Abstract
Background
The emergence and rapid spread of gram-negative bacteria resistant to carbapenems among newborns is concerning on a global scale. Nonetheless, the pooled estimate of gram-negative bacteria resistant to carbapenem that cause neonatal sepsis in developing nations remains unknown. Thus, this study aimed to determine the combined prevalence of gram-negative bacteria resistant to carbapenem in African newborns who were suspected of having sepsis.
Methods
All studies published from January 1, 2010, up to December 30, 2023, from PubMed, Science Direct, Scopus electronic databases, and the Google Scholar search engine were researched. Isolates tested for carbapenem from neonates with sepsis, English language papers conducted in Africa, and cross-sectional and cohort studies papers were included. Using PRISMA guidelines, we systematically reviewed and meta-analyzed studies that assessed the prevalence of carbapenem-resistant gram-negative bacteria. The “Joanna Briggs Institute” was used critically to evaluate the quality of the included studies. The data analysis was carried out using STATA™ version 17. Heterogeneity across the studies was evaluated using Q and I 2 tests. The subgroup analysis was done and, funnel plot and Egger’s regression test were used to detect publication bias. A sensitivity analysis was conducted.
Results
All 36 studies were included in the meta-analysis and systematic review. The pooled prevalence of carbapenem resistance in Africa was 30.34% (95% CI 22.03–38.64%). The pooled estimate of gram-negative bacteria resistant to imipenem, and meropenem was 35.57% (95% CI 0.67–70.54%) and 34.35% (95% CI 20.04% – 48.67%), respectively. A. baumannii and Pseudomonas spp. had pooled prevalence of 45.9% (95% CI 33.1–58.7%) and 43.0% (95% CI 23.0–62.4%), respectively. Similarly, Pseudomonas spp. and A. baumannii also exhibited strong meropenem resistance, with a pooled prevalence of 29.2% (95% CI 4.8–53.5%) and 36.7% (95% CI 20.1–53.3%), respectively. E. coli and K. pneumoniae were the two most common isolates.
Conclusion
There should be urgent antimicrobial stewardship practices, strengthened surveillance systems and effective treatment for neonates with sepsis. There was remarkable variation in resistance across the continent.
Similar content being viewed by others
Introduction
Sepsis is a serious inflammatory condition marked by fever and shock, which occurs when bacteria enter the bloodstream [1]. Neonatal septicemia continues to be a significant concern for newborn patients in neonatal intensive care units globally [2]. As a result of shifting antibiotic usage patterns and lifestyle modifications, the range of microorganisms responsible for newborn sepsis varies geographically and changes over time [3]. Approximately ten cases of probable severe bacterial infection are reported for every neonatal mortality, and there is a yearly population of two million that requires treatment for suspected infections [4]. There could be various reasons for neonatal mortality but septicemia continues to be a major cause and its incidence varies from country to country [5].
Epidemiological studies on neonatal sepsis reported an estimated 3.9 million annual cases [6] which is a primary cause of neonatal mortality within low and middle-income countries, bearing 99% of global neonatal mortality [7]. Every year, an estimated 2.5 million neonates die in their first month of life, accounting for nearly one-half of deaths in children under 5 years of age [8]. Adhering to established standards for managing neonatal sepsis and promptly initiating more potent drugs would greatly decrease the morbidity and death rate of babies caused by sepsis [9].
Antibiotic resistance is prioritized by the World Health Organization (WHO) as a pressing public health issue that requires immediate attention [10]. Due to the high rate of disease progression and the difficulty in accessing laboratory evaluations in the African nations [11], WHO recommended empirical treatment which are ampicillin and gentamicin or amikacin, and third-generation cephalosporins [12, 13]. In general, empirical antibiotic regimens should be guided by the local antimicrobial resistance patterns of bacterial isolates commonly detected in neonatal intensive care unit (NICU) or in the community setting [14].
Carbapenems are listed in the ‘watch group’ of WHO Access, Watch, Reserve classification as critically important antimicrobials for human medicine with a higher resistance potential [15]. Carbapenem-resistant gram-negative bacilli (CRGNB) have recently evolved and are spreading rapidly, raising global concerns [16]. Bacteria can break down β-lactam antibiotics, including penicillin, cephalosporin, and monobactams, using carbapenemase, an enzyme that hydrolyzes carbapenems [17]. Infections caused by CRGNB are known to be associated with significant morbidity and mortality, and these pathogens are now reported to be on the increase in children and neonates [18]. Carbapenem-resistant infections result in longer hospital admissions, higher healthcare costs, and increased mortality than carbapenem-susceptible bacterial infections [19]. Since the first CRGNB epidemic, many nations have seen a sharp rise in the prevalence of CRGNB infections [20]. Due to the extremely high rates of extended-spectrum beta-lactamase synthesis among gram-negative bacteria, carbapenem antibiotics are used extensively, which has led to the evolution of plasmid-mediated resistance to carbapenems [21].
It is estimated that at least 5,880 deaths can be attributed to these illnesses [22]. The emergence of bacteria resistant to carbapenem puts treatment strategy in jeopardy. Few drug options, like polymyxins, tigecycline, and fosfomycin, may be effective to treat infections by CRGNB. However, these antibiotics are rarely used as a monotherapy to treat these infections, either because of its complex pharmacokinetics or toxicity or unknown optimal therapeutic doses [23]. The fact that carbapenemase-producing bacteria are so prevalent around the globe makes them clinically important [24]. Several nations do not have prevalence estimates of carbapenem resistance in neonates, even though this age group is extremely susceptible. The pooled estimates of common gram-newgative bacteria (GNB) that cause neonatal sepsis in developing countries that are resistant to carbapenem medications are presently unknown [25]. Therefore this systematic review and meta-analysis aimed to provide an update on the rising of these CRGNB.
Methods
The systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines [26]. The protocol for this study was submitted to the International Prospective Register of Systematic Reviews (PROSPERO) and was assigned the identification number (CRD42024547715).
Data source and searching strategy
Electronic databases and search engines were used to gather important data about carbapenem-resistant Enterobacteriaceae causing neonatal sepsis. The search was done from data available from January 1, 2010, up to December 30, 2023 and the search was conducted from January 15, 2024- March 1, 2024. A systematic search was carried out using PubMed/Medline, Scopus, and Science Direct electronic databases. Additionally, articles available on Google Scholar and online repositories/registers of different institutions were also retrieved as part of the search process. We strictly followed the PRISMA flow diagram to report this study [26]. Appropriate MeSH (Medical Subject Headings) terms and key searching words were employed to retrieve relevant articles published in the English language. Our search string was developed using the following keywords: “prevalence”, “Carbapenem”, “carbapenem-resistant”, “carbapenemase-producers” “Enterobacteriaceae”, “gram-negative”, “Klebsiella”, “pseudomonas”, “E.coli”, “neonates”, “newborn”, “Sepsis”, and “bloodstream infection”, “Africa. These search words/phrases were further paired with each other or combined using “AND” and “OR” Boolean operators. Additionally, the remaining papers were screened at the reference lists to identify additional relevant data. The complete search strategy and searching strings for the PubMed/MEDLINE database are depicted in the supplementary file (Table S1). Furthermore, we reviewed the reference lists of primary studies and review papers to identify grey literature.
Study selection and quality assessment
All retrieved studies were exported into the EndNote reference manager software (Tomson Reuters, London), and duplicated studies were removed. Reviewers (AS, GK, ZA, YG, EG, and MAR) independently screened the titles, abstracts, and full texts to determine the eligibility of each study. Where there was disagreement, a decision was reached after discussion and consensus among all reviewers. Five reviewers (AS, ZA, GK, YG, EG, and MAR) independently assessed the quality of the full-text articles. The discrepancy was resolved through discussion to reach on consensus and to include articles in the final analysis. The critical quality assessment checklist recommended by the “Joanna Briggs Institute (JBI)” was used to evaluate the quality of the included studies studies [27]. The domain paper quality assessment criteria for prevalence studies were clearly stated (Table S2). Disagreements between the reviewers were resolved by taking the mean score of the two reviewers’ evaluations. Studies with a final quality score of 50% or higher were considered for inclusion in this systematic review and meta-analysis.
Eligibility criteria
After retrieving potential papers from the databases, they were subjected to eligibility screening. Thus, papers were included if they report; (a) GNB isolates tested for carbapenem, (b) CRGNB isolates from neonates, (c) neonates suspected of sepsis, (d) English language papers, (e) papers conducted in Africa, (f) and cross-sectional, and cohort studies. Papers were excluded if they reported antibiotic sensitivity other than carbapenem drugs. Review studies, letters, case reports, case control, and conference papers were excluded. Also, we excluded studies that have methodological problems and flaws (lack of clear measurement, incomplete diagnostic criteria, selection bias, and unclear presentation of the study population).
Data extraction
All articles included in the final analysis were reviewed and relevant data were recorded by two reviewers independently using standardized data extraction tools prepared in the Microsoft Excel sheet. The following data were extracted from each original article: author’s name, year of publication, study country, study design, sample size, number of bacteria isolates tested for carbapenem-resistant, and the prevalence of carbapenem resistance.
Outcome measurement
Molecular and culture confirmed sepsis with carbapenem resistant gram negative bacteria was considered. Carbapenem-resistance was defned as resistance to any one of meropenem, imipenem, or ertapenem according to the US Central Laboratory Standards Institute.
Data processing and analysis
The extracted relevant data were exported to STATA 17 for final analysis. The overall pooled prevalence of GNB which was tested for carbapenem resistance among neonates suspected of sepsis in Africa was calculated using the random effect model, due to the existence of heterogeneity across studies. The presence of heterogeneity across studies was examined using the Q and the I2 statistics. In this study, the I2 statistic value of zero indicates true homogeneity, whereas the values 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively [28]. A p-value of less than 0.05 was used to declare the presence of heterogeneity. Subgroup analysis was done by the study country, year of publication, and methods of isolation to assess the difference in the pooled estimates. Publication bias was checked by the funnel plot and more objectively through Egger’s regression test [29]. Sensitivity analysis was employed to examine the effect of a single study on the overall estimation of the pooled prevalence. To calculate the pooled prevalence of carbapenem resistance, the continuity correction was made for zero and one hundred% carbapenem resistance values which resulted in zero standard error [30].
Results
Searching results
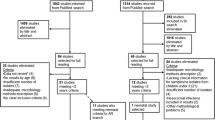
As illustrated in Fig. 1, we identified a total of 7,365 potentially relevant studies from searched electronic databases and search engines, and 1,623 articles were excluded due to duplication. After reviewing the titles and abstracts, 5,361 articles were excluded because they did not meet the objectives and the inclusion criteria of the review. Accordingly, 381 full-text articles were reviewed in-depth based on the preset inclusion criteria, of which 345 articles were excluded due to full-text inaccessibility, lack of carbapenem susceptibility testing, studies outside Africa, and failure to include the study subject’s age of interest. Finally, 36 studies were included and used for the final quantitative analysis (meta-analysis) (Fig. 1).
Results of quality assessment
The critical quality assessment checklist recommended by the “Joanna Briggs Institute (JBI)” Studies with a final quality score of 50% or higher were considered in this systematic review and meta-analysis.
Characteristics of included studies
As illustrated in Table 1, for all the included studies (n = 36), the final quantitative analysis was done. From these, 24 studies were cross-sectional [18, 31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53], and 12 were retrospective [54,55,56,57,58,59,60,61,62,63,64,65] by study design. The included studies were conducted in eleven countries in Africa. These were eleven studies from Egypt, one study from Equatorial Guinea, five studies from Ethiopia, two studies from Ghana, one study from Morocco, two studies from Nigeria, two studies from Sudan, six studies from South Africa, four studies from Tanzania, one study from Uganda, and one study from Zambia (Table 1).
Meta-analysis
The pooled prevalence of carbapenem resistant isolates
The resistance test used in this meta-analysis included 7,116 isolates for carbapenem (2,946, 2,618, and 1,552 isolates for meropenem, imipenem, and, ertapenem respectively). The pooled prevalence of carbapenem resistance in GNB was 30.34% (95%CI 22.03–38.64) (Fig. 2). Whereas the meta-analysis produced the pooled estimates of isolates with resistance to meropenem, imipenem, and ertapenem of 26.11% (95% CI: 15.82–36.40), 34.35% (95% CI: 20.04–48.67%) and 35.57 (95% CI 0.60-70.54) (Figs. 3, 4 and 5) respectively. In this review, the most frequently tested isolate for carbapenem drugs was K. pneumoniae which accounts for 2,719 (38.2%) of all isolates. In this study, a total of 1,231 A. baumannii isolates were tested for carbapenem resistance. The results indicated a significant prevalence of carbapenem resistance, with 45.9% (95%CI 33.1–58.7) of the isolates demonstrating strong resistance. Similarly, this bacterium exhibited the highest pooled prevalence of meropenem resistance, with 36.7% (95% CI: 20.1–53.3) of the 565 tested isolates showing resistance, In contrast, E. coli exhibited the lowest pooled prevalence of meropenem resistance, with only 6.9% (95% CI: 2.0–11.8) of the 318 tested isolates showing resistance (Table 2).
Level of heterogeneity
The included studies’ level of heterogeneity was evaluated. Thus, 99.7% of the studies showed considerable heterogeneity for carbapenem (Fig. 2). The I2 test results for each drug were 99.37% for meropenem (Figs. 3), 99.73% for imipenem (Fig. 4), and 99.9% for ertapenem (Fig. 5). Furthermore, notable variations were observed between the investigations for every isolated species. The I2 revealed 99.8% for pseudomonas spp., 98.8% for A. baumannii, 96.3% for Citrobacter spp., 98.7% for E. coli, 99.0% for Enterobacter spp., 99.5% for K. pneumoniae, 99.8% for Klebsiella spp., and 99.8% for Serratia spp. for carbapenem resistance (Table 2).
Sub-group analysis for carbapenem
Having significant heterogeneity for carbapenem resistance across the included studies, a sub-group analysis was carried out by year of publication, study country and methods of isolation. The pooled prevalence of carbapenem-resistant bacterial isolates among newborns suspected of sepsis was reported to be 20.8% (95% CI 12.2% - 29.4%), 29.1% (95% CI 13.9% − 44.3%), and 32.7% (95% CI 21.0% − 44.5%), with I2 of 77.7%, 99.8% and 99.6 for the years 2010–2015, 2016–2020, and 2021–2023, respectively. There is also a variation of a pooled estimate of carbapenem resistance across countries ranging from 0.5% (95% CI -1.2-2.2) in Sudan to 57.9% (95% CI -3.7-28.7) in Egypt (Table 3).
Sub-group analysis for meropenem
The pooled prevalence of meropenem-resistant bacteria among newborns suspected of sepsis was reported to be 14.3% (95% CI 7.6–21.0), 31.3% (95% CI 11.3–51.3), and 24.8% (95% CI 11.3–38.2) for the years 2010–2015, 2016–2020, and 2021–2023, respectively, based on the year of publication. Our meta-analysis found that there was a variation in the pooled prevalence of carbapenem-resistant GNB isolates among neonates suspected of sepsis across countries, from 2.25% (95% CI -1.2- 5.7) in Ghana to 57.1% (95% CI 33.4, 80.7) in Egypt (Table 3).
Sub-group analysis for imipenem
The pooled prevalence of imipenem-resistant GNB from neonates with suspected bloodstream infections were found 54.5% (95% CI 35.6% -73.3%) in Egypt, 49.0% (95% CI -45.4% − 43.5%) in Ethiopia, 11.5% (95% CI -3.7 -26.7%) in South Africa, and 0.5% (95% CI -1.7 -2.7%) in Sudan according to the study country. Our meta-analysis revealed a variation in the pooled prevalence of CRGNB isolates among neonates suspected of sepsis. This variation was attributed to differences in isolation methods. The prevalence was 30.0% (95% CI: 10.0–42.9) using automated methods and 39.6% (95% CI: 18.5–60.7) using conventional culture methods (Table 3).
Publication bias
To assess the prevalence of publication bias in the included studies, a variety of methods were utilized. The funnel plot showed an uneven distribution of studies for carbapenem and for specific carbapenem drug groups (meropenem, imipenem, and ertapenem) (Figure S 1, 2, 3, and 4). However, Egger’s regression test for publication bias revealed marginally insignificant for carbapenem (p = 0.22) and also for each carbapenem group of drugs (meropenem (p = 0.24), imipenem (p = 0.52) and ertapenem (p = 0.72) (Figure S 5, 6, 7,8).
Sensitivity analysis
A leave-one-out sensitivity analysis was employed to identify the potential source of heterogeneity in the analysis of the pooled prevalence of resistance of bacteria that cause neonatal sepsis in Africa. The sensitivity analysis showed the effect of individual studies on the pooled estimate was insignificant, suggesting the robustness of the aggregated estimate. Therefore, the pooled prevalence of resistance of bacterial isolates for carbapenem, meropenem and imipenem was steady when examined by neglecting one study at a time (Figure: S 9. 10, 11).
Discussion
Estimating national and global prevalence data for neonatal sepsis, particularly cases caused by CRGNB, is crucial for prioritizing and implementing effective control measures [11]. In settings without microbiology capacity, treating newborns with clinical symptoms of sepsis with “big gun” antibiotics is harmful by increasing antibiotic resistance or adverse side effects [66]. This study aimed to present the updated pooled resistance estimate of GNB to carbapenem drugs among neonates with sepsis in African nations. Knowledge gaps on the population-based epidemiology of neonatal sepsis remain in most of low and middle income countries due the lack of a robust research infrastructure, formal healthcare systems or prioritisation of other important healthcare issues [67]. This study aimed to present the updated pooled resistance estimate of GNB to carbapenem drugs among neonates with sepsis in African nations.
In this systematic review and meta-analysis, the overall pooled prevalence of carbapenem-resistant isolates was 30.34%. This finding aligns with results from a study conducted across twelve African and Asian countries [68]. However, this result was higher compared to several other studies, including those from Asia and Africa on the burden of antibiotic resistance in neonates from developing societies (BARNARDS) [69], studies conducted across thirty nations [22], a systematic review from Iran [70], and research from China [71], a study focusing on low- and lower-middle-income countries [72], and a study conducted from seven Asian and African countries [73]. The elevated rate of pooled resistance could be attributed to the population’s growing resistance to antibiotics that are currently effective against GNB [74]. Additionally, globalization may contribute to the spread of antibiotic-resistant strains to regions where certain antibiotics have not yet been introduced which underscores considering regional variations in antibiotic sensitivity when developing treatment guidelines for neonatal sepsis and selecting new empirical antibiotics [75]. Furthermore, inadequate dosing or incomplete courses of treatment may also play a significant role in the emergence of antimicrobial resistance [76].
The overall pooled prevalence of meropenem resistance among isolates was 26.11%. This rate is higher compared to findings from a study across seven countries in Asia and Africa on antibiotic resistance in neonates from developing societies [69], as well as another study conducted in Asian and African countries [73]. Elevated rates of this condition are commonly observed and can vary significantly worldwide for various reasons. In Africa, the higher prevalence is often attributed to factors such as inadequate healthcare infrastructure, socioeconomic challenges, limited maternal health services, suboptimal infection control practices, insufficient diagnostic stewardship, and cultural practices that influence essential newborn care [77].
In this systematic review and meta-analysis, the overall pooled prevalence of imipenem resistance was 34.35%. This rate was comparable to findings from a systematic review and meta-analysis conducted in Iran [78]. However, it was higher than results from studies involving seven countries in Asia and Africa on antibiotic resistance in neonates from developing societies (BARNARDS) [69], nother systematic review from Iran [70],, and studies from various Asian and African countries [73]. This higher prevalence may be attributed to factors such as increased self-medication, overuse of antibiotics, and the use of insufficient doses or incomplete treatment courses [76]. Additionally, a global point prevalence survey of antimicrobial prescribing in neonatal and pediatric sepsis found that less than a quarter of neonates received WHO-recommended first- or second-line empirical antibiotics [79].
To address this global health threat, it is essential to implement robust infection prevention measures, antimicrobial stewardship, and strict surveillance of infections and antimicrobial resistance (AMR). This is particularly crucial as third-line medications and carbapenems are increasingly losing their effectiveness [80]. This finding highlights a persistently high pooled prevalence of carbapenem-resistant bacterial isolates, with A. baumannii exhibiting the highest resistance rate at 45.9%, followed by Pseudomonas spp. at 43.0%, and Klebsiella spp. at 36.7%. These results are consistent with studies conducted in low- and middle-income countries [72], across thirty nations [22], and Iran [81]. In contrast, other studies have reported different patterns of carbapenem resistance. For instance, research conducted in Sub-Saharan African countries identified K. pneumoniae as the most resistant, followed by E. coli [82]. In Iran, Enterobacter spp. was most resistant, followed by Pseudomonas spp. [70], while another study in Sub Sharan Africa found Klebsiella spp. to be the most resistant, followed by E. coli [83]. Additionally, a study from China reported E. coli as the most resistant, with K. pneumoniae following [84]. The variation might be attributed to differences in the implementation of policies related to the control of drug-resistant bacteria. These policies can influence factors such as infection prevention measures, antimicrobial stewardship practices, and surveillance systems, which in turn affect the prevalence and types of resistant strains observed in different regions.
In this study, K. pneumoniae was identified as the most common isolate causing neonatal sepsis. This predominance of K. pneumoniae as the primary causative agent of neonatal sepsis is more pronounced in studies from Iran [70, 78], seven countries of Asia and Africa on the burden of antibiotic resistance in neonates from developing societies [69], Sub-Saharan African countries [82], studies conducted from Asian and African countries [73]. In contrast to this studies from China [84] E. coli followed by Klebsiella spp and Iran [85] Enterobacter spp followed by K. pneumoniae were the main causative pathogens of neonatal sepsis. National disparities in microbiology may result from changes in community flora, varying incidence of maternal and neonatal risk factors, and differences in neonatal healthcare practices [82]. Additionally, the observed dissimilarities could be due to epidemiological variations in bacterial strains and shifts in the incidence and etiology of bloodstream infections over time [86].
The examined studies displayed significant heterogeneity regarding publication year, isolation methods, and study country. The percentage of carbapenem-resistant isolates varied widely between countries, from 0.5% in Sudan to 57.9% in Egypt. This variability is supported by studies from diverse settings across 12 countries [68], in sub-Saharan Africa [82], and other studies conducted across thirty nations [22]. These variations can be attributed to several factors, including the fact that some pooled estimates were derived from studies with small sample sizes and highly variable blood culture positivity rates. Additionally, differences in detection capacities, population density, malnutrition prevalence, health-seeking behaviors, and the effectiveness of infection prevention and control measures in healthcare facilities can also contribute to the observed variability in carbapenem resistance rates [87]. These limitations are crucial when interpreting our findings. Additionally, factors such as the preventive measures implemented in each country, the clinical criteria used to diagnose sepsis, the sensitivity and specificity of culture methods in various laboratories, the hygienic conditions in delivery areas, and the social and economic conditions of the nations may all contribute to explaining these regional differences [88].
Limitations of the study
There were various restrictions on our review. Initially, the analysis in this study was limited to papers written in the English language. Second, there may be variances in interpretations and conclusions due to changes in antimicrobial susceptibility standards and interpretive criteria over time, and the pooled estimates of carbapenem resistance were based on studies with few isolates and highly fluctuating carbapenem resistance rates.
Conclusions
Overall, this meta-analysis showed a broad range of gram-negative bacilli. Furthermore, there was a significant difference in the carbapenem resistance patterns of the isolates across these countries. Stronger microbiology laboratory capacity to diagnose drug resistance is required in countries with a high burden of neonatal sepsis. This inturn will enable more accurate detection, timely diagnosis, and effective monitoring of these resistant strains which lead to more targeted and effective treatments and informed treatment decisions.
Feature perspective
The feature perspective involves identifying and analyzing the specific traits and mechanisms that contribute to this resistance. This approach helps in understanding how resistance develops, how it can be detected, and what strategies might be effective in combating it. Key features include: Mechanisms of resistance, genetic elements, phenotypic characteristics and environmental and contextual factors.
The added value of this study
This is the first comprehensive review of carbapenem-resistant gram-negative bacterial infections in neonates in Africa, as far as we are aware. Our analysis evaluates geographical variations in pathogen dominance and carbapenem resistance patterns in addition to filling in the information gap about carbapenem-resistant bacteria that cause invasive bacterial infections.
Recommendation
Platforms that provide data in a timely and useful manner should be created since the types of GNB infections and the corresponding carbapenem resistances are altered over time. The observed increase in GNB resistance to carbapenem drugs indicates that there should be an urgent need for enhanced infection control measures, careful antimicrobial stewardship practices, and strengthened surveillance systems to curb the spread of resistant strains and ensure effective treatment of GNB infections in Africa. Additionally, collaborative efforts at local, national, and international levels are warranted to address the multifaceted factors contributing to AMR and mitigate its impact on public health.
Data availability
All generated data and research materials used during this systematic review and meta analysis are available from paper and supplementary material.
Abbreviations
- AMR:
-
Antimicrobial Resistance
- CI:
-
Confidence Interval
- CLSI:
-
Clinical and Laboratory Standards Institute
- CRGNB:
-
Carbapenem-Resistant Gram-Negative Bacilli
- EOS:
-
Early Onset Sepsis
- GNB:
-
Gram-Negative Bacteria
- JBI:
-
Joanna Briggs Institute
- LOS:
-
Late-Onset Sepsis
- MeSH:
-
Medical Subject Headings
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- WHO:
-
World Health Organization
References
Randolph AG, McCulloh RJ. Pediatric sepsis: important considerations for diagnosing and managing severe infections in infants, children, and adolescents. Virulence. 2014;5(1):179–89.
Gomaa HH, Udo EE, Rajaram U. Neonatal septicemia in Al-Jahra Hospital, Kuwait: etiologic agents and antibiotic sensitivity patterns. Med Principles Pract. 2001;10(3):145–50.
Tsering DC, Chanchal L, Pal R, Kar S. Bacteriological profile of septicemia and the risk factors in neonates and infants in Sikkim. J Global Infect Dis. 2011;3(1):42.
Seale AC, Blencowe H, Manu AA, Nair H, Bahl R, Qazi SA, et al. Estimates of possible severe bacterial infection in neonates in sub-saharan Africa, south Asia, and Latin America for 2012: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):731–41.
Kaistha N, Mehta M, Singla N, Garg R, Chander J. Neonatal septicemia isolates and resistance patterns in a tertiary care hospital of North India. J Infect Developing Ctries. 2010;4(01):055–7.
Cassini A, Allegranzi B, Fleischmann-Struzek C, Kortz T, Markwart R, Saito H et al. Global Report on the epidemiology and burden on sepsis: current evidence, identifying gaps and future directions. Global Report on the epidemiology and burden on sepsis: current evidence, identifying gaps and future directions. 2020.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of Disease Study. Lancet. 2020;395(10219):200–11.
Unicef. Levels and trends in child mortality report 2018. 2018.
Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376(9749):1339–46.
Organization WH. Global action plan on antimicrobial resistance. 2015.
Weltgesundheitsorganisation. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. World Health Organization; 2013.
Fuchs A, Bielicki J, Mathur S, Sharland M, Van Den Anker J. Antibiotic use for sepsis in neonates and children: 2016 evidence update. WHO Reviews. 2016.
Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30(14):2068–9.
Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet. 2017;390(10104):1770–80.
Umair M, Walsh TR, Mohsin M. A systematic review and meta-analysis of carbapenem resistance and its possible treatment options with focus on clinical Enterobacteriaceae: thirty years of development in Pakistan. Heliyon. 2024.
Lee C-R, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase-producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016:895.
Logan LK. Carbapenem-resistant enterobacteriaceae: an emerging problem in children. Clin Infect Dis. 2012;55(6):852–9.
Kabwe M, Tembo J, Chilukutu L, Chilufya M, Ngulube F, Lukwesa C, et al. Etiology, antibiotic resistance and risk factors for neonatal sepsis in a large referral center in Zambia. Pediatr Infect Dis J. 2016;35(7):e191–8.
Lutgring JD. Carbapenem-resistant Enterobacteriaceae: an emerging bacterial threat. Semin Diagn Pathol. 2019;36(3):182–6.
Vardakas KZ, Matthaiou DK, Falagas ME, Antypa E, Koteli A, Antoniadou E. Characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. J Infect. 2015;70(6):592–9.
Friedman ND, Carmeli Y, Walton AL, Schwaber MJ. Carbapenem-resistant Enterobacteriaceae: a strategic roadmap for infection control. Infect Control Hosp Epidemiol. 2017;38(5):580–94.
Hu Y, Yang Y, Feng Y, Fang Q, Wang C, Zhao F, et al. Prevalence and clonal diversity of carbapenem-resistant Klebsiella pneumoniae causing neonatal infections: a systematic review of 128 articles across 30 countries. PLoS Med. 2023;20(6):e1004233.
Thomas R, Velaphi S, Ellis S, Walker AS, Standing JF, Heath P, et al. The use of polymyxins to treat carbapenem resistant infections in neonates and children. Expert Opin Pharmacother. 2019;20(4):415–22.
Patel JB, Rasheed JK, Kitchel B. Carbapenemases in Enterobacteriaceae: activity, epidemiology, and laboratory detection. Clin Microbiol Newsl. 2009;31(8):55–62.
Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respiratory Med. 2018;6(3):223–30.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
Munn Z, Moola S, Lis K, Riitano D, C T. Systematic reviews of prevalence and incidence. In: Aromataris E, Z. M, editors. JBI Manual for evidence synthesis. JBI; 2020.
Borenstein M, Cooper H, Hedges L, Valentine J. Heterogeneity in meta-analysis. Handb Res Synthesis meta-analysis. 2019;3:453–70.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). 1997;315(7109):629–34.
Sweeting J, Sutton MJ, Lambert AC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med. 2004;23(9):1351–75.
Braima OA, Ali MA, Abdulla EM. Bacteriological profile and antibiotic resistance in newborn infants with possible community-acquired neonatal sepsis in Khartoum State, Sudan. Sudan J Paediatrics. 2021;21(1):13.
Kayange N, Kamugisha E, Mwizamholya DL, Jeremiah S, Mshana SE. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatr. 2010;10:1–9.
Majigo M, Makupa J, Mwazyunga Z, Luoga A, Kisinga J, Mwamkoa B, et al. Bacterial aetiology of neonatal Sepsis and Antimicrobial Resistance Pattern at the Regional Referral Hospital, Dar Es Salam, Tanzania; a call to strengthening antibiotic stewardship program. Antibiotics. 2023;12(4):767.
Onyedibe KI, Bode-Thomas F, Nwadike V, Afolaranmi T, Okolo M, Uket O. High rates of bacteria isolates of neonatal sepsis with multidrug resistance patterns in Jos, Nigeria. Ann Pediatr Child Health. 2015;3(2):1052.
Gaballah AH, Shawky S, Amer AN. Microbiological profiles of neonatal sepsis in Northern Egypt. Microbes Infect Dis. 2022;3(3):645–56.
Silago V, Kovacs D, Msanga DR, Seni J, Matthews L, Oravcová K et al. Bacteremia in critical care units at Bugando Medical Centre, Mwanza, Tanzania: The role of colonization and contaminated cots and mothers’ hands in cross-transmission of multidrug resistant Gram-negative bacteria. Antimicrob Resist Infect Control. 2020;9(1).
Weldu Y, Naizgi M, Hadgu A. Neonatal septicemia at intensive care unit, Ayder Comprehensive Specialized Hospital, Tigray, North Ethiopia: bacteriological profile, drug susceptibility pattern, and associated factors. 2020;15(6):e0235391.
Worku E, Fenta DA, Ali MM. Bacterial etiology and risk factors among newborns suspected of sepsis at Hawassa, Ethiopia. Sci Rep. 2022;12(1):20187.
Tumuhamye J, Sommerfelt H, Bwanga F, Ndeezi G, Mukunya D, Napyo A, et al. Neonatal sepsis at Mulago national referral hospital in Uganda: etiology, antimicrobial resistance, associated factors and case fatality risk. PLoS ONE. 2020;15(8):e0237085.
Ghaith DM, Zafer MM, Said HM, Elanwary S, Elsaban S, Al-Agamy MH, et al. Genetic diversity of carbapenem-resistant Klebsiella Pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur J Clin Microbiol Infect Dis. 2020;39:583–91.
Panwar K, Dutta S, Sarkar R, Sinha E, Arun A, Venu G, et al. Int J Curr Microbiol Appl Sci Int J Curr Microbiol App Sci. 2023;12(01):261–74.
Shatalov A, Awwad F, Mangue P, Foqahaa RJ. Predominance of multi-drug resistant Klebsiella pneumonia and other Gram negative bacteria in neonatal sepsis in Equatorial Guinea. Open J Med Microbiol. 2015;5(04):254.
Uwe NO, Ezenwa BN, Fajolu IB, Oshun P, Chukwuma ST, Ezeaka VC. Antimicrobial susceptibility and neonatal sepsis in a tertiary care facility in Nigeria: a changing trend? JAC-antimicrobial resistance. 2022;4(5):dlac100.
Fenta GM, Woldemariam HK, Metaferia Y, Seid A, Gebretsadik D. Admission outcome and Antimicrobial Resistance Pattern of Bacterial isolates among neonates with suspected Sepsis in neonatal intensive care unit at Dessie Comprehensive Specialized Hospital, Dessie, Northeastern Ethiopia. Interdisciplinary perspectives on infectious diseases. 2022;2022.
Msanga DR, Parpia F, Konje ET, Hokororo A, Mshana SE. High mortality among premature neonates with positive blood culture neonatal sepsis in a tertiary hospital, Tanzania: a call for action. Children. 2021;8(11):1037.
Babiker W, Ahmed A, Babiker T, Ibrahim E, Almugadam B. Prevalence and Causes of Neonatal Sepsis in Soba University Hospital, Sudan. Med Microbiol Rep 1: 2. of. 2018;3:11 – 3.
Gashaw M, Ali S, Tesfaw G, Eshetu B, Workneh N, Berhane M et al. Bacterial profile and drug resistance patterns in neonates admitted with sepsis to a tertiary teaching hospital in Ethiopia. 2019.
Hassan F, Shawky A, Ahmed AAA, Hagras AM, Fahim NAE, Abdelkader H. Molecular Identification of Multi Drug-resistant Klebsiella pneumoniae isolated from neonates with Sepsis in Egypt.
Solomon S, Akeju O, Odumade OA. Prevalence and risk factors for antimicrobial resistance among newborns with gram-negative sepsis. 2021;16(8):e0255410.
Raafat RM, Dwedar RA, Bassyouni RH, Emira AS, Abd El-Hmid RG, Dowidar MA et al. Alarming antibiotic resistance pattern of bacterial isolates in neonatal Sepsis: A Study from Egypt.
Shehab El-Din EMR, El-Sokkary MMA, Bassiouny MR, Hassan R. Epidemiology of neonatal sepsis and implicated pathogens: a study from Egypt. Biomed Res Int. 2015;2015.
Aamir R, Ateya RM, Arafa M, Yahia S. Ceftazidime/avibactam efficiency tested in vitro against carbapenem-resistant Klebsiella pneumoniae isolated from neonates with sepsis. Microbes Infect Dis. 2021;2(3):529–40.
Aamir MM, Ali E, Hamouda M, Mourad F. Prevalence of multidrug resistant bacteria causing late-onset neonatal sepsis. Int J Curr Microbiol App Sci. 2015;4(5):172–90.
Almohammady MN, Eltahlawy EM, Reda NM. Pattern of bacterial profile and antibiotic susceptibility among neonatal sepsis cases at Cairo University Children Hospital. J Taibah Univ Med Sci. 2020;15(1):39–47.
Awad HA, Mohamed MH, Badran NF, Mohsen M, Abd-Elrhman AS. Multidrug-resistant organisms in neonatal sepsis in two tertiary neonatal ICUs, Egypt. J Egypt Public Health Assoc. 2016;91(1):31–8.
Ballot DE, Bandini R, Nana T, Bosman N, Thomas T, Davies VA et al. A review of -multidrug-resistant Enterobacteriaceae in a neonatal unit in Johannesburg, South Africa. BMC Pediatr. 2019;19(1).
Pillay D. Microbial profile and antimicrobial susceptibility patterns of neonatal blood stream infections in Durban, South Africa. 2020.
Thomas R, Wadula J, Seetharam S, Velaphi S. Prevalence, antimicrobial susceptibility profiles and case fatality rates of acinetobacter baumannii sepsis in a neonatal unit. J Infect Developing Ctries. 2018;12(4):211–9.
Tetteh FKM, Fatchu R, Ackah K, Philips TJ, Shewade HD, Fenny AP, et al. Sepsis among neonates in a Ghanaian Tertiary Military Hospital: culture results and Turnaround Times. Int J Environ Res Public Health. 2022;19(18):11659.
Perez-Palacios P, Girlich D, Soraa N, Lamrani A, Maoulainine FMR, Bennaoui F, et al. Multidrug-resistant Enterobacterales responsible for septicaemia in a neonatal intensive care unit in Morocco. J Global Antimicrob Resist. 2023;33:208–17.
van Staaden H, Hendricks C, Spicer K. Bacteraemia and antibiotic sensitivity in a tertiary neonatal intensive care unit. South Afr J Infect Dis. 2021;36(1):195.
Acheampong EN, Tsiase JA, Afriyie DK, Amponsah SK. Neonatal Sepsis in a Resource-Limited setting: causative microorganisms and Antimicrobial Susceptibility Profile. Interdisciplinary perspectives on infectious diseases. 2022;2022.
Fahmey SS. Early-onset sepsis in a neonatal intensive care unit in Beni Suef, Egypt: bacterial isolates and antibiotic resistance pattern. Korean J Pediatr. 2013;56(8):332.
Lebea MM, Davies V. Evaluation of culture-proven neonatal sepsis at a tertiary care hospital in Johannesburg, South Africa. South Afr J Child Health. 2017;11(4):170–3.
Reddy K, Bekker A, Whitelaw AC, Esterhuizen TM, Dramowski A. A retrospective analysis of pathogen profile, antimicrobial resistance and mortality in neonatal hospital-acquired bloodstream infections from 2009–2018 at Tygerberg Hospital, South Africa. PLoS ONE. 2021;16(1):e0245089.
Solomon S, Akeju O, Odumade OA, Ambachew R, Gebreyohannes Z, Van Wickle K, et al. Prevalence and risk factors for antimicrobial resistance among newborns with gram-negative sepsis. PLoS ONE. 2021;16(8):e0255410.
Diaz JV, Riviello ED, Papali A, Adhikari NK, Ferreira JC. Global critical care: moving forward in resource-limited settings. Annals Global Health. 2019;85(1).
Li G, Bielicki JA, Ahmed ANU, Islam MS, Berezin EN, Gallacci CB, et al. Towards understanding global patterns of antimicrobial use and resistance in neonatal sepsis: insights from the NeoAMR network. Arch Dis Child. 2020;105(1):26–31.
Thomson KM, Dyer C, Liu F, Sands K, Portal E, Carvalho MJ, et al. Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy (BARNARDS). Lancet Infect Dis. 2021;21(12):1677–88.
Akya A, Rostamian M, Rezaeian S, Ahmadi M, Janatolmakan M, Sharif SA et al. Bacterial causative agents of neonatal sepsis and their antibiotic susceptibility in neonatal intensive care units (NICUs) and neonatal wards in Iran: a systematic review. Archives Pediatr Infect Dis. 2020;8(2).
Ding Y, Wang Y, Hsia Y, Sharland M, Heath PT. Systematic review of carbapenem-resistant Enterobacteriaceae causing neonatal sepsis in China. Ann Clin Microbiol Antimicrob. 2019;18:1–8.
Wen SC, Ezure Y, Rolley L, Spurling G, Lau CL, Riaz S, et al. Gram-negative neonatal sepsis in low-and lower-middle-income countries and WHO empirical antibiotic recommendations: a systematic review and meta-analysis. PLoS Med. 2021;18(9):e1003787.
Sands K, Carvalho MJ, Portal E, Thomson K, Dyer C, Akpulu C, et al. Characterization of antimicrobial-resistant Gram-negative bacteria that cause neonatal sepsis in seven low-and middle-income countries. Nat Microbiol. 2021;6(4):512–23.
Crichton H, O’Connell N, Rabie H, Whitelaw A, Dramowski A. Neonatal and paediatric bloodstream infections: pathogens, antimicrobial resistance patterns and prescribing practice at Khayelitsha District Hospital, Cape Town, South Africa. South Afr Med J. 2018;108(2):99–104.
Munita JM, Arias CA. Mechanisms of antibiotic resistance. Virulence Mech Bacterial Pathogens. 2016:481–511.
Worku S, Gelaw A, Aberra Y, Muluye D, Derbie A, Biadglegne F. Bacterial etiologies, antibiotic susceptibility patterns and risk factors among patients with ear discharge at the University of Gondar Hospital, Northwest Ethiopia. Asian Pac J Trop Dis. 2017;7(1):36–42.
Alemu AY, Endalamaw A, Belay DM, Mekonen DK, Birhan BM, Bayih WA. Healthcare-associated infection and its determinants in Ethiopia: a systematic review and meta-analysis. PLoS ONE. 2020;15(10):e0241073.
Moftian N, Rezaei-Hachesu P, Arab-Zozani M, Samad-Soltani T, Esfandiari A, Tabib MS, et al. Prevalence of gram-negative bacteria and their antibiotic resistance in neonatal sepsis in Iran: a systematic review and meta-analysis. BMC Infect Dis. 2023;23(1):534.
Jackson C, Hsia Y, Basmaci R, Bielicki J, Heath PT, Versporten A, et al. Global divergence from World Health Organization treatment guidelines for neonatal and pediatric sepsis. Pediatr Infect Dis J. 2019;38(11):1104–6.
Laxminarayan R, Bhutta ZA. Antimicrobial resistance—a threat to neonate survival. Lancet Global Health. 2016;4(10):e676–7.
Aletayeb SMH, Dehdashtian M, Malakian A, Aramesh MR, Kouti L, Aletayeb F, Frequency. Bacteriological Profile, and outcome of neonatal Sepsis with Carbapenem-Resistant Gram-negative Bacteria at the Tertiary neonatal intensive care unit, Ahvaz, Iran. Jundishapur J Microbiol. 2023;16(4).
Okomo U, Akpalu EN, Le Doare K, Roca A, Cousens S, Jarde A, et al. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect Dis. 2019;19(11):1219–34.
Kowalski M, Obama BM, Catho G, Dewez JE, Merglen A, Ruef M et al. Antimicrobial resistance in Enterobacterales infections among children in sub-saharan Africa: a systematic review and meta-analysis. Eclinicalmedicine. 2024;70.
Li J-y, Chen S-q, Yan Y-y, Hu Y-y, Wei J, Wu Q-p, et al. Identification and antimicrobial resistance of pathogens in neonatal septicemia in China—A meta-analysis. Int J Infect Dis. 2018;71:89–93.
Akbarian-Rad Z, Riahi SM, Abdollahi A, Sabbagh P, Ebrahimpour S, Javanian M, et al. Neonatal sepsis in Iran: a systematic review and meta-analysis on national prevalence and causative pathogens. PLoS ONE. 2020;15(1):e0227570.
Guimarães R, Cunha T, Oliveira A, de Araujo L, Pedroso R. Bloodstream infection: the influence of risk factors, etiology and antimicrobial therapy on mortality rates. J Nurs Care. 2017;6(391):2167–11681000391.
Agnche Z, Yenus Yeshita H, Abdela Gonete K. Neonatal sepsis and its associated factors among neonates admitted to neonatal intensive care units in primary hospitals in central gondar zone, northwest Ethiopia, 2019. Infect Drug Resist. 2020:3957–67.
Schuchat A, Zywicki SS, Dinsmoor MJ, Mercer B, Romaguera J, O’Sullivan MJ, et al. Risk factors and opportunities for prevention of early-onset neonatal sepsis: a multicenter case-control study. Pediatrics. 2000;105(1):21–6.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AS: led the systematic review and meta-analysis, overseeing the study’s conceptualization, article selection, data extraction, statistical analysis, and manuscript writing. AS, ZA, MAR, EG, ST, and YG: involved in searching for relevant articles, conducting data extraction, performing statistical analysis, and involved in manuscript drafting. BBA, MAR, and AAK were involved in statistical analysis, consultation of the overall process of this systematic review, and meta-analysis. WA, TM, AA, MG, CM, AAL, GK, MN, SA, AJ, SG, ZD and WK, were involved in data extraction, statistical analysis, manuscript writing, editing, and ensuring the accuracy and completeness of the data. Additionally, all authors actively engaged in critically reviewing the study’s progress, data analysis, and manuscript writing, involved in the approval of the final manuscript for submission, thereby affirming their endorsement of its content and findings.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In this study, ethics approval and consent to participate were not required as it exclusively utilized publicly available aggregated data.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sisay, A., Asmare, Z., Kumie, G. et al. Prevalence of carbapenem-resistant gram-negative bacteria among neonates suspected for sepsis in Africa: a systematic review and meta-analysis. BMC Infect Dis 24, 838 (2024). https://doi.org/10.1186/s12879-024-09747-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09747-6