is associated with reduced neuromuscular excitability as a result of increased interstitial Ca2+ levels [12]. This was mediated by CaSRs on the myenteric plexus regulating smooth muscle function and coordination [10, 11]. Intestinal motility is modulated in a highly complex manner with Ca2+ playing a fundamental role in regulation of muscular contraction and neuronal excitation. Contraction of smooth muscle cells is controlled by the interstitial Ca2+ levels and the sensitivity of the contractile elements to Ca2+ in response to changes in the interstitial space [13]. Activation of CaSRs causes Ca2+ release from intracellular Ca2+ stores such as the endoplasmic reticulum by initializing intracellular second messenger cascades and consequently increasing interstitial Ca2+ levels [6, 14]. Consequently, a dysfunctional CaSR causing disturbed intestinal Ca2+ absorption may have effects on intestinal motility, secretion [7, 8], neuromuscular excitability, colonic fluid transport, peptide release and contribute to the symptoms seen in functional GI disorders, such as Irritable Bowel Syndrome (IBS).
IBS patients present with abdominal pain associated with a change in bowel habit [15]. Depending on predominant bowel habit, IBS is further sub-typed into IBS-C (constipation), IBS-D (diarrhea), IBS-M (mixed constipation/ diarrhea) and IBS-U (unspecified) [16–21]. Despite intense research, its pathophysiology remains unclear but is thought in part to be related to disordered motility, secretion and visceral sensation. Dysfunction within the ENS [22] and/or central nervous system, along with impaired mucosal barrier function have all been implicated in its pathophysiology [23].
Interestingly, a large meta-analysis including 12.865 individuals of European and Indian descent correlated serum Ca2+ level with single nucleotide polymorphisms (SNPs) in the CASR gene [24]. In particular, the minor allele of the CASR SNP rs1801725 was reported to be strongly associated with increased serum Ca2+ levels (p < 0.001). This SNP encodes a missense variant which leads to a non-conservative amino acid change: (c.2956 G > T, p.Ala986Ser = A986S) residing in the cytoplasmic tail of the CaSR [24]. In vitro studies showed that mutations within the respective region of the receptor may influence CaSR function, impair signal transduction, intracellular trafficking and/or cell surface expression, respectively [25–27]. The functions and influences of a dysfunctional CaSR have not been explored in IBS previously. We hypothesized that this common CASR polymorphism may be relevant in the pathophysiology of IBS and evaluated rs1801725 in patients with IBS from three case–control cohorts from the UK, Belgium and the USA.
Methods
Genotyped study subjects
Case–control cohort from Manchester (UK)
SNP genotyping was performed in a cohort of 86 patients with IBS-D (aged 18–66 years; mean age 41.7 years; 60 female, 26 male), 88 with IBS-C (aged 18–65 years; mean age 39.9 years; 82 female, 6 male) and 86 healthy controls (aged 18–63 years; mean age 35.0 years; 55 female, 31 male) (Table 1). IBS patients with mixed bowel habit (IBS-M) were not included. Recruitment of IBS patients was via the Out Patients Departments of the University Hospital of South Manchester, local general practices, advertisement in regional newspapers and an existing departmental volunteer pool of patients. All patients satisfied the Rome II criteria for IBS and predominant bowel habit subtype [28]. Moreover, they underwent appropriate investigations to exclude organic disease [15] and did not show any functional disorder of the upper GI tract that was more prominent than their IBS. In addition, no subject had a history of major psychiatric disorder or history of alcohol or substance abuse. Healthy controls were recruited by advertisement. All subjects were Caucasian and drank below the recommended safe alcohol limit (<21 units/week) smoked <5 cigarettes per day. Written consent was obtained from all subjects and the study was approved by the South Manchester Medical Research Ethics Committee. DNA was extracted from peripheral blood cells taken from both the patients and healthy controls using standard protocols [29].
Case–control cohort from St. Louis, Missouri (USA)
The second genotyped cohort included 28 patients with IBS-D (aged 20–83 years; mean age 49.9 years; 18 female, 10 male), 16 IBS-C patients (aged 28–64 years; mean age 45.9 years; 10 female, 6 male); and 86 healthy controls (aged 18–81 years; mean age 55.9 years; 45 female, 41 male) (Table 1). IBS patients with mixed bowel habit (IBS-M) were not included. The group was composed of adult subjects (≥18 years old) who carried a clinical functional GI disease (FGID) diagnosis, and met Rome III criteria for IBS. Prior to enrolment in the study, all FGID subjects had organic bowel disease excluded with a comprehensive evaluation, including but not limited to laboratory, endoscopic and radiographic testing, performed at the discretion of the treating gastroenterologist. The control group consisted of patients undergoing screening endoscopy in the absence of on going GI diagnoses or symptoms at the time of enrolment, and without evidence for FGID using Rome III criteria. All participants provided written informed consent prior to collection of biological specimens and clinical data. The study protocol was approved by the Human Research Protection Office (institutional review board) at Washington University School of Medicine and Barnes-Jewish Hospital, St. Louis, Missouri. Sputum and/or blood samples were collected for DNA analysis at the time of subject enrolment. Blood was collected in cases where the participant was to undergo phlebotomy for clinical purposes. If a blood specimen was not required for other laboratory testing, a sputum sample was collected. DNA was extracted using Qiagen Gentra Puregene Reagents (Qiagen Inc., Valencia, CA). Following DNA extraction, samples were frozen and banked by the Biobank Core of the Washington University Digestive Diseases Research Center.
Case–control cohort from Leuven (Belgium, BEL)
The third genotyped cohort included 420 patients with IBS-D (aged 18–83 years; mean age 41.0 years; 305 female, 115 male), 313 IBS-C patients (aged 18–82 years; mean age 43.6 years; 288 female, 25 male) and 622 healthy controls (aged 18–82 years; mean age 43.5 years; 470 female, 152 male) (Table 1). IBS patients with mixed bowel habit (IBS-M) were not included. Further details can be found in Wouters et al. [30].
Genotyping
The SNP genotyping was performed with the KASPar® assay system (KBiosciences, Ltd, Hoddesdon, UK) as recommended by the manufacturer using a customized assay. An initial 15-min cycle at 94 °C was followed by 20 cycles consisting of 10 s at 94 °C, 5 s at 57 °C, and 10 s at 72 °C; 28 cycles consisting of 10 s at 94 °C, 20 s at 57 °C, and 40 s at 72 °C; and a cool down at 10 °C. After the thermal cycling, KASPar® analysis was performed using the fluorescence plate reader TaqMan7500 system (Applied Biosystems, Foster City, CA).
Statistical analysis
Chi-squared tests were used to assess deviations of observed genotype frequencies from Hardy-Weinberg-Equilibrium (HWE). Genotype relative risks of IBS were quantified by odds ratios (ORs) with corresponding 95 % confidence intervals (CIs) based on a logistic regression model under additive genetic penetrance. Tests for deviation from Hardy-Weinberg equilibrium and tests for association were performed using an online tool provided by the Institute of Human Genetics, Technical University of Munich (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl). In addition, results were stratified by IBS subtypes. Probability values were adjusted for multiplicity using the Holm-Bonferroni method.
Results
In our study we analyzed the SNP rs1801725 (p. A986S) of the CASR gene, which has not been examined in IBS previously. We investigated three case–control samples with 1745 individuals of Caucasian origin (Table 1). Genotype frequencies did not deviate from Hardy-Weinberg equilibrium and showed no association in the three genotyped case–control samples, neither with IBS nor with IBS subtypes (Table 2). In addition, no significant differences between the three case–control cohorts could be identified.
Discussion
This is the first study to investigate whether the CASR SNP rs1801725 associates with the functional GI disorder IBS. The investigated SNP is known to be associated with increased serum Ca2+ levels [24] and has therefore a potentially inactivating effect on the CaSR. The set point (= onset of activation) is increased for inactivating mutations or polymorphisms compared to CASR wild-type. For receptor activation, the concentration of the CaSR agonist Ca2+ needs to be higher in comparison to the CaSR wild-type. Consequently, with respect to intestinal motility, an increased frequency of the CASR p.986S allele in the IBS-D group compared to control individuals would be conceivable. Although our study showed no association overall or in any of the three genotyped cohorts or with respect to the established bowel symptom based IBS-subtypes, association to changed motility and transit cannot be excluded. In fact, disturbances in the motility of the GI tract have been shown to be an important feature of IBS, with these varying with sub-type studied. In this sense, IBS-D patients have been shown to have increased colonic motility, particularly high amplitude propagating contractions (HAPCs) and accelerated transit [31]. In contrast, IBS-C patients exhibit reduced motility, less HAPCs and slower transit compared with healthy volunteers [32]. Furthermore, IBS-D patients presented with an increased basal motility index (contractions per minute) compared to patients with IBS-C [33]. Although in general these various motor patterns associate with a particular sub-type of IBS, not all IBS patients in those subtypes will exhibit them. Stratification for any of these traits would have increased the likelihood to correlate a functional SNP with in a particular subset of patients. However, additional analysis stratifying for total serum Ca2+ levels, disturbed motility or secretion were not possible since this data were lacking. This underlines the importance of a more detailed patient characterization and assessment of relevant traits and laboratory parameters. Colonic motility for example could be assessed by oroanal transit time measurement [34, 35]. Stool scale form could be taken into account as measure for transit time implementing the Bristol Stool Scale into the phenotyping pipeline of the patients and control individuals [36].
Conclusions
Although we could not associate the SNP to any of the established bowel symptom based IBS subtypes we cannot rule out association to altered Ca 2+ levels and gut motility which were unfortunately not assessed in the patients genotyped. Further investigation of CASR-related genetic variations in IBS and relevant intermediate traits such as Ca2+ serum levels in addition to the assessment of motility, transit and secretion are needed for a detailed characterization of the gene function and a better understanding of the correlation between CASR variants and the aetiology of IBS.
References
Brown EM, Gamba G, Riccardi D, Lombardi M. Cloning and characterization of an extracellular Ca2 + −sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–80.
Brown EM, Pollak M, Riccardi D, Hebert SC. Cloning and characterization of an extracellular Ca2+ − sensing receptor from parathyroid and kidney: new insights into the physiology and pathophysiology of calcium metabolism. Nephrol Dial Transplant. 1994;9:1703–6.
Chattopadhyay N, Cheng I, Rogers K. Identification and localization of extracellular Ca2 + −sensing receptor in rat intestine. Am J Physiol. 1998;274:G122–30.
Chattopadhyay N, Brown EM. Cellular “sensing” of extracellular calcium (Ca(2+)(o)): emerging roles in regulating diverse physiological functions. Cell Signal. 2000;12(6):361–6.
Hebert SC, Cheng S, Geibel J. Functions and roles of the extracellular Ca2 + −sensing receptor in the gastrointestinal tract. Cell Calcium. 2004;35:239–47.
Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81(1):239–97.
Cheng SX, Geibel JP, Hebert SC. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology. 2004;126(1):148–58.
Cheng SX. Calcium-sensing receptor inhibits secretagogue-induced electrolyte secretion by intestine via the enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2012;303(1):G60–70.
Geibel J, Sritharan K, Geibel R. Calcium-sensing receptor abrogates secretagogue-induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. PNAS. 2006;103:9390–7.
Kirchhoff P, Geibel JP. Role of calcium and other trace elements in the gastrointestinal physiology. World J Gastroenterol. 2006;12(20):3229–36.
Geibel JP, Hebert SC. The Functions and Roles of the Extracellular Ca 2 + −Sensing Receptor along the Gastrointestinal Tract. Annu Rev Physiol. 2009;71:205–17.
Ebert EC. The Parathyroids and the Gut. J Clin Gastroenterol. 2010;44:479.
Karaki H, Ozaki H, Hori M. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49:157–230.
Pollak MR, Brown EM, Estep HL, McLaine PN, Kifor O, Park J, et al. Autosomal dominant hypocalcaemia caused by a Ca(2+)-sensing receptor gene mutation. Nat Genet. 1994;8(3):303–7.
Drossman DA, Camilleri M, Mayer EA. AGA Technical Review on Irritable Bowel Syndrome. Gastroenterology. 2002;123:2108–31.
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional Bowel Disorders. Gastroenterology. 2006;130:1480–91.
Fukudo S, Kanazawa M. Gene, environment, and brain‐gut interactions in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26:110–5.
Kanazawa M, Hongo M, Fukudo S. Visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26:119–21.
Gunnarsson J, Simrén M. Peripheral factors in the pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2009;41(11):788–93.
Öhman L, Simrén M. New insights into the pathogenesis and pathophysiology of irritable bowel syndrome. Dig Liver Dis. 2007;39(3):201–15.
Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. Gut. 1973;14(2):125–32.
Scalera A, Loguercio C. Focus on irritable bowel syndrome. Eur Rev Med Pharmacol Sci. 2012;16(9):1155–71.
Andresen V, Keller J, Pehl C. Irritable bowel syndrome—the main recommendations. Deutsches Ärzteblatt. 2011;108:751–60.
Kapur K, Johnson T, Beckmann ND. Genome-Wide Meta-Analysis for Serum Calcium Identifies Significantly Associated SNPs near the Calcium-Sensing Receptor (CASR) Gene. PLoS Genet. 2010;6, e1001035.
Bai M, Trivedi S, Brown EM. Dimerization of the Extracellular Calcium-sensing Receptor (CaR) on the Cell Surface of CaR-transfected HEK293 Cells. J Biol Chem. 1998;273:23605–10.
Bai M, Trivedi S, Lane CR, Yang Y, Quinn SJ, Brown EM. Protein Kinase C Phosphorylation of Threonine at Position 888 in Ca2+ o -Sensing Receptor (CaR) Inhibits Coupling to Ca2+ Store Release. J Biol Chem. 1998;273:21267–75.
Gama L, Breitwieser GE. A Carboxyl-terminal Domain Controls the Cooperativity for Extracellular Ca2+ Activation of the Human Calcium Sensing Receptor. A study with receptor-green fluorescent protein fusions. J Biol Chem. 1998;273(45):29712–8.
Thompson WG, Longstreth GF, Drossman DA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45 Suppl 2:II43–7.
Green MR, Sambrook J. Molecular Cloning: a Laboratory Manual. Cold Spring Harbor Lab Press. 2012;4(1):2–5.
Wouters MM, Lambrechts D, Knapp M. Genetic variants in CDC42 and NXPH1 as susceptibility factors for constipation and diarrhoea predominant irritable bowel syndrome. Gut. 2013;63:304570–1111.
Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499–506.
Bassotti G, Chistolini F, Morelli A. Pathophysiological aspects of diverticular disease of colon and role of large bowel motility. World J Gastroenterol. 2003;9(10):2140–2.
Kanazawa M, Palsson OS, Thiwan SIM, Turner MJ, van Tilburg MAL, Gangarosa LM, et al. Contributions of Pain Sensitivity and Colonic Motility to IBS Symptom Severity and Predominant Bowel Habits. Am J Gastroenterol. 2008;103:2550–61.
Sadik R, Björnsson E, Simrén M. The relationship between symptoms, body mass index, gastrointestinal transit and stool frequency in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2010;22:102–8.
Törnblom H, Van Oudenhove L, Sadik R, Abrahamsson H, Tack J, Simrén M. Colonic Transit Time and IBS Symptoms: What's the Link|[quest]|. Am J Gastroenterol. 2012;107:754–60.
Lewis SJ, Heaton KW. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand J Gastroenterol. 2009;32:920–4.
Acknowledgements
We thank Cristina Martinez for helpful discussion. This study was supported by the Medical Faculty of the University of Heidelberg. We are grateful to the patients and healthy volunteers for participating in the study. This manuscript results in part from collaboration and network activities promoted under the frame of the international network GENIEUR (Genes in Irritable Bowel Syndrome Research Network Europe), which is currently funded by the COST program (BM1106, www.GENIEUR.eu). We acknowledge financial support by Deutsche Forschungsgemeinschaft and Ruprecht-Karls-Universität Heidelberg within the funding programme Open Access Publishing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
No financial competing interests
Authors’ contributions
PR and SS performed the genotyping. PR, MMW, LAH, BC, GSS, GEB, PG, SHC and BN conceived and designed the study. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Romero, P., Schmitteckert, S., Wouters, M.M. et al. No association between the common calcium-sensing receptor polymorphism rs1801725 and irritable bowel syndrome. BMC Med Genet 16, 110 (2015). https://doi.org/10.1186/s12881-015-0256-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-015-0256-0