Abstract
Background
The effect of nirmatrelvir/ritonavir on preventing post-COVID condition (PCC) in the BA4, BA5, and XBB Omicron predominant periods is not well understood. The purpose of this study was to assess how nirmatrelvir/ritonavir treatment affected both PCC and health-related quality of life.
Methods
This retrospective cohort study enrolled 2,524 adults aged 18 years and older who were eligible for nirmatrelvir/ritonavir between July 14 to November 14, 2022. All outcomes were observed from the patient’s first visit to the primary health clinic, 1 week, 1 month, 3 months, and 6 months after testing positive for COVID-19. The primary outcome was the presence of PCC. Secondary outcomes included the effects on health-related quality of life, such as walking, bathing and dressing, activities, cause adverse emotions or signs that prevent individuals from leading normal lives over a 180-day observation period.
Results
There were no significant differences observed between the nirmatrelvir/ritonavir and those not administered (control group) in terms of PCC symptoms at 3 months (OR 0.71 95% CI 0.31, 1.64) and 6 months (OR 1.30 95% CI 0.76, 2.21). At 3 months, the use of nirmatrelvir/ritonavir was associated with a 26% reduction in symptoms causing negative emotions (OR 0.74 95% CI 0.60, 0.92) and an increased likelihood of symptoms limiting walking (OR 1.58 95% CI 1.10, 2.27). However, there were no significant differences between the nirmatrelvir/ritonavir and the control group in terms of the impact of PCC on health-related quality of life at 6 months.
Conclusions
Our study indicates that the administration of nirmatrelvir/ritonavir does not significantly reduce PCC after 3 months and 6 months in a population with high vaccination coverage.
Similar content being viewed by others
Introduction
COVID-19 is an infectious disease caused by the SARS-CoV-2 virus. As of April 2024, Malaysia has reported over 5.2 million cases and 37,350 deaths. [1] While most people recover and symptoms resolve within a short period, certain high-risk groups may require hospitalization. Vaccination against COVID-19 has been shown to be beneficial in reducing infection, hospitalization, and mortality. [2, 3] Malaysia began offering COVID-19 vaccination in February 2021. [4] As of October 2022, the national COVID-19 vaccination coverage for the adult population was 98.2%. [5] However, despite the high vaccination coverage, a small portion of the population, particularly the elderly and those with underlying comorbidities such as diabetes, hypertension, hypercholesterolemia, chronic kidney disease and chronic pulmonary disease were at a higher risk of severe COVID-19 and mortality. [6, 7] In addition to acute symptoms, a subset of patients who survived the initial acute phase of COVID-19 reported persisting symptoms across a wide spectrum. [8] These symptoms include fatigue, myalgia, rashes, respiratory symptoms, cardiac problems, neurological issues and digestive symptoms. [9, 10]
Post-COVID condition (PCC) or long COVID is broadly defined as signs, symptoms, and conditions that continue to develop after the acute COVID-19 infection. [9] There is currently no consensus on when PCC can be identified. The Center for Disease Control and Prevention (CDC) identifies the start of PCC as four weeks after the first infection, while the World Health Organization and the National Institute for Health and Care Excellence (NICE) define it as 12 weeks after the first infection [4, 11]. Some countries classified PCC according to acute and chronic phases. The acute phase refers to persistent symptomatic COVID-19 in people with symptoms lasting between 4 and 12 weeks after the onset of acute illness. [9, 11,12,13] The chronic phase is defined as PCC symptoms lasting longer than 12 weeks. [9, 11,12,13] PCC symptoms can last for varying durations, ranging from weeks to months or even years. They may appear, persist, resolve, and reappear over different periods. [9] Due to the differences in definition and type of patients (hospitalized or non-hospitalized) used in published studies, the reported prevalence of PCC ranges from 13.6 to 45.0%. [10, 14]
Nirmatrelvir/ritonavir (Paxlovid™) received emergency use authorization from the US Food and Drug Administration (FDA) in December 2021 for the treatment of mild to moderate COVID-19 patients, followed by full approval in May 2023. [15] Real-world studies have demonstrated the effectiveness of nirmatrelvir/ritonavir in reducing the progression to severe COVID-19 in high-risk patients. [16,17,18,19] Nirmatrelvir/ritonavir functions by inhibiting viral replication of the SARS-CoV-2 virus, with the aim of reducing viremia during active COVID-19. [20]. [21] Various real world studies have shown mixed results on the effectiveness of nirmatrelvir/ritonavir in preventing PCC. (21–22) Congdon et al. analyzed eleven common long COVID symptoms (e.g., dyspnea, palpitations, changes in smell or taste, generalized fatigue or tiredness, headache, dizziness or light-headedness, chest pain, brain fog, nausea and vomiting, and paresthesia) four months after individuals tested positive for SARS-CoV-2. [21] Their findings indicated that nirmatrelvir/ritonavir did not significantly reduce overall PCC symptoms but did show a notable decrease in brain fog and chest pain/pressure. [21] In contrast, Xie et al. investigated 13 post-acute COVID-19 sequelae (e.g., deep vein thrombosis, acute kidney injury, muscle pain, neurocognitive impairment, dysautonomia, shortness of breath, and cough) up to 180 days post infection and reported a 26% reduction in PCC. [22]
In Malaysia, nirmatrelvir/ritonavir was conditionally approved by the National Pharmaceutical Regulatory Agency (NPRA) for the treatment of COVID-19 in high-risk patients. [23] Several studies have shown that nirmatrelvir/ritonavir reduces 30-day hospitalization rates of high-risk patients during different COVID-19 periods, regardless of vaccination status. [24,25,26] A real-world study in Malaysia also demonstrated a 36% reduction in hospitalization among highly vaccinated patients, when nirmatrelvir/ritonavir was administered in the early stages of the disease. [27] The effectiveness of nirmatrelvir/ritonavir in the acute phase of COVID-19 raises the question of whether it can also reduce the risk of PCC beyond 12 weeks. Although current clinical trials have not extended the evaluation period of nirmatrelvir/ritonavir beyond 30 days, real-world studies suggest its potential effectiveness in reducing PCC. [22, 28] Therefore, the aim of our study was to assess the impact of nirmatrelvir/ritonavir in preventing PCC among high-risk outpatients during the predominant periods of BA4, BA5, and XBB Omicron, as well as its influence on individual PCC and the overall health related-quality of life.
Methods
This study was approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR ID-22-00938-2YN). Study findings are reported based on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. [29]
Settings
MySejahtera application, owned by the Government of Malaysia, was developed to support the management of COVID-19 pandemic in the country. It includes an electronic medical record component utilized by the public health clinics (eCOVID system) and a mobile application used by the general public. After testing positive for COVID-19, individuals must access the MySejahtera application to notify the positive test results to the Ministry of Health (MOH) in compliance with the Malaysian Prevention and Control of Infectious Diseases Act 1988.
Nirmatrelvir/ritonavir treatment was administered to all eligible patients with COVID-19 at designated public health clinics across Malaysia. [30] The patient’s encounter and nirmatrelvir/ritonavir prescription will be recorded in the eCOVID system.
Additionally, the MySejahtera application was used to monitor symptoms of COVID-19 patients who have consented to self-report their conditions at home. This was done through push notification from the application on COVID-19 symptoms and health-related quality of life, using MyLongCOVID questionnaire from day 7 to day 180. The questionnaire was developed by incorporating findings from a literature review on the frequent symptoms of COVID-19 and consensus from local experts in infectious diseases, epidemiology, public health, and clinical research. It consists of three Sect. [31] The first section collects information on demographic characteristics and underlying medical comorbidities. The second section captures the presence of COVID-19-related symptoms. The third section assesses the impact of COVID-19 on the health-related quality of life of individuals, including aspects such as mobility, self-care, activities of daily living, emotions, and the ability to return to work. Notifications occurred at 1 week, 1 month, 3 months, and 6 months after their COVID-19 diagnosis and participation was voluntary. The MyLongCOVID study questionnaire is included in the supplementary materials.
Data source
Electronic datasets from Malaysia’s MySejahtera eCOVID system, which records COVID-19 patients’ encounters and antiviral prescriptions, were retrieved from 647 public health clinics from July 14 to November 14, 2022. Next, the eCOVID datasets were merged with MySejahtera’s MyLongCOVID database starting from July 14, 2022.
Study design and population
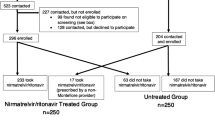
Adult patients aged 18 years and older who tested positive for COVID-19 and visited a public health clinic within 5 days and were eligible for nirmatrelvir/ritonavir treatment were included in the study. Data analysis was conducted from July 14 to November 14, 2022 for those who completed the MyLongCOVID symptoms questionnaire at 1 week, 1 month, 3 months, and 6 months. A full description of the eCOVID cohort is detailed elsewhere. [27] The final cohort consisted of 2,524 individuals, given the limited number of individuals who had completed the questionnaire at all time points. (See Fig. 1 for study selection criteria)
Exposure and outcomes
We defined the exposed groups as patients with COVID-19 who were administered nirmatrelvir/ritonavir, while those who did not receive the drug were designated as the control group. We examined individual post COVID-19 symptoms at intervals of 1 week, 1 month, 3 months and 6 months following a positive test for COVID-19. These symptoms include fatigue, myalgia, muscle weakness, shortness of breath, chest pain, cough, headache, insomnia, anxiety, depression, difficulty to focus, forgetfulness, dizziness upon standing, anosmia, ageusia (loss of taste), loss of appetite, diarrhea, stomach pain, rash and palpitation.
In our study, the primary outcome was PCC, defined by the clinical case definition provided by the World Health Organization. PCC is identified in individuals who continue to experience symptoms or develop new ones 3 months after testing positive for SARS-CoV-2 infection, with these symptoms lasting for at least 2 months. [32] Secondary outcomes were on health-related quality of life, including any symptoms that limit walking, bathing/cleaning/dressing, activities of daily living, emotions, and symptoms that prevent the individual from living a normal life without the support of others. All outcomes were observed from the patient’s first encounter at the primary health clinic until completion of the MyLongCOVID questionnaire (1 week, 1 month, 3 months and 6 months) on day 180 of testing positive for COVID-19.
Covariates
We included baseline characteristics such as age in years, sex, date of registration in the health clinic, day of illness onset, vaccination status (partially vaccinated or fully vaccinated/receive booster dose), the presence of comorbid conditions (cancer, heart disease, hypertension, diabetes, rheumatic disease, retroviral disease, liver disease, respiratory disease, gastrointestinal disease, and kidney disease) and presence of symptoms during acute phase (within 1 week of COVID-19 diagnosis).
Statistical analysis
Baseline characteristics and frequency of each of the PCC symptoms were reported as mean and standard deviation or as frequency and percentage for both groups. Chi-square tests or t-tests were applied to compare the demographics between the two groups. Multivariable logistic regression was used to estimate the association of the receipt of nirmatrelvir/ritonavir with PCC, adjusted for the aforementioned covariates. However, symptoms during the acute phase were not included due to confounding by indication. The adjusted odds ratio (aOR) were estimated for PCC at both 3 months and 6 months post COVID-19 diagnosis. The impact of receiving nirmatrelvir/ritonavir on health-related quality of life at 3 months and 6 months were also evaluated.
In addition, we also calculated the propensity score based on age group, sex, registration date, day of illness, vaccination status (partially vaccinated or fully vaccinated/received booster dose) and the presence of comorbidities using logistic regression model. We used inverse probability weighting (IPTW) for the difference in baseline characteristics of the nirmatrelvir/ritonavir and control groups. The absolute standardized differences, where a difference of less than 0.1 is considered good balance, were used to assess the covariate balance between groups. We then created the inverse probability weights by assigning subjects in the nirmatrelvir/ritonavir group a value of 1 divided by propensity score (1/propensity score) and using 1 divided by 1 minus the propensity score (1/1-propensity score) for those in the control group. This approach allowed us to estimate the association between nirmatrelvir-ritonavir and PCC in a population with identical baseline characteristics. Weights above 10 were truncated to reduce the influence of extreme weights (2 weights were larger than 10 and were truncated). Subsequently, we applied these weights to the logistic regression model to estimate the association of nirmatrelvir/ritonavir with PCC at 3 months and 6 months. Besides, we conducted a series of separate multivariable logistic regression models to assess the association of nirmatrelvir/ritonavir with PCC, using propensity score as covariates adjustment.
To ensure the robustness of findings, sensitivity analyses were conducted using the definition of PCC as the presence of one or more PCC symptoms at 3 months and 6 months.
The statistical analyses were performed using SAS 9.4 (SAS Institute) and R 4.2.4 (R project for statistical computing). A statistically significant risk on the absolute scale was defined as a 95% CI that does not cross 0 (zero). The P values were 2-sided, and P < 0.05 were considered statistically significant. The data were analyzed from July 14, 2022 to May 14, 2023.
Results
The analysis included 2,524 individuals who tested positive for COVID-19. Among them, 1,289 were categorized under the nirmatrelvir/ritonavir group, while 1,235 were assigned to the control group. In the nirmatrelvir/ritonavir group, there were more females (696 (54.0%) females vs. 593 (46.0%) males) whereas males were slightly more prevalent in the control group (533 (43.2%) females vs. 702 (56.8%) males). A higher proportion of individuals in the treatment group had hypertension (nirmatrelvir/ritonavir vs. control group, 559 (43.4%) vs. 310 (25.1%) and diabetes (nirmatrelvir/ritonavir vs. control group, 317 (24.6%) vs. 168 (13.6%)) compared to the control group. Additionally, a higher number of the individuals were symptomatic during the acute phase in the control group compared to the nirmatrelvir/ritonavir group (nirmatrelvir/ritonavir vs. control group: 337 (26.1%) vs. 406 (32.9%) (Table 1).
Table 2 summarizes the PCC symptoms in both the nirmatrelvir/ritonavir group and the control group at 3 months and 6 months. In addition, it includes the total count of symptoms per person, and the presence of specific PCC in each respective group. There was no statistically significant difference observed between the nirmatrelvir/ritonavir group and the control group in terms of PCC at 3 months (p = 0.196) and 6 months (p = 0.931). At 6 months, 2.48% (n = 32) of the individuals in the nirmatrelvir/ritonavir group experienced PCC, whereas 2.43% (n = 30) of individuals in the control group experienced PCC. Additionally, there was no significant difference in the total number of symptoms between the two groups at 3 months and 6 months. Fatigue, cough, and forgetfulness were the top 3 most common individual symptoms at 6 months post COVID-19 positive in both groups, but the differences were not statistically significant.
Table 3 presents a summary of the health-related quality of life findings amongst the individual participants. After 6 months of testing positive for COVID-19, a lower percent of individuals in the nirmatrelvir/ritonavir group reported experiencing symptoms associated with negative emotions such as sadness, anxiety and depression compared to the control group (5.74% vs. 7.77%, p = 0.050). There was no statistically significant difference between the groups in terms of the impact of PCC on symptoms that limit walking, bathing/cleaning/dressing, the ability for self-care and hygiene, limitation in performing usual activities, inability to live alone without any assistance from another person and affecting the ability to work.
Table 4 summarizes the association of nirmatrelvir/ritonavir with PCC. The study found no significant difference in the prevention of PCC at 3 months (OR 0.71 95% CI 0.31, 1.64) and 6 months (OR 1.30 95% CI 0.76, 2.21) between nirmatrelvir/ritonavir group and the control group. However, the study did find that being male (3 months: OR 3.27 95% CI 1.19, 8.97; 6 months: OR 2.39, 95% CI 1.33, 4.31) and having chronic pulmonary disease (3 months: OR 3.17, 95% CI 1.33, 7.57; 6 months: OR 2.15, 95% CI 1.16, 3.97) were associated with an increased risk of PCC at both 3 months and 6 months.
Table 5 summarizes the association of nirmatrelvir/ritonavir with health-related quality of life. After 3 months, the use of nirmatrelvir/ritonavir showed a 26% decrease in symptoms causing negative emotions (OR 0.74, 95%CI 0.60, 0.92) and an increased likelihood of symptoms limiting walking (OR 1.58, 95% CI 1.10, 2.27). However, no difference was found between nirmatrelvir/ritonavir and the control group in terms of the impact of PCC on symptoms limit walking, bathing/cleaning/dressing, the ability for self-care and hygiene, limitation in performing usual activities, inability to live alone without assistance, and the ability to work at 6 months.
Sensitivity and subgroup analysis
Based on the sensitivity analysis using IPTW, no significant difference was found between the nirmatrelvir/ritonavir group and control group in terms of PCC at 3 months. However, there was an increased risk of PCC at 6 months risk in the nirmatrelvir/ritonavir group compared to control group (OR 1.47, 95% CI 1.03, 2.09). Using the propensity score as covariate adjustment, no significant difference was found on the association between nirmatrelvir/ritonavir group and control group with PCC at both 3 months and 6 months. (Table 6)
Using an alternative definition of presence of one more symptom, no significant difference was observed between the nirmatrelvir/ritonavir group and the control group at both 3 months and 6 months using IPTW. The finding remained consistent when using the propensity score as covariate adjustment.
Discussion
In this retrospective cohort study, we observed that the administration of nirmatrelvir/ritonavir within 5 days of a positive SARS-CoV-2 infection did not show a decreased likelihood of PCC compared to the control group within a population with high vaccination coverage. Our findings are consistent with the study by Congdon et al. which indicated no significant difference between nirmatrelvir/ritonavir and the control group in reducing PCC in COVID-19 patients. [21] Similarly, Magnusson et al. in a study conducted in the United States also found that COVID-19 patients treated with nirmatrelvir/ritonavir did not demonstrate a reduction in PCC symptoms within a similar time frame. [33] However, our findings contrast with a different US study that showed the beneficial effects of nirmatrelvir/ritonavir in reducing 10 of 13 PCC symptoms [22]. These variations could be due to varying vaccination rates (16.73% of the US cohort remained unvaccinated) and differences in study methodologies (utilization of electronic medical records for PCC assessment versus use of push-notification questionnaire through the MySejahtera application).
One possible reason for the lack of long-term benefit of nirmatrelvir/ritonavir against PCC could be the high vaccination rate in our population, as almost everyone (99%) in our cohort was fully vaccinated against COVID-19. These findings align with similar results from the UK, Spain and Estonia, where COVID-19 vaccinations were found to reduce the risk of long COVID-19 symptoms, especially among adults. [14]
Another potential reason for the low PCC could be the impact of the Omicron variant. Our study was conducted during the period when the Omicron variant was predominant, and during this time, the risk of being diagnosed with PCC was lower in comparison to earlier SARS-CoV-2 variants, such as the wild-type, Alpha or Delta periods. [34] Furthermore, the population vaccination rate was higher during the Omicron period compared to the earlier variants. Thus, the potential reduction in the risk of PCC can be attributed to both vaccination and the variant effect.
PCC is a complex condition characterized by a combination of biological, psychological factors and social factors. [35] Our study is among the first to investigate the impact of COVID-19 on quality of life, as well as the potential indirect improvement of patient’s daily activities through the reduction of PCC with nirmatrelvir/ritonavir. Notably, we observed that individuals administered nirmatrelvir/ritonavir experienced a significant reduction in symptoms associated with negative emotions like sadness, anxiety or depression in the short term. These findings indicate that using nirmatrelvir/ritonavir during acute phase reduce neuropsychiatric outcomes, aligning with the study by Liu et al., which also showed the benefits of nirmatrelvir/ritonavir treatment in reducing long-term risk of neuropsychiatric consequences. [36] Furthermore, the increased likelihood of symptoms limiting walking in the nirmatrelvir/ritonavir group may be influenced by factors such as the baseline health status of the participants or variations in post-treatment care. The low event rate and the resulting sensitivity of the regression analysis further add complexity to interpreting these results.
Strengths and limitations of this study
Our study has various strengths. We analyzed a wide range of PCC symptoms over different periods, unlike most studies that only look at one time point. We also studied how PCC affects daily activities. In addition, we used IPTW to balance differences in baseline characteristics, reducing bias in the study. There are a few limitations to this study. Firstly, it was conducted in a population with high vaccination rates, thus we were unable to investigate the effect of nirmatrelvir/ritonavir in those who were not vaccinated. Secondly, we only focused on 20 COVID-19 symptoms, potentially overlooking other symptoms related to COVID-19. Since the data relied on questionnaire responses provided by individuals with COVID-19, there is a potential for recall bias. We also assumed all individuals who were administered nirmatrelvir/ritonavir followed the treatment as recommended.
Conclusions
In conclusion, our study suggests that the combination of nirmatrelvir/ritonavir alongside COVID-19 vaccination does not appear to lead to a reduction of PCC at 3 months and 6 months. Future studies with large sample sizes and in individuals without vaccination are needed to assess how effective nirmatrelvir/ritonavir is in lowering PCC symptoms and for periods longer than 6 months.
Data availability
The databases consist of individual-level information. The deidentified data that support the findings of this study are available upon reasonable request, and with protocol approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia.
References
Ministry of Health Malaysia. COVID-19: What is latest situation in Malaysia? 2024 https://data.moh.gov.my/dashboard/covid-19. Accessed 15 Dec 2023.
Mohammed I, Nauman A, Paul P, Ganesan S, Chen KH, Jalil SMS, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother. 2022;18(1):2027160.
Centers for Disease Control and Prevention. Benefits of Getting https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html. Accessed 15 Dec 2023.
Anand R. PM Muhyiddin receives first Covid-19 vaccine as Malaysia kicks off mass innoculation campaign. Straits Time. 2021.
Ministry of Health Malaysia. COVIDNOW: Vaccination Progress 2023. https://covidnow.moh.gov.my/vaccinations/?refresh=1720781109523. Accessed 1 January 2024.
Sim BLH, Chidambaram SK, Wong XC, Pathmanathan MD, Peariasamy KM, Hor CP, et al. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study. Lancet Reg Health West Pac. 2020;4:100055.
Centers for Disease Control and Prevention. Underlying Medical Conditions 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed 15 Dec 2023.
Dryden M, Mudara C, Vika C, Blumberg L, Mayet N, Cohen C, et al. Post-COVID-19 condition 3 months after hospitalisation with SARS-CoV-2 in South Africa: a prospective cohort study. Lancet Glob Health. 2022;10(9):e1247–56.
Centers for Disease Control and Prevention, Long COVID. 2023. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html. Accessed 15 Dec 2023.
O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of long covid among hospitalised and non-hospitalised populations: a systematic review and meta-analysis. EClinicalMedicine. 2023;55:101762.
National Istitue for Health and Care Excellence (NICE). Royal College of General Practitioner (RCGP); Scottish intercollegiate Guidelines Network (SIGN); COVID-19 rapid guidelines: managing the long term effects of COVID-19. 2022. Accessed 15 Dec 2023.
Government of Canada. Post COVID-19 condition (long COVID) 2023 [updated March 9. 2023. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/symptoms/post-covid-19-condition.html. Accessed 15 Dec 2023.
Department of Health and Aged Care, Long COVID. 2023 https://www.health.gov.au/topics/covid-19/long-covid. Accessed 15 Dec 2023.
Woodrow M, Carey C, Ziauddeen N, Thomas R, Akrami A, Lutje V, et al. Systematic review of the prevalence of long COVID. Open Forum Infect Dis. 2023;10(7):ofad233.
FDA. FDA Approves First Oral Antiviral for Treatment of COVID-19 in Adults. 2023 https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults. Accessed 15 Nov 2023.
Aggarwal NR, Molina KC, Beaty LE, Bennett TD, Carlson NE, Mayer DA et al. Real-world use of nirmatrelvir-ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. The Lancet Infectious Diseases.
Arbel R, Wolff Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, et al. Nirmatrelvir use and severe Covid-19 outcomes during the Omicron Surge. N Engl J Med. 2022;387(9):790–8.
Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of Paxlovid in reducing severe coronavirus Disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2022;76(3):e342–9.
Wai AK-C, Chan CY, Cheung AW-L, Wang K, Chan SC-L, Lee TT-L et al. Association of Molnupiravir and Nirmatrelvir-Ritonavir with preventable mortality, hospital admissions and related avoidable healthcare system cost among high-risk patients with mild to moderate COVID-19. Lancet Reg Health – Western Pac. 2023;30.
Marzi M, Vakil MK, Bahmanyar M, Zarenezhad E, Paxlovid. Mechanism of action, synthesis, and in Silico Study. Biomed Res Int. 2022;2022:7341493.
Congdon S, Narrowe Z, Yone N, Gunn J, Deng Y, Nori P, et al. Nirmatrelvir/ritonavir and risk of long COVID symptoms: a retrospective cohort study. Sci Rep. 2023;13(1):19688.
Xie Y, Choi T, Al-Aly Z. Association of Treatment with Nirmatrelvir and the risk of Post–COVID-19 Condition. JAMA Internal Medicine; 2023.
National Pharmaceutical Regulatory Agency. SENARAI PRODUK – PRODUK YANG TELAH DILULUSKAN OLEH PIHAK BERKUASA KAWALAN DADAH (PBKD.) DALAM MESYUARAT PBKD KALI KE – 370. 2022.
Lewnard JA, McLaughlin JM, Malden D, Hong V, Puzniak L, Ackerson BK, et al. Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. The Lancet Infectious Diseases; 2023.
Lai CC, Wang YH, Chen KH, Chen CH, Wang CY. The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network Meta-analysis of Randomized controlled trials. Viruses. 2022;14(8).
Wong CKH, Lau KTK, Leung GM. Real-world effectiveness of nirmatrelvir/ritonavir against BA.4 and BA.5 omicron SARS-CoV-2 variants. Lancet Infect Dis. 2023;23(6):639–40.
Low EV, Pathmanathan MD, Chidambaram SK, Kim WR, Lee WJ, Teh ZW, et al. Real-world nirmatrelvir-ritonavir outpatient treatment in reducing hospitalization for high-risk patients with COVID-19 during Omicron BA.4, BA.5 and XBB subvariants dominance in Malaysia: a retrospective cohort study. Int J Infect Dis. 2023;135:77–83.
Bajema KL, Berry K, Streja E, Rajeevan N, Li Y, Mutalik P, et al. Effectiveness of COVID-19 Treatment with Nirmatrelvir-Ritonavir or Molnupiravir among U.S. veterans: Target Trial Emulation Studies with one-Month and Six-Month outcomes. Ann Intern Med. 2023;176(6):807–16.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7.
Ministry of Health Malaysia. Clinical Management of Confirmed COVID-19 Case in Adult and Paediatric 2022. https://covid-19.moh.gov.my/garis-panduan/garis-panduan-kkm/ANNEX-2E-CLINICAL-MANAGEMENT-OF-CONFIRMED-COVID-19-31052022.pdf. Accessed 15 Nov 2023.
Tok PSK, Kang KY, Ng SW, Ab Rahman N, Syahmi MA, Pathmanathan MD, et al. Post COVID-19 condition among adults in Malaysia following the Omicron wave: a prospective cohort study. PLoS ONE. 2024;19(1):e0296488.
World Health Organization. Post COVID-19 condition (COVID-19) 2022 https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition. Accessed 15 November 2023.
Magnusson K, Kristoffersen DT, Dell’Isola A, Kiadaliri A, Turkiewicz A, Runhaar J, et al. Post-covid medical complaints following infection with SARS-CoV-2 omicron vs Delta variants. Nat Commun. 2022;13(1):7363.
Hedberg P, Nauclér P. Post-COVID-19 Condition after SARS-CoV-2 infections during the Omicron Surge vs the Delta, Alpha, and wild type periods in Stockholm, Sweden. J Infect Dis. 2024;229(1):133–6.
Saunders C, Sperling S, Bendstrup E. A new paradigm is needed to explain long COVID. Lancet Respiratory Med. 2023;11(2):e12–3.
Liu TH, Wu JY, Huang PY, Tsai YW, Lai CC. The effect of nirmatrelvir-ritonavir on the long-term risk of neuropsychiatric sequelae following COVID-19. J Med Virol. 2023;95(7):e28951.
Acknowledgements
We would like to thank the Director General of Health Malaysia for his permission to publish this article. We also acknowledge the contributions of all personnel involved in the study, national COVID-19 surveillance in Malaysia and all healthcare professionals who have worked tirelessly in managing the patients with COVID-19, without whom this work would not have been possible.
Funding
This research was funded by the Ministry of Health Malaysia Research Grant for Communicable Diseases (NIH/800-3/2/2 Jilid 13 (182)). The funders had no role in the design and conduct of the study or the collection, management, analysis, and interpretation of the data.
Author information
Authors and Affiliations
Contributions
EVL, MDP and KP designed the study. WRK, WJL, MR and ZWT collected the data. EVL and YYT acquired and analyzed the data. The data was interpreted by all authors. EVL wrote the first draft of the manuscript. All authors reviewed and edited the manuscript. EVL, MD, SC, MI, AAS and KP performed the critical revision of the manuscript for intellectual content. All authors had full access to all data in the studies and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Ethical approval
This study was registered in the National Medical Research Register and approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR ID-22-00938-2YN).
Data sharing statement
The databases consist of individual-level information. The deidentified data that support the findings of this study are available upon reasonable request, and with protocol approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia.
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Low, E.V., Pathmanathan, M.D., Ten, Y.Y. et al. Nirmatrelvir/ritonavir treatment and the risk of post-COVID condition over 180 days in Malaysia. BMC Infect Dis 24, 780 (2024). https://doi.org/10.1186/s12879-024-09679-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09679-1