Abstract
Background
Understanding the factors influencing disease progression and severity in pediatric COVID-19 cases is essential for effective management and intervention strategies. This study aimed to evaluate the discriminative ability of clinical and laboratory parameters to identify predictors of COVID-19 severity and mortality in hospitalized children.
Methods
In this multicenter retrospective cohort study, we included 468 pediatric patients with COVID-19. We developed a predictive model using their demographic, clinical, and laboratory data. The performance of the model was assessed using various metrics including sensitivity, specificity, positive predictive value rates, and receiver operating characteristics (ROC).
Results
Our findings demonstrated strong discriminatory power, with an area under the curve (AUC) of 0.818 for severity and 0.873 for mortality prediction. Key risk factors for severe COVID-19 in children include low albumin levels, elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), and underlying medical conditions. Furthermore, ROC curve analysis highlights the predictive value of CRP, LDH, and albumin, with AUC values of 0.789, 0.752, and 0.758, respectively.
Conclusion
Our study indicates that laboratory values are valuable in predicting COVID-19 severity in children. Various factors, including CRP, LDH, and albumin levels, demonstrated statistically significant differences between patient groups, suggesting their potential as predictive markers for disease severity. Implementing predictive analyses based on these markers could aid clinicians in making informed decisions regarding patient management.
Similar content being viewed by others
Background
The Coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2), has significantly impacted populations worldwide since its emergence in late 2019. Initially, the focus of attention was predominantly on adults, particularly those with underlying health conditions, who were deemed at higher risk for severe illness and mortality. However, as the pandemic evolved, it became evident that children could also be affected by this viral infection, albeit with different clinical manifestations and outcomes compared to adults [1,2,3,4,5].
In general, children infected with SARS-CoV-2 tend to experience milder symptoms or may even be asymptomatic carriers of the virus. However, a distinct and concerning entity known as Multisystem Inflammatory Syndrome in Children (MIS-C) emerged as a complication associated with COVID-19 infection in pediatric populations [6, 7]. While children generally experience milder cases of COVID-19 compared to adults, there are instances where they require hospitalization, intensive care unit (ICU) admission, or mechanical ventilation to manage severe respiratory distress [3, 8,9,10].
Based on current evidence, various laboratory parameters such as white blood cell (WBC) count, polymorphonuclear (PMN) count, lymphocyte count, neutrophil-lymphocyte ratio (NLR), D-dimer, albumin, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), troponin, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and the presence of acute respiratory distress syndrome (ARDS) have been associated with disease severity in COVID-19 patients [11]. Additionally, the presence of underlying medical conditions has been linked to an increased risk of severe disease [12].
Understanding factors that influence disease progression and severity in pediatric COVID-19 cases is crucial for effective management and intervention strategies. This study aimed to evaluate the discriminative ability of clinical and laboratory parameters to identify predictors of COVID-19 severity and mortality in hospitalized children.
Methods
This was a multicenter retrospective cohort study based on a large dataset of hospitalized patients with COVID-19. This study received ethical approval from Tehran University of Medical Sciences in Tehran, Iran (IR.TUMS.VCR.REC.1399.060), and all procedures were conducted in compliance with relevant guidelines and regulations. The data for this study were collected from four pediatric referral hospitals located in Tehran, Qom, Hamadan, and Hormozgan provinces in Iran, spanning from April 2020 to March 2021. These hospitals, strategically situated in the North-central, West, and South regions of Iran, served as key referral centers for pediatric COVID-19 cases. Among them, the Children’s Medical Center in Tehran particularly stood out as a highly specialized center for providing exceptional care to pediatric patients, including those affected by COVID-19. The need for informed consent was waived by the “Tehran University of Medical Sciences” due to the retrospective nature of the study. The inclusion criteria comprised patients aged 18 or younger, who were admitted as inpatients and tested positive for SARS-CoV-2 via real-time reverse transcription polymerase chain reaction (rRT-PCR) at any of the hospitals. Patient demographic data, along with clinical and laboratory findings such as white WBC, PMN count, lymphocyte count, NLR ratio, albumin levels, lactate dehydrogenase (LDH) levels, ESR, CRP levels, SGOT levels, SGPT levels, prothrombin time (PT), and partial thromboplastin time (PTT) were collected from medical records. Additionally, data on chest computed tomography (CT) scan results and the duration of hospital stay were also retrieved.
The severity of COVID-19 was classified into two groups: severe/critical and mild/moderate. This categorization was defined by specific criteria, including respiratory failure, shock, other organ failures, severe pneumonia, hypoxia (with oxygen saturation, SpO2 ≤ 93%), elevated respiratory rate (RR) of ≥ 70/min for children aged one year or younger, RR of ≥ 50/min for children older than one year, and abnormal blood gas analysis results (PaO2 < 60 mmHg, PaCO2 > 50 mmHg) [2, 13, 14].
Statistical analysis
The SPSS software version 22 was used for data analysis. Categorical variables were represented as frequencies and percentages, while continuous variables were described using medians and interquartile ranges (IQR). Statistical analyses involved comparing variables between the mild/moderate and severe/critical as well as deceased/recovered groups using Fisher’s exact test and nonparametric tests. Two-sided p-value ≤ 0.05 were considered statistically significant.
To predict the severe manifestations and mortality due to COVID-19, logistic regression models were employed. These models incorporated age, gender, underlying conditions, and laboratory data from 468 pediatric COVID-19 patients. Receiver operating characteristic (ROC) curves were generated to evaluate the model’s performance in predicting severity and mortality, with the area under the ROC curve (AUC) calculated for each parameter. Additionally, sensitivity and specificity analyses were conducted across various thresholds between 0 and 1 to determine a cut-off point for identifying individuals at high risk of severity and death.
Results
The research involved 468 patients, distributed across different regions as follows: Qom (n = 161), Hamadan (n = 14), Hormozgan (n = 101), and Tehran (n = 192) (Table 1). The median age of the patients was 4.2 years (IQR, 1.3-9), with a median duration of hospital stay of 6 days (IQR, 4–10). Among these cases, 171 (36.5%) had underlying diseases. Within the severe/critical group, which comprised 67 cases (14.3%), the median hospitalization stay was 12 days (IQR, 8–21). Furthermore, among the deceased group, which consisted of 23 cases (4.9%), the median hospital stay was 15 days (IQR, 12–27), with 19 of them (82.6%) having underlying conditions. The laboratory parameters among the patient population across different regions are indicated in Table 1.
The study involved 401 patients (85.7%) categorized as mild/moderate and 67 patients (14.3%) classified as severe/critical. The presence of underlying conditions was significantly higher among patients with severe/critical conditions compared to those with mild/moderate conditions (80% vs. 36.5%, p-value < 0.001). The prevalence of abnormal chest CT findings did not significantly differ between the two groups (p-value = 0.448). Regarding laboratory parameters, the median WBC count, and PMN count did not show significant differences between the groups. However, the NLR was significantly higher in the severe/critical group compared to the mild/moderate group (p-value = 0.042). The median lymphocyte count was significantly lower in the severe/critical group compared to the mild/moderate group (p-value < 0.001). Serum albumin levels were significantly lower in the severe/critical group compared to the mild/moderate group (p-value = 0.002). Similarly, LDH levels were significantly higher in the severe/critical group (p-value = 0.004) (Table 2).
When comparing the recovered and deceased groups, significant differences were found in the presence of underlying conditions (p-value < 0.001), WBC count (p-value = 0.05), albumin levels (p-value = 0.004), LDH levels (p-value = 0.025), and CRP levels (p-value < 0.001) (Table 2).
The most frequently reported symptoms among the patients were fever (64.5%) and cough (41.5%). Additionally, 28% experienced vomiting, 24% had shortness of breath, 23.5% experienced respiratory distress, and 21% presented with tachypnea.
Notably, respiratory distress (p-value < 0.001), tachypnea (p-value < 0.001), hypotension (p-value < 0.001), acute kidney damage (p-value < 0.001), intubation requirement (p-value < 0.001), and oxygen requirement (p-value < 0.001) were significantly more prevalent in the severe/critical group and were associated with higher mortality rate. Additionally, hands and feet edema (p-value = 0.036) was significantly associated with severity of disease and mortality (Table 3).
To predict patient outcomes, including severity and mortality, we initially employed a linear model to calculate the impact of each variable on the probability of disease severity and death. After estimating the effects of these variables based on available data, particularly data where the death status of individuals is known, we further estimated the probability of death using the following approach:
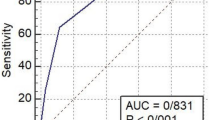
The performance of the prediction models for COVID-19 severity and mortality in children is detailed in Fig. 1.
Performance of the prediction model for COVID-19 severity and mortality in children using the Receiver-operating characteristics curve, plotting the sensitivity against one minus specificity. (a) COVID-19 severity model, (b) COVID-19 mortality model. The area under the curve, with p-value, is shown on the bottom-right
The AUC for severity and death was calculated to be 0.818 and 0.873, respectively, indicating good discrimination ability of the models. The AUC values for independent risk factors associated with COVID-19 mortality are indicated in Table 4. Albumin exhibited a strong predictive ability with an AUC of 0.758 (95% CI: 0.651–0.865, p-value < 0.001), followed closely by CRP with an AUC of 0.789 (95% CI: 0.717–0.862, p-value < 0.001). LDH also showed notable predictive power with an AUC of 0.752 (95% CI: 0.604–0.899, p-value < 0.001). Additionally, PT and PTT demonstrated moderate predictive capacity with AUCs of 0.655 (95% CI: 0.520–0.791, p-value = 0.024) and 0.658 (95% CI: 0.519–0.797, p-value = 0.022), respectively. The optimal cut-off point for predicted death values was determined to be 0.046. At this threshold, the sensitivity and specificity values were found to be 82.61% (95% confidence interval: 61.2–95.0) and 80.22% (95% confidence interval: 76.2–83.8), respectively. The likelihood ratio positive (LR+) and likelihood ratio negative (LR-) were calculated as 4.18 and 0.22, respectively.
The optimal cut-off point for predicted severity values was determined to be 0.2368. At this threshold, the sensitivity and specificity values were 88.68% (95% CI: 77.0–83.8) and 64.29% (95% CI: 55.3–72.6), respectively. Additionally, the LR + was calculated as 2.48, while the LR- was 0.18.
Discussion
Identifying potential predictors that can aid in improving the clinical management of children with COVID-19 is indeed an urgent task that requires immediate attention. By understanding factors that influence disease progression and severity in pediatric cases, healthcare providers can better tailor treatment strategies, allocate resources effectively, and improve outcomes for children affected by the virus [9, 15, 16].
Several studies have investigated risk factors associated with moderate to severe COVID-19 in children and adolescents, aiming to develop clinical prediction models for predicting disease severity [9, 16,17,18,19]. The implementation of predictive models offers a pathway to providing timely assistance to individuals at heightened risk of severe illness or mortality from COVID-19 [20]. Given the multitude of risk factors associated with COVID-19 severity, predictive models can be particularly valuable in forecasting clinical outcomes. In this study, we developed a prediction model utilizing readily available variables extracted from medical records. Our model demonstrated strong performance in distinguishing between outcomes, with high accuracy for predicting both mortality (AUC = 0.873, sensitivity 82.61%, specificity 80.22%) and severity (AUC = 0.818, sensitivity 88.68%, specificity 64.29%).
The main laboratory characteristics commonly observed in COVID-19 cases include decreased WBC and lymphocyte counts, as well as increased levels of CRP, ESR, and liver enzymes. The NLR has garnered attention for its potential predictive value in assessing disease severity, as evidenced in studies involving both influenza virus and coronavirus disease [21, 22]. Our findings also support this finding, indicating that NLR serves as a notable marker of severe COVID-19 in children.
Notably, our study revealed that over 80% of severely ill or deceased COVID-19 patients had underlying conditions, highlighting the role of underlying conditions as a primary risk factor for severe COVID-19 in pediatric patients. This observation is consistent with previous reports, which have similarly indicated that children with underlying conditions face an increased risk of COVID-19-associated severity and mortality [9, 15, 17, 18, 23].
In our study, we identified albumin as another important risk predictor for COVID-19 mortality in pediatric patients, with an AUC of 0.758. We observed a decrease in albumin level in the severely ill and deceased groups compared to patients in the mild/moderate group. Previous research on pediatrics has also reported hypoalbuminemia, suggesting its potential role in predicting the progression of COVID-19 cases towards severe conditions [4, 24, 25]. Low albumin levels may indicate impaired organ function, and pre-existing liver and kidney damage could exacerbate organ dysfunction in the presence of SARS-CoV-2 infection [26].
CRP as an inflammatory marker has been correlated with COVID-19 mortality in pediatric patients [9, 13, 15, 27]. We found significantly higher CRP levels in the deceased group compared to the recovered group, with an AUC of 0.789. This aligns with other research indicating that elevated CRP levels may serve as an indicator of cardiac injury, development of ARDS, and mortality [27,28,29,30]; therefore, the detection of CRP levels proves to be highly beneficial in assessing the severity of COVID-19 patients.
Furthermore, our study observed a lower lymphocyte count in the severe/deceased groups, which aligns with previous reports [29, 31, 32]. Elevated levels of LDH, indicating tissue damage and serving as a biomarker for lung damage in COVID-19 patients [30], were significantly higher in the severe/deceased groups compared to the mild/moderate group, consistent with findings from other studies [1, 5, 30].
Liver enzymes are regarded as additional biomarkers for assessing disease severity in children [31, 33]. While both SGPT and SGOT levels are considered, differences in SGPT levels have been reported more frequently in studies [25, 34]. Our study also found elevated SGPT levels in the severe/deceased groups, suggesting a potential association between liver enzyme abnormalities and disease severity in pediatric COVID-19 cases.
The AUC values for severity and death demonstrate good discrimination. Using a cut-off point of 0.046 for the predicted values of death, the sensitivity and specificity were 82.61% (95% CI: 61.2–95.0) and 80.22% (95% CI: 76.2–83.8), respectively. Conversely, for the predicted values of severity, the sensitivity and specificity were 88.68% (95% CI: 77.0–83.8) and 64.29% (95% CI: 55.3–72.6), respectively. These results indicate that the prediction model performs well in distinguishing between severe and non-severe cases, as well as between fatal and non-fatal outcomes in pediatric COVID-19 patients.
Although this study was based on collection of a large number of children with COVID-19 across the country, it is important to acknowledge its limitations. Firstly, due to the retrospective nature of the study, only a limited number of variables were included, as researchers were constrained to those available in the dataset. Secondly, the inclusion of children with complex conditions referred to referral pediatric hospitals may skew the representation of COVID-19 severity, potentially biasing the findings towards a more severe manifestation of the disease. Therefore, caution is warranted when interpreting the results.
Conclusion
Our study indicates that laboratory values are valuable in predicting severe cases of COVID-19 in children. Various factors, including CRP, LDH, and albumin levels, demonstrated statistically significant differences between patient groups, suggesting their potential as predictive markers for disease severity. Implementing predictive analyses based on these markers could aid clinicians in making informed decisions regarding patient management. To reduce the fatality rate associated with COVID-19, early and accurate disease assessment is imperative. Clinicians must prioritize timely detection and intervention, swiftly initiating appropriate treatment measures to effectively manage and potentially improve outcomes for young patients with COVID-19.
Data availability
The data presented in this study are available upon request from the corresponding author.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS‑CoV‑2:
-
Severe acute respiratory syndrome coronavirus 2
- MIS-C:
-
Multisystem Inflammatory Syndrome in Children
- ICU:
-
intensive care unit
- WBC:
-
white blood cell
- PMN:
-
polymorphonuclear
- NLR:
-
neutrophil-lymphocyte ratio
- CRP:
-
C-reactive protein
- ESR:
-
erythrocyte sedimentation rate
- SGOT:
-
serum glutamic oxaloacetic transaminase
- SGPT:
-
serum glutamic pyruvic transaminase
- ARDS:
-
acute respiratory distress syndrome
- PT:
-
prothrombin time
- PTT:
-
partial thromboplastin time
- rRT-PCR:
-
real-time reverse transcription polymerase chain reaction
- CT:
-
computed tomography
- RR:
-
respiratory rate
- IQR:
-
interquartile ranges
- ROC:
-
Receiver operating characteristic
- AUC:
-
area under the ROC curve
References
Mahmoudi S, Mehdizadeh M, Badv RS, Navaeian A, Pourakbari B, Rostamyan M, et al. The coronavirus disease 2019 (COVID-19) in children: a study in an Iranian children’s referral hospital. Infect drug Resist. 2020;13:2649.
Mahmoudi S, Yaghmaei B, Sharifzadeh Ekbatani M, Pourakbari B, Navaeian A, Parvaneh N, et al. Effects of coronavirus disease 2019 (COVID-19) on peripheral blood lymphocytes and their subsets in children: imbalanced CD4+/CD8 + T cell ratio and disease severity. Front Pead. 2021;9:267.
Mamishi S, Heydari H, Aziz-Ahari A, Shokrollahi MR, Pourakbari B, Mahmoudi S, et al. Novel coronavirus disease 2019 (COVID-19) outbreak in children in Iran: atypical CT manifestations and mortality risk of severe COVID-19 infection. J Microbiol Immunol Infect. 2021;54(5):839–44.
Mamishi S, Movahedi Z, Mohammadi M, Ziaee V, Khodabandeh M, Abdolsalehi MR et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect. 2020;148.
Pourakbari B, Mahmoudi S, Mahmoudieh Y, Eshaghi H, Navaeian A, Rostamyan M, et al. SARS-CoV‐2 RNAaemia in children: an Iranian referral hospital‐based study. J Med Virol. 2021;2021:1–6.
Sancho-Shimizu V, Brodin P, Cobat A, Biggs CM, Toubiana J, Lucas CL et al. SARS-CoV-2-related MIS-C: a key to the viral and genetic causes of Kawasaki disease? J Exp Med. 2021;218(6).
Mamishi S, Olfat M, Pourakbari B, Eshaghi H, Abdolsalehi MR, Shahbabaie MA, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in children: update and new insights from the second report of an Iranian referral hospital. Epidemiol Infect. 2022;150:e179.
Mohammadpour M, Hassani SA, Sharifzadeh M, Tahernia L, Mamishi S, Yaghmaie B, et al. COVID-19 pandemic experiences in Pediatric Intensive Care Unit: an Iranian Referral Hospital-based study. Int J Clin Pract. 2022;2022:1682986.
Shi Q, Wang Z, Liu J, Wang X, Zhou Q, Li Q, et al. Risk factors for poor prognosis in children and adolescents with COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2021;41:101155.
Mahmoudi S, Pourakbari B, Benvari S, Hosseinpour Sadeghi R, Abdolsalehi MR, Shahbabaie MA, et al. Clinical and laboratory features of SARS-CoV-2 variants across multiple rounds of pandemic waves in hospitalized children in an Iranian referral hospital. BMC Pediatr. 2023;23(1):241.
Sugiyama M. Tools and factors predictive of the severity of COVID-19. Glob Health Med. 2023;5(2):78–84.
Navaeian A, Mahmoudi S, Pourakbari B, Bakhtiari M, Khodabandeh M, Abdolsalehi MR, et al. COVID-19 infection in children with underlying malignancies in Iran. J Basic Clin Physiol Pharmacol. 2021;33(1):79–84.
Mamishi S, Pourakbari B, Mehdizadeh M, Navaeian A, Eshaghi H, Yaghmaei B, et al. Children with SARS-CoV-2 infection during the novel coronaviral disease (COVID-19) outbreak in Iran: an alarming concern for severity and mortality of the disease. BMC Infect Dis. 2022;22(1):382.
Pourakbari B, Mahmoudi S, Mahmoudieh Y, Eshaghi H, Navaeian A, Rostamyan M, et al. SARS-CoV-2 RNAaemia in children: an Iranian referral hospital-based study. J Med Virol. 2021;93(9):5452–7.
Zhou B, Yuan Y, Wang S, Zhang Z, Yang M, Deng X, et al. Risk profiles of severe illness in children with COVID-19: a meta-analysis of individual patients. Pediatr Res. 2021;90(2):347–52.
Ng DC, Liew CH, Tan KK, Chin L, Ting GSS, Fadzilah NF, et al. Risk factors for disease severity among children with Covid-19: a clinical prediction model. BMC Infect Dis. 2023;23(1):398.
Domínguez-Rodríguez S, Villaverde S, Sanz-Santaeufemia FJ, Grasa C, Soriano-Arandes A, Saavedra-Lozano J, et al. A bayesian model to Predict COVID-19 severity in children. Pediatr Infect Dis J. 2021;40(8):e287–93.
Tagarro A, Cobos-Carrascosa E, Villaverde S, Sanz-Santaeufemia FJ, Grasa C, Soriano-Arandes A, et al. Clinical spectrum of COVID-19 and risk factors associated with severity in Spanish children. Eur J Pediatr. 2022;181(3):1105–15.
Gao J, Zhu Y, Wang W, Wang Y, Tang W, Harrison EM et al. A Comprehensive Benchmark for COVID-19 Predictive Modeling Using Electronic Health Records in Intensive Care. arXiv Preprint arXiv:220907805 2022.
Kappen TH, van Klei WA, van Wolfswinkel L, Kalkman CJ, Vergouwe Y, Moons KG. Evaluating the impact of prediction models: lessons learned, challenges, and recommendations. Diagn Prognostic Res. 2018;2(1):1–11.
Han Q, Wen X, Wang L, Han X, Shen Y, Cao J, et al. Role of hematological parameters in the diagnosis of influenza virus infection in patients with respiratory tract infection symptoms. J Clin Lab Anal. 2020;34(5):e23191.
Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Translational Med. 2020;18(1):1–12.
Tsankov BK, Allaire JM, Irvine MA, Lopez AA, Sauvé LJ, Vallance BA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–56.
Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324(3):294–6.
Mansourian M, Ghandi Y, Habibi D, Mehrabi S. COVID-19 infection in children: a systematic review and meta-analysis of clinical features and laboratory findings. Archives de Pédiatrie. 2021;28(3):242–8.
Zhang C, Shi L, Wang F-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–30.
Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, et al. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2021;143(1):21–32.
Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389–99.
Badal S, Bajgain KT, Badal S, Thapa R, Bajgain BB, Santana MJ. Prevalence, clinical characteristics, and outcomes of pediatric COVID-19: a systematic review and meta-analysis. J Clin Virol. 2021;135:104715.
Isoldi S, Mallardo S, Marcellino A, Bloise S, Dilillo A, Iorfida D, et al. The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID-19: a 6‐months prospective study. J Med Virol. 2021;93(5):3122–32.
Han X, Li X, Xiao Y, Yang R, Wang Y, Wei X. Distinct characteristics of COVID-19 infection in children. Front Pead. 2021;9:130.
Singh K, Mittal S, Gollapudi S, Butzmann A, Kumar J, Ohgami RS. A meta-analysis of SARS-CoV-2 patients identifies the combinatorial significance of D-dimer, C-reactive protein, lymphocyte, and neutrophil values as a predictor of disease severity. Int J Lab Hematol. 2021;43(2):324–8.
Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med (CCLM). 2020;58(7):1135–8.
Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169–74.
Acknowledgements
The work of SM1 was partially supported by the European Commission- European Research Executive Agency (REA) under grant agreement No. 101130873.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SM1, BP, and SM2 conceived and designed the study. HE, ZM, HH, MM, MBR, MT, ZS, and AN participated in data extraction. SM1 conducted data analysis. EJ wrote the first draft of the manuscript, which was subsequently revised by SM1. All authors critically reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The need for informed consent was waived by the “Tehran University of Medical Sciences” due to the retrospective nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahmoudi, S., Pourakbari, B., Jafari, E. et al. Predictive factors for COVID-19 severity and mortality in hospitalized children. BMC Infect Dis 24, 757 (2024). https://doi.org/10.1186/s12879-024-09675-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09675-5