Abstract
This study presents the clinical profile of a 74-year-old male patient admitted to the hospital due to a 20-day history of coughing, chest tightness, and dyspnea. Upon admission, the patient presented with fever, tachycardia, and tachypnea. Clinical examination revealed evidence of lung infection, sepsis, and multi-organ dysfunction, alongside abnormal blood gas analysis and elevated C-reactive protein (CRP) levels. Pathogen testing confirmed Chlamydia psittaci (C. psittaci), infection. Throughout the treatment course, the patient developed concurrent fungal and viral infections, necessitating a comprehensive approach involving combined antibiotic and antifungal therapy. Despite encountering treatment-related complications, the patient demonstrated clinical improvement with aggressive management. This case underscores the importance of recognizing immune suppression subsequent to Chlamydia infection, emphasizing the critical role of early diagnosis, intervention, and standardized treatment protocols in enhancing patient prognosis.
Similar content being viewed by others
Introduction
C. psittaci is a zoonotic pathogen primarily hosted by birds. Human infection can occur through brief contact with feathers, feces, or carcasses of infected birds, or inhalation of aerosolized bacteria. Following infection, patients may exhibit a range of symptoms from asymptomatic to multiple manifestations. The virulence of C. psittaci depends on the host’s immune response; when the host’s immunity is compromised, the chlamydial virulence increases and leads to organ damage. By reviewing this case’s diagnosis and treatment, we aim to emphasize the significance of immune dysfunction following C. psittaci infection that can result in severe complications. Early diagnosis, intervention, and standardized treatment are crucial for improving patient prognosis.
Case report
A 74-year-old male patient was admitted to our hospital due to a 20-day history of productive coughing accompanied by chest tightness and shortness of breath which did not improve significantly after treatment at a local hospital. As a result, he was transferred to our Intensive Care Medicine Department for acute respiratory failure and lung infection. Upon admission physical examination: body temperature 39.6℃, heart rate 112 beats/min, respiratory rate 25 breaths/min, blood pressure 159/79 mmHg. The patient remained conscious but presented profuse sweating along with dyspnea. Auscultation revealed coarse respiratory sounds with audible phlegm and wet rhonchi in both lower lungs, while the heart rhythm was synchronized without any apparent murmurs. Meanwhile, blood gas analysis showed the following results: PaO2 48.3 mmHg, PaCO2 29.8 mmHg, pH 7.446, blood glucose 8.5 mmol/L, lactate 2.0 mmol/L, and oxygenation index 121. Upon admission, the patient received mask oxygenation, however, adequate oxygen saturation could not be maintained. Therefore, endotracheal intubation was performed followed by mechanical ventilation using a ventilator set to A/C mode with VT of 400 ml and PEEP of 8 cmH2O.
The patient’s admission test results indicated ultrasensitive CRP levels of 268.21 mg/L, creatinine levels of 340.8 umol/L, leukocyte count of 16 × 109/L with N% at 95.2%. Additionally, tests for calcitoninogen (PCT) and interleukin-6(IL-6) as well as other inflammatory indicators were conducted. Considering the patient’s medical history and clinical manifestations, the doctor empirically administered moxifloxacin (0.4 g qd IVgtt) for anti-infection purposes along with gastric protection measures to maintain circulatory function stability, internal environment balance, and nutritional support. The patient had a history of hypertension and hyperlipidemia for over 30 years which had been effectively managed using amlodipine and atorvastatin.
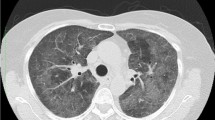
After admission, lung infection rapidly progressed to acute respiratory distress syndrome (ARDS), and chest CT revealed patchy hyperdense images in the left lower lung (Fig. 1 4th day in hospital). In order to improve the patient’s condition, a lung-protective ventilation strategy combined with prone position ventilation therapy was employed. Furthermore, based on information provided by the patient’s family, it was discovered that prior to disease onset, the patient had visited flower and bird markets. Therefore, prompt alveolar lavage fluid collectionand Metagenome next-generation sequen-cing (mNGS) of pathogenic microorganisms in both blood samplesand lavage fluidwere conducted. The diagnosis of C. psittaci was ultimately confirmed, and treatment for the patient (4th day in hospital) involved a combination of antibiotics and doxycycline administered at a dosage of 0.1 g q12h. After one week of this treatment, the patient’s oxygenation improved; however, intermittent high fever persisted for an additional week after admission. At the same time, the bacterial culture and drug sensitivity results of the patient’s sputum suggested ceftazidime sensitivity, so ceftazidime was added to anti-infective treatment. To address this, ceftazidime was added to the anti-infective therapy regimen at a dosage of 2 g q8h IV gtt.
4th day in hospital shows the patient’s chest CT at the time of admission, which showed a patchy dense shadow in the left lower lung, after aggressive anti-infective treatment, a review on 21st day in hospital showed a partial cavity formation with a small amount of peripheral exudate, showing the air crescent sign, which was considered to be an invasive pulmonary Aspergillus infection
During subsequent treatment, chest CT revealed partial cavity formation with minimal peripheral exudate, indicating invasive pulmonary Aspergillus infection (Fig. 1 21st day in hospital). Consequently, voriconazole was introduced as antifungal therapy by administering it intravenously at a dosage of 0.2 g q12h IV gtt. However, despite improved oxygenation after one week in the hospital, the patient experienced recurrent black stools and persistent hematocrit decrease even after hemostasis measures and blood transfusion were implemented. Gastrointestinal endoscopy identified multiple hemorrhages in the gastric sinus area as well as in the angle and body of the stomach necessitating titanium clip application to control bleeding (Fig. 2A); additionally, a large ulcer was observed in the stomach body (Fig. 2B). A tissue biopsy obtained during endoscopy indicated cytomegalovirus gastritis as the primary cause for gastric ulcer development worldwide (Fig. 3). Subsequent administration of ganciclovir at a dosage of 50 mg qd IV gtt led to improvement in the patient’s condition. At a later stage, Herpesvirus infection manifested with ulcers around the mouth (Fig. 4), prompting topical application of ganciclovir gel for treatment purposes. After an active treatment period lasting for approximately 50 days, repeat chest CT demonstrated partial absorption within the cavity (Fig. 1 47th day in hospital), signifying improvement in overall condition. The patient was transferred to the rehabilitation department for further treatment after receiving intermittent ventilator-assisted ventilation until withdrawal. Detailed records of changes in relevant infection indicators and specific antimicrobial adjustments throughout the treatment course can be found in Fig. 5; Table 1.
Histopathological biopsies at the time of gastroscopy are reported below: A; Pathology suggestive of chronic atrophic gastritis, moderate with activity, enterocytosis and lymphoid follicle formation; B; a few intrinsic glands were seen with large nuclei, deeply stained, cytoplasmic vacuolated cells protruding into the lumen, whose immunohistochemical markers showed CMV nuclear positive (white arrow) and chromogenic in situ hybridization (CISH) EBER (-). Cytomegalovirus gastritis was considered
Discussion
C. psittaci, a Gram-negative intracellular bacterium, is known to cause severe psittacosis in humans [1]. Upon invasion of the host, C. psittaci primarily affects the lungs and leads to the development of atypical pneumonia. It accounts for approximately 1% of pathogenic microorganisms causing community-acquired pneumonia (CAP) [2]. the difficulty in diagnosing psittacosis due to diagnostic tests for C. psittaci rarely done when patients present with CAP. The patient in this case had a history of exposure to a flower and bird market and the infectious factors were not effectively controlled at the time of admission. After identifying the pathogen as C. psittaci, we administered doxycycline [3], leading to gradual control over lung infection. Furthermore, post-admission tests revealed immunosuppression in the patient based on lymphocyte subpopulation counts on 10–22. However, based on the patient’s past medical history and test results suggest that the patient has no immune-related diseases. The virulence of C. psittaci towards its host depends on host immunity [4], weaker autoimmunity enhances chlamydial virulence and facilitates faster multiplication within the host system while increasing susceptibility to other pathogens [5] and exacerbating organ damage. In a recent Study, mNGS results indicated that 41.3% of patients with C.psittaci infection(19/46) had suspected coinfections. The most common bacterial co-infection was mainly related to Acinetobacter baumannii, and the most common co-infection of virus and fungus were Candida albicans and EB virus [6]. Heterogeneous immunological responses may occur during psittacosis, including a high-level expression of a variety of chemokines, which promote the rapid proliferation of neutrophils and facilitate the migration to the infection sites within the lung tissue, consistent with the monocyte and lymphocyte infiltrates in the lung tissues of C. psittaci patients, hypercytokinemia caused by the combined action of a variety of cytokines led to strong inflammatory responses [7], and a suppression of immune response by inhibiting humoral responses and altering Th1/Th2 balance [5], leading to an increase susceptibility to other pathogens. We detected both C. psittaci and EVB virus in bronchoalveolar lavage fluid (BALF) and mNGS analysis one day after admission. Additionally, tests conducted 20 days after admission identified sequences related to Hordeum neoformans bloggeri, Anopheles mosquitoes elizabethiae, Acinetobacter baumannii bacteria species along with human α-herpesvirus type I (HSV-1), EBV virus strain(s), human β-herpesvirus type 5 (human cytomegalovirus or HCMV), alongside C. psittaci itself. These findings provide compelling evidence that C. psittaci is a cause of secondary multiple infections.
The patient presented with papules and ulcers around the mouth on October 23rd, indicating a suspected herpes virus infection. Chest X-ray results on October 20th showed improvement in lung infection-related imaging features; however, subsequent review revealed cavity formation in certain areas of both lungs, suggesting a potential fungal infection caused by Aspergillus spp. It is worth noting that Aspergillus spp are widely distributed in the environment [8]. The risk of infection increases when patients have underlying lung disease, chronic hormone or drug use, and airway endothelial cell damage [9, 10]. ICU patients are particularly susceptible to Aspergillus spp infections [11]. Although invasive pulmonary aspergillosis does not exhibit specific pathognomonic features on imaging, the presence of a halo sign strongly suggests the disease [12]. A follow-up chest X-ray conducted on October 20th clearly displayed a cavity resembling a halo sign which eventually resolved completely after appropriate treatment (Fig. 1 47th day in hospital).
However, during the course of treatment, the patient experienced recurrent gastrointestinal bleeding leading to hemorrhagic shock. Initial endoscopy revealed an extensive irregular ulcerated infiltrative lesion located at the gastric sinus and angle to the body of stomach with active bleeding observed at its margins. Subsequent endoscopy demonstrated further development of an ulcer neoplasm characterized by a white moss-covered hard surface. Biopsy histopathologic findings indicated cytomegalovirus gastritis for this patient. Previous studies have shown that greater immune system suppression significantly increases susceptibility to cytomegalovirus-mediated diseases and clinical presentations can range from transient fever to involvement of multiple organ systems [13, 14].
In clinical practice, when a patient with a history of bird exposure presents symptoms such as cough, sputum production, fever, and hemoptysis without apparent triggers, along with chest imaging indicating lung infection, conventional antibiotic therapy fails to yield significant results. Therefore, it is crucial to remain vigilant about the possibility of C. psittaci infection under these circumstances and promptly conduct relevant pathogenicity tests. Metagenomic next-generation sequencing (mNGS) detection has higher accuracy and can effectively detect pathogens that cannot be detected by traditional method [6]. If necessary, mNGS testing can be performed on alveolar lavage fluid and blood samples to accurately identify the pathogen before initiating appropriate medication promptly and precisely. However, patients with confirmed infections may experience secondary viral and fungal infections if immunosuppression occurs during treatment; hence early detection measures should be implemented alongside interventions aimed at improving humoral and cellular immune function to mitigate multiple infections’ occurrence. Additionally, active nutritional supportive therapy combined with suitable rehabilitation training can expedite physical recovery while enhancing therapeutic outcomes. In summary, the timely and accurate diagnosis, as well as the correct treatment of potential complications, are crucial strategies for effectively managing immunosuppression following chlamydia infection.
Data availability
All data supporting the results of this study can be found in the paper and its Supplementary Information. Data related to metagenomic next-generation sequencing (mNGS) of pathogenic microorganisms in blood samples and lavage fluids are available at the following links:http://www.ncbi.nlm.nih.gov/bioproject/1113407.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- BALF:
-
Bronchoalveolar lavage fluid
- C. psittaci:
-
Chlamydia psittaci
- CAP:
-
Community-acquired pneumonia
- CRP:
-
C-reactive protein
- IL-6:
-
Interleukin-6
- mNGS:
-
Metagenome next-generation sequen-cing
- PCT:
-
Calcitoninogen
References
Su S, Su X, Zhou L, et al. Severe Chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med. 2021;10(7):8051–60.
Hogerwerf L, DE Gier B, Baan B, Hoek VANDER. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–105.
Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
Knittler MR, Sachse K. Chlamydia psittaci: update on an underestimated zoonotic agent. Pathog Dis. 2015;73(1):1–15.
Chu J, Zhang Q, Zhang T, et al. Chlamydia psittaci infection increases mortality of avian influenza virus H9N2 by suppressing host immune response. Sci Rep. 2016;6:29421.
Yuan L, Chen Q, Zhu XY, Lai LM, Zhao R, Liu Y. Evaluation of clinical characteristics and risk factors associated with Chlamydia psittaci infection based on metagenomic next-generation sequencing. BMC Microbiol. 2024;24(1):86.
Zhang Z, Wang P, Ma C, et al. Host inflammatory response is the major factor in the progression of Chlamydia psittaci pneumonia. Front Immunol. 2022;13:929213.
Cadena J, Thompson GR 3rd, Patterson TF. Aspergillosis: epidemiology, diagnosis, and treatment. Infect Dis Clin North Am. 2021;35(2):415–34.
Koulenti D, Garnacho-Montero J, Blot S. Approach to invasive pulmonary aspergillosis in critically ill patients. Curr Opin Infect Dis. 2014;27(2):174–83.
Meersseman W, Vandecasteele SJ, Wilmer A, Verbeken E, Peetermans WE, Van Wijngaerden E. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170(6):621–5.
Lat A, Bhadelia N, Miko B, Furuya EY, Thompson GR 3rd. Invasive aspergillosis after pandemic (H1N1) 2009. Emerg Infect Dis. 2010;16(6):971–3.
Alexander BD, Lamoth F, Heussel CP, et al. Guidance on imaging for invasive pulmonary aspergillosis and mucormycosis: from the imaging working group for the revision and update of the consensus definitions of fungal disease from the EORTC/MSGERC. Clin Infect Dis. 2021;72(Suppl 2):S79–88.
Griffiths P, Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat Rev Microbiol. 2021;19(12):759–73.
Fulkerson HL, Nogalski MT, Collins-McMillen D, Yurochko AD. Overview of human cytomegalovirus pathogenesis. Methods Mol Biol. 2021;2244:1–18.
Acknowledgements
Not applicable.
Funding
This work was supported, in part, by the Anhui Provincial Natural Science Foundation (2108085MH300) and the Anhui Province Clinical Medical Research Translation Special Project (202304295107020006, 202304295107020001).
Author information
Authors and Affiliations
Contributions
MZ, QX and WL designed this study and protocol development; YC, HZ, and WL were responsible for the data collection; MZ and QX conducted the manuscript writing; YC and WL revised the manuscript; WL provided final approval for this version to be published; All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted under ethical principles (Declaration of Helsinki). Written consent was obtained from her authorized representatives.
Consent for publication
Written informed consent was obtained from the patient for publication of this report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, M., Xu, Q., Zhang, H. et al. Multiple infections secondary to immunosuppression after Chlamydia psittaci infection: a case report. BMC Infect Dis 24, 752 (2024). https://doi.org/10.1186/s12879-024-09663-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09663-9