Abstract
Background
Hospital infections with SARS-CoV-2 continued during the initial waves of the pandemic worldwide. So far, Data on the dynamics of these infections and the economic burden of outbreaks are rare.
Methods
We retrospectively analysed SARS-CoV-2 infections in patients, hospital employees and nosocomial infections resulting in outbreaks in two hospitals of a secondary care hospital network in Germany during the initial 3 pandemic waves (03/2020–06/2021). In addition to hospital infections, we evaluated infection prevention strategies and the economic burden of hospital outbreaks.
Results
A total of 396 patients with SARS-CoV-2 infection were hospitalized in both hospitals. The risk factors for severe disease and death increased with age, male sex and a CRB-65 score > 0. The most frequent symptom was dyspnoea (30.1%). Sixty-five patients died, most of whom were in the 2nd wave. A total of 182 (12.5%) hospital employees were infected, 63 (34.6%) of whom were involved in outbreaks. An occupational risk of infection during outbreaks was particularly common among nurses and HCWs working on regular wards. Eleven hospital outbreaks led to high economic impact on both hospitals through the loss of manpower as result of infected employees, temporary locked wards, blocked beds, a reduced number of total hospitalized patients and increased personnel costs.
Conclusion
Continuously adaptation of infection prevention strategies is a valuable tool to keep hospitals safe places for patients and employees. We do need more analyses of the different pandemic waves and applied infection prevention strategies to learn from weak points.
Trial registration
This research was conducted in accordance with the Declaration of Helsinki and national standards. The study protocol was approved by the relevant ethics committee of the Chamber of Physicians Westphalia-Lippe and University of Münster (no. 2021–475-f-S). The study was registered on 25th August 2021 at the German Clinical Trials Register (DRKS00025865).
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged as the cause of coronavirus disease 2019 (COVID-19) [1]. The WHO declared a global health emergency on 31 January 2020; subsequently, on 11 March 2020, there was a pandemic [2]. The first pandemic year in 2020 in Germany was dominated by the wild type virus (up to March 2021), followed by the alpha type virus (up to June 2021) [3].
The SARS-CoV-2 pandemic has become a worldwide challenge for the medical sector. Healthcare workers (HCWs) across the world are at specific risk for SARS-CoV-2 infection. At the beginning of the pandemic, HCWs were approximately ten times more likely to acquire a SARS-CoV-2 infection than was the general population [4,5,6]. Data on the dynamics of hospital infections in different pandemic waves are rare. Understanding and evaluating the risk factors for HCWs to become infected are essential for developing targeted protection strategies and ensuring adequate healthcare provision during pandemics.
Despite an increasing understanding of transmission mechanisms and infection prevention strategies, outbreaks have continued to occur in hospitals and care facilities during the initial pandemic waves [7,8,9,10,11]. Most hospital outbreaks in Germany occurred in the 1st and 2nd waves of the pandemic [12]. Infection prevention strategies have been adapted continuously, and now, it is time to evaluate them to be prepared for further challenges related to SARS-CoV-2 and other infections.
The primary objective of this study was to analyse SARS-CoV-2 infections in two hospitals in a secondary care hospital network in North Rhine-Westphalia (NRW), Germany, during the first 3 pandemic waves (from March 2020 to June 2021). We retrospectively evaluated the data of infected patients and hospital employees with respect to age, risk of infection, source of infection, course of the disease and outcome. Secondary aims were (i) the analysis of hospital outbreaks to evaluate the infection prevention and control strategies to identify possible weak points to learn from, and (ii) to obtain an impression of the economic impact of nosocomial transmissions.
Methods
Study design
The study was a single center longitudinal cohort study conducted at the “Vestische Caritas Kliniken GmbH” in Germany. The following two hospitals in the secondary care hospital network were included: (i) St. Vincenz-Hospital Datteln (VHD): 1085 employees, 316 beds and the main departments internal medicine, surgery, gynecology, obstetrics, and urology; and (ii) St.-Laurentius-Stift Waltrop (LSW), with 375 employees and 172 beds (geriatric and psychiatric departments). Both hospitals have one management structure, so that raising data took place as single center study. The study time was retrospectively chosen from March 2020 to June 2021 to cover the first 3 pandemic waves: 1st wave: March 2020-September 2020; 2nd wave: October 2020-January 2021; and 3rd wave: February 2021-June 2021.
Data management
We retrospectively analysed the data collected in our electronic data system with the following program: HyBASE (version V6.2022.04.R14), JiveX Enterprise PACS (Picture Archiving and Communication System, version 5.2.0.43 RC01), Meierhofer-Krankenhaus-Informationssystem (M-KIS, version M-KIS 2020 SP10 Hotfix 07), and Swisslab (version 2.23.3.00).
Data from infected patients
We included all patients with the International Classification of Disease (10th edition Clinical Modification: ICD-10-CM) code U7.01 (SARS-CoV-2 virus detected) as main or secondary diagnosis. We retrospectively evaluated patient-specific data in the clinical data system M-KIS: sex, age, social background, date of COVID-19 diagnosis (including diagnosis-specific information such as positive antigen test, PCR), number of medical risk factors according to the Robert Koch-Institute (RKI) definition [13], number of prescribed drugs on admission, possible source of infection, symptoms, including X-ray findings and signs of bacterial superinfection, CRB-65 score (confusion, respiratory rate, blood pressure), COVID-19-specific therapy, duration of stay, stay in regular or intensive care units, artificial respiration, and status at discharge.
According to the endpoints of several analyses [13, 14], a severe course of COVID-19 was defined if any kind of artificial respiration, including high-flow oxygen, intensive care treatment or death, was temporally related to SARS-CoV-2 infection.
Data from infected employees
The Department of Hygiene documented and included all SARS-CoV-2-positive employees in their own registers and in the clinical data system M-KIS. In this study, we analysed these data according to sex, age, profession, occupational risk for SARS-CoV-2 infection, reason for PCR testing, possible source of infection, clinical symptoms and course of COVID-19.
Outbreaks
An outbreak was defined according to the German Infection Protection Law IfSG §6 (3), by two or more positive SARS-CoV-2 test results with temporal and spatial correlation, e.g., in patients who were hospitalized and/or employees working on the same ward. In outbreak situations, data from the involved patients and employees were collected in the clinical data system M-KIS. We analysed the location, ward, date, involved department, number and symptoms of involved patients and employees, profession of employees, days of isolation, and number of blocked beds or days the wards were closed in total.
Statistical analysis
In descriptive analyses, patient and employee demographics, employee professions, patient and employee symptoms and other attributes of COVID-19 were determined and compared for the whole cohort and stratified by the 1st, 2nd and 3rd waves of the pandemic using absolute and relative frequencies. Clinical characteristics and test results were compared by Pearson’s chi-square test or Fisher’s exact test. Characteristics that appeared more frequently in the 2nd or 3rd pandemic wave were estimated by univariate logistic regression, and odds ratios (ORs) and 95% confidence intervals (CIs) were calculated versus the reference level for each main category of the characteristics. The risk of a severe course of the disease was estimated by multivariate logistic regression with a combined endpoint of the need for artificial respiration, intensive care unit stay or death. We applied a significance level of 0.05. The data were analysed with the statistical software R [15].
Results
Characteristics of SARS-CoV-2-infected patients
Table 1 summarizes the characteristics of SARS-CoV-2-infected patients from March 2020 to June 2021. Overall, 396 patients were treated in both hospitals: 357 patients (90.2%) in the VHD and 39 patients (9.9%) in the LSW. The majority (76.5%) of the hospitalized patients were older than 60 years (166 females, 137 males), and 71.6% (130 females, 87 males) of them were admitted to hospitals in the 2nd pandemic wave.
Among the hospitalized patients with SARS-CoV-2 females were significantly more common than males comparing the 2nd and the 3rd wave (OR 0.57, 95% CI 0.35; 0.93) (Table 1).
Medical situation at admission
A total of 171/396 patients (43.2%) were admitted with diagnoses other than SARS-CoV-2 infection, 194 patients (49%) had 1–3 medical risk factors, and 85 patients (21.5%) had more than 3 medical risk factors according to the RKI definition [13]. Only 62 patients (15.7%) had no prescribed drugs on admission, whereas the majority of patients (52.8%) had more than 3 drugs.
More than half of the patients (51%) acquired SARS-CoV-2 infection in private contacts. The difference between the 2nd and 3rd wave was statistically significant for hospital-acquired infection (OR 0.20, 95% CI 0.09; 0.41) and for infection acquired in residential care homes for the elderly (OR 0.20, 95% CI 0.08; 0.44) (Table 1). Among the infected patients, 16.9% (67/396) were involved in hospital outbreaks..
The risk of undiagnosed infected patients being the source of nosocomial transmissions is difficult to estimate. Two hundred twenty-one patients (55.8%) were hospitalized with the suspicion of COVID-19 or already known COVID-19, 171 patients (43.2%) had other diagnoses on admission. We diagnosed 88 patients with SARS-CoV-2 infection during the hospital stay (Table 1), 67 of them were PCR-positive tested in outbreaks (Supplementary Table 1).
Since we adapted our infection prevention strategy continuously (e.g. testing of patients on admission, on 3rd day after admission, then once a week since November 2020), it is difficult to say if and what proportion of incidentally diagnosed infections led to nosocomial transmissions.
Symptoms
The majority of patients (60.6%) were clinically symptomatic, and 53% had 1–3 symptoms. Among the symptomatic patients, two groups of symptoms were the most common: flu-like symptoms (72.1%), especially dyspnoea (49.6%) and unspecific symptoms (67.9%). Fewer patients had gastrointestinal symptoms (11.7%) or taste or smell disorders (11.3%) (Table 1).
Course of disease
Of the 396 positive patients, 192 (48.5%) received COVID-19-specific drugs according to the current recommendations, which changed over time. The specific treatment used included hydroxychloroquine at the beginning of the pandemic and subsequently administered remdesivir, dexamethasone, tocilizumab and vitamin D.
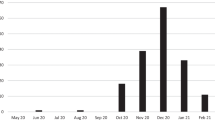
Of all infected patients, 37 (9.3%) needed intensive care; in 30 (7.6%) patients artificial respiration, including high-flow oxygen, was necessary. The duration of hospitalization was up to 14 days for the majority of patients (59.1%), and 112 patients (28.3%) were hospitalized longer. Fifty patients were outpatients, i.e., they did not need admission. In the initial three pandemic waves, 65 COVID-19-positive patients (16.4%) died, 72.3% of whom (47/65) died in the 2nd wave. Hospitalized male patients (36/172, 20.9%) had a greater risk of death than female patients did (29/224, 12.9%) (Fig. 1).
Risk factors for severe disease. Age, sex and CRB-65 score without age were analysed in a multivariate logistic regression model as risk factors for severe disease (combined endpoint of artificial respiration, intensive care unit admission or death). The results are displayed in a forest plot showing odds ratios with 95% confidence limits. Effect estimates for severe disease were a 29% higher risk per 10 years of age, 47% a higher risk for males, and a 98% risk per point in the CRB-65 score
Among the 65 patients who died, 81.5% were older than 70 years, 83.1% had cardiovascular disease, and 50.8% had 3 or more than 3 medical risk factors according to the RKI definitions of 32 comorbidities for a severe course of COVID-19 [13]. Twenty-six patients who died (40%) needed intensive care, 20 of whom (76.9%) needed artificial ventilation, including high-flow oxygen (Table 2).
Risk factors for severe disease and death
The risk factors for hospitalized patients to develop severe COVID-19 disease (need for artificial respiration or intensive care unit stay or death) were age, male sex and CRB-65 score. The results of the multivariate logistic regression are shown in the forest plot in Fig. 1. Patients had a 29% greater risk for having a severe course of disease per additional 10 years of age (OR 1.29, 95% CI 1.09; 1.57), a 47% greater risk for male patients than for female patients (OR 1.47, 95% CI 0.86; 2.54), and a 98% greater risk for each additional point in the CRB-65 score (OR 1.98, 95% CI 1.21; 3.27). The results for the risk factor age and CRB-65 score were statistically significant (Fig. 1).
Characteristics of SARS-CoV-2-infected employees
Altogether, 1460 employees were working in both hospitals at the beginning of the study. During the first 3 pandemic waves, 182 employees (12.5%) were tested positive according to the SARS-CoV-2 PCR, 157 (86.3%) of whom tested positive in the 2nd wave. Infections were distributed equally among all age groups. Approximately 1/3 of the infected employees (34.6%) were involved in hospital outbreaks (Table 3). In outbreaks, employees working in hospital VHD and in the intermediate-risk group were more frequently infected than employees working in hospital LSW, in the low-risk or high-risk group. Nurses had the highest risk of becoming infected during outbreaks (Supplementary Table 2).
Among the professionals, 107 (58.8%) infected employees were nurses. The occupational risk for infection was significantly greater for HCWs in the intermediate-risk group (regular wards with patient contact) than for those in both the low-risk and high-risk groups (p = 0.017).
Among all infected employees, 108 (59.3%) were symptomatic, most frequently with flu-like symptoms (n = 97), unspecific symptoms (n = 50), taste and smell disorders (n = 20) and gastroenterological symptoms (n = 4). For 3 infected employees, hospital admission was necessary (Table 3). The total number of infected employees in isolation were at least 2548 days. The exact duration of health-related absence was longer than the time of isolation in employees with prolonged periods of COVID-19 associated illness.
Characteristics of outbreaks
1st pandemic wave: March 2020 – September 2020
During the 1st pandemic wave we only diagnosed SARS-CoV-2-positive patients in the VHD, not in the LSW (Table 1). Only 4 of 1460 employees had positive PCR results (Table 3). In a seroprevalence study that was running during the same time we found 13 employees with antibodies against SARS-CoV-2 [16]. We had no outbreaks in either hospital (Fig. 2a), and we did not document transmission of the infection that clearly resulted from contact with SARS-CoV-2-positive patients.
2nd pandemic wave: October 2020 – January 2021
With ≥ 2 positive SARS-CoV-2 test results in patients and/or employees on the same ward, the health authority imposed an admission stop for new patients and closed the affected ward for new admittances to prevent further transmissions.
Overall, 274 COVID-19-positive patients were hospitalized in the 2nd pandemic wave (Table 1). In addition, PCR-positive employees were detected (Table 3).
Nine documented outbreaks occurred in both hospitals on different wards: 8 outbreaks on non-COVID wards (4 × surgical ward, 2 × geriatric ward, 1 × urological ward, 1 × psychiatric ward) and 1 outbreak on the COVID-19 ward (Fig. 2b). Sixty of the 274 patients (21.9%) were detected PCR-positive in outbreaks, and 33 (55%) of them had COVID-19-specific symptoms. Among the employees, 63/157 were positive HCWs (40.1%) in outbreaks, and 39 (61.9%) of them were symptomatic. The involved HCWs were in isolation or, if still symptomatic, stayed at home longer. Altogether, these outbreaks resulted in at least 896 days of isolation for employees. Affected wards were closed for 137 days, resulting in 2582 free beds that were blocked for admission of new patients (Fig. 3).
Impact of SARS-CoV-2 hospital outbreaks on individual wards and employees. The percentage of PCR-positive hospital employees (nurses, care workers and cleaning service) who were involved in outbreaks is shown in relation to all employees in %. Additionally, the sum of days resulting from the isolation of positive employees, the duration of ward closure in days and the percentage of blocked beds during ward closure are shown as indicators of the impact of SARS-CoV-2 hospital outbreaks
3rd pandemic wave: February 2021 – June 2021
Altogether, 103 COVID-19-positive patients were hospitalized at this time (Table 1). In the 3rd pandemic wave, we only registered 2 outbreaks: 1 × geriatric day clinic, and 1 × internal ward (Fig. 2c).
A total of 7/103 patients (6.8%) were involved in outbreaks, and only 1 (14.3%) of them was symptomatic. Fortunately, we had no PCR-positive employees involved in either outbreak. Affected wards were closed for 17 days, resulting in 140 free beds that were blocked for admission of new patients (Fig. 3).
Epidemiological context
In the 1st pandemic wave both hospitals had low exposure to SARS-CoV-2-positive patients, and the surrounding region itself was not a SARS-CoV-2 hotspot (Fig. 4).
Hospitalized patients and infected employees in the epidemiological context. The percentages of all PCR-positive patients in both hospitals and employees are compared to the 7-day incidence of SARS-CoV-2 infections in the district of Recklinghausen (RE), in North Rhine-Westphalia (NRW) and throughout Germany
In the 2nd and 3rd waves, the 7-day incidence in the general population of the city and district of Recklinghausen (RE), in NRW and in Germany increased markedly. Moreover, we had a rising number of hospitalized SARS-CoV-2-positive patients and a rising number of SARS-CoV-2-positive employees (Fig. 4). Both community-acquired and nosocomial transmission were present at that time.
Discussion
In this retrospective analysis of the initial 3 waves of the SARS-CoV-2 pandemic, we analysed hospital infections of patients and employees, infection prevention measures, the epidemiological context of infections, hospital outbreaks and their economic impact.
Age and male sex were identified as factors associated with severe COVID-19 disease in many previous studies in Germany [22, 23], England [24], Denmark [25] and in a worldwide meta-analysis [26]. Patients with medical risk factors were more frequently hospitalized than patients without comorbidities in our hospitals. Other studies have described specific risk factors, such as cardiovascular disease [27], elevated C-reactive protein [22], smoking [28] and diabetes [29], as predictors of severe COVID-19. Scoring systems are often used as predictors for severe COVID-19, mainly as prospective methods. The COVID-GRAM score, which has been validated to predict the risk of critical illness or death in the Chinese population, was one of the first reported scores [30]. The CURB-65 score includes urea as an additional predictor compared to the CRB-65 score. It is widely used for predicting 30-day mortality in patients with community-acquired pneumonia [31] and has been proposed as a reference prognostic tool for SARS-CoV-2 pneumonia [32]. The Charlson Comorbidity Index (CCI) was introduced in 1987 as a standardized score to estimate the likelihood of death in various medical situations, taking into account the impact of coexisting medical conditions on the outcome [33]. To date, the association between the CCI and the severity of pneumonia caused by SARS-CoV-2 has not been widely explored. The International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) Coronavirus Clinical Characterisation Consortium (4C) Mortality Score was developed in an ongoing prospective study in 260 hospitals across England, Scotland, and Wales [34]. Parameters for the mentioned scores, for instance, serum urea for the CURB-65 score, were not available for any of our patients. Therefore, we retrospectively analysed our data using the CRB-65 score with age as separate risk factor in combination with the RKI definition of 32 possible comorbidities that are associated with severe COVID-19 [13]. The 2nd pandemic wave had the highest number of hospitalized patients and the highest mortality rate (17.2%), which was also reported in another study in Germany in which different time periods were evaluated [27].
The percentage of employees with a positive SARS-CoV-2 PCR test was 0.3% in the 1st wave, 10.8% in the 2nd wave and 1.4% in the 3rd pandemic wave. Employees definitely had an additional occupational risk of contracting SARS-CoV-2. All 63 PCR-positive employees who were involved in outbreaks were detected in the 2nd wave. Nurses had an increased occupational risk of infection in our study (58.8%) compared to other professionals (e.g., 10.4% medical doctors, 2.2% therapists). This result is consistent with the findings of other studies investigating hospital employees [6, 35, 36] and may be attributed in part to their more frequent contact with and longer contact times with COVID-19 patients than physicians and other occupational groups.
We were looking for reasons why employees working on the COVID-19 ward acquired infection. As they were wearing personnel protective equipment in all patient contacts, other structural factors were likely responsible for nosocomial transmission. During the 2nd wave, the COVID-19 ward was relocated within the hospital. Additionally, we had a high turnover of patients on this ward. Patients who died had longer retention times on the ward due to a temporary lack of storage capacity. After adapting the screening strategy for employees with high occupational risk in November 2020, nosocomial transmissions on the COVID-19 ward stopped.
Furthermore, infected high-risk HCWs have led to personnel shortages, personnel shifts, a high workload, less time for correct self-protection and a shortage of manpower for carefully instructing new personnel [37]. Ongoing infections on regular wards in both hospitals led to an additional occupational risk of infection in intermediate-risk HCWs. The identification of colleagues as important sources for nosocomial transmission resulted in the recommendation to limit the number of colleagues sitting together at breaks to a smaller amount and, if possible, always with the same colleagues. While eating, drinking and smoking, employees should minimize speaking and increase the distance between each other. The use of lifts was limited to 2 people per cabin at the same time. Similar recommendations were described in the university hospital in Jena [35].
Both hospitals included in the study were also included in the seroprevalence study of employees during the first year of infection. The seroprevalence rates of COVID-19-positive employees increased sharply from 1.1% (first wave) to 13.2% in the second wave, and to 29.3% in the third wave [37]. PCR was used to detect the highest infection rate in HCWs working at intermediate-risk in this study, but the seroprevalence study revealed a greater occupational risk of infection in HCWs working at intermediate and high-risk than in non-HCWs in low-risk group [37]. Occupational risk in other studies varies according to the investigated period of infection. A nationwide seroepidemiological study in Germany, called the “RKI-SOEP-Study” revealed a clearly increased risk of SARS-CoV-2 infection for employees in healthcare professions (4.6%) compared to non-healthcare employees (1.8%) from October 2020 to February 2021 [38]. In contrast, Bahrs et al. (2022) described non-patient-related SARS-CoV-2 exposure from colleagues and household members as the highest risk of infection for hospital employees in the University Hospital Jena, Germany, in the first pandemic year [35]. The main limitation of all seroepidemiological studies in contrast to PCR testing is that the exact time of infection could not be determined.
We found a clear correlation between the infection rate in the general population, hospitalized patients and nosocomial transmission. Among all the hospitalized COVID-19-positive patients, 21.9% were involved in outbreaks in the 2nd wave, and 6.8% (7/103) were involved in the 3rd wave. A study that analysed outbreaks in hospitals and long-term facilities in Germany up to September 2021 supported this finding. Suwono et al. (2023) described the strongest association of outbreaks with weekly cases of SARS-CoV-2 infections in the general population in the 2nd wave [12]. Accordingly, the majority of the outbreaks (9/11) occurred on different wards in both hospitals during this time period. The risk factors for severe disease and death in hospitalized patients were age, male sex and a CRB-65 score (without age) > 0 on admission.
It is difficult to evaluate the total costs of the SARS-CoV-2 pandemic in Germany for the health system, all hospitals and the individual hospital itself. The economic burdens that resulted from nosocomial transmissions, such as the isolation time of employees, ward closures and blocked beds, were politically predetermined by health authorities. Therefore, infection prevention urgently needs to be improved to avoid nosocomial transmission. A national observation study investigated SARS-CoV-2 outbreaks in hospitals and long-term care facilities in Germany. The authors reported that hospitals experienced a learning curve throughout the pandemic. As a result, outbreaks could be stopped efficiently, and fewer people were involved in outbreaks if they occurred compared to long-term care facilities [12].
Strengths of our study are the observation of SARS-CoV-2 infections in two hospitals in the first 3 pandemic waves and the determination of the exact time of SARS-CoV-2 infection with PCR testing compared to serological studies. Furthermore, only few analyses of hospital outbreaks with respect to the economic impact have been published so far.
The main limitation of the study is its retrospective nature. As single center study with a small number of infected individuals, data is not representative to the general population due to differences in patient and employee profiles, healthcare structures and resources. Unfortunately, the study period was too short to get meaningful information on the effect of general vaccination and different variants of SARS-CoV-2 viruses. Infection prevention and restriction measures, based on RKI recommendations, were valid in Germany and not necessarily applicable to other countries. Nevertheless, impact of nosocomial transmissions was probably similar in most hospitals in Germany since the health authority rules were uniform.
Conclusions
Nosocomial transmission of SARS-CoV-2 was strongly associated with infection dynamics in the general population but also with structural limitations in the hospital, such as the availability of personnel protective equipment in the early pandemic, personnel shortages and high workloads. The lessons learned will help us to manage further waves of SARS-CoV-2 but also similar infections. We must adapt our infection prevention concept continuously to avoid nosocomial infections, keep hospitals safe for patients and employees and minimize the economic impact on the health system.
Availability of data and materials
Data is provided within the manuscript and supplementary tables. Further datasets and materials analysed in this study are available from the corresponding author upon reasonable request.
References
Velavan TP, Meyer CG. The COVID-19 epidemic. Trop Med Int Health. 2020;25(3):278–80. https://doi.org/10.1111/tmi.13383.
Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ. Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev. 2020;33(4). https://doi.org/10.1128/CMR.00028-20.
Robert Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19).31.03.2022. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2022-03-31.pdf. Accessed 05.04.2022.
Sahu AK, Amrithanand VT, Mathew R, Aggarwal P, Nayer J, Bhoi S. COVID-19 in health care workers - A systematic review and meta-analysis. Am J Emerg Med. 2020;38(9):1727–31. https://doi.org/10.1016/j.ajem.2020.05.113.
Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Impact of personal protective equipment use on health care workers’ physical health during the COVID-19 pandemic: A systematic review and meta-analysis. Am J Infect Control. 2021;49(10):1305–15. https://doi.org/10.1016/j.ajic.2021.04.084.
Dzinamarira T, Nkambule SJ, Hlongwa M, Mhango M, Iradukunda PG, Chitungo I, Dzobo M, Mapingure MP, Chingombe I, Mashora M, Madziva R, Herrera H, Makanda P, Atwine J, Mbunge E, Musuka G, Murewanhema G, Ngara B. Risk Factors for COVID-19 Infection Among Healthcare Workers. A First Report From a Living Systematic Review and meta-Analysis. Saf Health Work. 2022;13(3):263–8. https://doi.org/10.1016/j.shaw.2022.04.001.
Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers: A Living Rapid Review. Ann Intern Med. 2020;173(2):120–36. https://doi.org/10.7326/M20-1632.
Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Update Alert 5: Epidemiology of and Risk Factors for Coronavirus Infection in Health Care Workers. Ann Intern Med. 2020;173(11):W154–5. https://doi.org/10.7326/L20-1227.
Khonyongwa K, Taori SK, Soares A, Desai N, Sudhanva M, Bernal W, Schelenz S, Curran LA. Incidence and outcomes of healthcare-associated COVID-19 infections: significance of delayed diagnosis and correlation with staff absence. J Hosp Infect. 2020;106(4):663–72. https://doi.org/10.1016/j.jhin.2020.10.006.
Zhao D, Wang M, Wang M, Zhao Y, Zheng Z, Li X, Zhang Y, Wang T, Zeng S, Hu W, Yu W, Hu K. Asymptomatic infection by SARS-CoV-2 in healthcare workers: A study in a large teaching hospital in Wuhan. China Int J Infect Dis. 2020;99:219–25. https://doi.org/10.1016/j.ijid.2020.07.082.
Bonsignore M, Hohenstein S, Kodde C, Leiner J, Schwegmann K, Bollmann A, Moller R, Kuhlen R, Nachtigall I. Burden of hospital-acquired SARS-CoV-2 infections in Germany: occurrence and outcomes of different variants. J Hosp Infect. 2022;129:82–8. https://doi.org/10.1016/j.jhin.2022.08.004.
Suwono B, Steffen A, Schweickert B, Schonfeld V, Brandl M, Sandfort M, Willrich N, Eckmanns T, Haller S. SARS-CoV-2 outbreaks in hospitals and long-term care facilities in Germany: a national observational study. Lancet Reg Health Eur. 2022;14:100303. https://doi.org/10.1016/j.lanepe.2021.100303.
Rößler M, Jacob J, Risch L, Tesch F, Enders D, Wende D, Jucknewitz R, Weidinger O, Batram M, Ballesteros P, Baßler S, Hertle D, Repschläger U, Richter N, Schulte C, Schramm A, Sobik F, Treskova-Schwarzbach M, Scholz S, Schmitt J, Walker J. Hierachisierung von Risikofaktoren für schwere COVID-19-Erkrankungsverläufe im Kontext der COVID-19-Schutzimpfungen - Eine gepoolte GKV-Routinedatenanalyse basierend auf 30 Mio. Versicherten Epidemiologisches Bulletin. 2021;19:3–12. https://doi.org/10.25646/8405.2.
Treskova-Schwarzbach M, Haas L, Reda S, Pilic A, Borodova A, Karimi K, Koch J, Nygren T, Scholz S, Schonfeld V, Vygen-Bonnet S, Wichmann O, Harder T. Pre-existing health conditions and severe COVID-19 outcomes: an umbrella review approach and meta-analysis of global evidence. BMC Med. 2021;19(1):212. https://doi.org/10.1186/s12916-021-02058-6.
R Core Team. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2021.
Hildebrandt A, Hokelekli O, Uflacker L, Rudolf H, Gatermann SG. COVID-19: Hotspot hospital?- seroprevalence of SARS-CoV-2 antibodies in hospital employees in a secondary care hospital network in Germany: Intermediate results of a prospective surveillance study. Int J Hyg Environ Health. 2021;235: 113771. https://doi.org/10.1016/j.ijheh.2021.113771.
Bundesministerium für Gesundheit. https://www.bundesgesundheitsministerium.de/coronavirus/chronik-coronavirus.html. Accessed 15.11.2023.
Robert Koch-Institut. https://www.rki.de/DE/Content/InfAZ/Neuartiges_Coronavirus/nCoV_node.html. Accessed 08.11.2023.
Tagesschau. https://www.tagesschau.de/newsticker/liveblog-corona.de. Accessed 26.10.2023.
Ministerium für Arbeit GuSdLN-W. The respective corona protection ordinance and corona test- and quarantine ordinances. https://www.mags.nrw/coronavirus-rechtlicheregelungen-nrw. Accessed 08.11.2023.
Kreis Recklinghausen. https://www.kreis-re.de. Accessed 26.10.2023.
Koppe U, Schilling J, Stecher M, Ruthrich MM, Marquis A, Diercke M, Haselberger M, Koll CEM, Niebank M, Ruehe B, Borgmann S, Grabenhenrich L, Hellwig K, Pilgram L, Spinner CD, Paerisch T, group Ls. Disease severity in hospitalized COVID-19 patients: comparing routine surveillance with cohort data from the LEOSS study in 2020 in Germany. BMC Infect Dis. 2023;23(1):89. https://doi.org/10.1186/s12879-023-08035-z.
Nachtigall I, Lenga P, Jozwiak K, Thurmann P, Meier-Hellmann A, Kuhlen R, Brederlau J, Bauer T, Tebbenjohanns J, Schwegmann K, Hauptmann M, Dengler J. Clinical course and factors associated with outcomes among 1904 patients hospitalized with COVID-19 in Germany: an observational study. Clin Microbiol Infect. 2020;26(12):1663–9. https://doi.org/10.1016/j.cmi.2020.08.011.
Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–6. https://doi.org/10.1038/s41586-020-2521-4.
Holler JG, Eriksson R, Jensen TO, van Wijhe M, Fischer TK, Sogaard OS, Israelsen SB, Mohey R, Fabricius T, Johnk F, Wiese L, Johnsen S, Soborg C, Nielsen H, Kirk O, Madsen BL, Harboe ZB. First wave of COVID-19 hospital admissions in Denmark: a Nationwide population-based cohort study. BMC Infect Dis. 2021;21(1):39. https://doi.org/10.1186/s12879-020-05717-w.
Pijls BG, Jolani S, Atherley A, Derckx RT, Dijkstra JIR, Franssen GHL, Hendriks S, Richters A, Venemans-Jellema A, Zalpuri S, Zeegers MP. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11(1):e044640. https://doi.org/10.1136/bmjopen-2020-044640.
Lampl BMJ, Edenharter B, Leitzmann MF, Salzberger B. COVID-19-related deaths: a 2-year inter-wave comparison of mortality data from Germany. Infection. 2023;51(4):1147–52. https://doi.org/10.1007/s15010-023-01982-4.
El-Shabasy RM, Nayel MA, Taher MM, Abdelmonem R, Shoueir KR, Kenawy ER. Three waves changes, new variant strains, and vaccination effect against COVID-19 pandemic. Int J Biol Macromol. 2022;204:161–8. https://doi.org/10.1016/j.ijbiomac.2022.01.118.
Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS ONE. 2020;15(12):e0243191. https://doi.org/10.1371/journal.pone.0243191.
Liang W, Liang H, Ou L, Chen B, Chen A, Li C, Li Y, Guan W, Sang L, Lu J, Xu Y, Chen G, Guo H, Guo J, Chen Z, Zhao Y, Li S, Zhang N, Zhong N, He J, China Medical Treatment Expert Group for C. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern Med. 2020;180(8):1081–9. https://doi.org/10.1001/jamainternmed.2020.2033.
Shah BA, Ahmed W, Dhobi GN, Shah NN, Khursheed SQ, Haq I. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci. 2010;52(1):9–17.
Demir MC, Ilhan B. Performance of the Pandemic Medical Early Warning Score (PMEWS), Simple Triage Scoring System (STSS) and Confusion, Uremia, Respiratory rate, Blood pressure and age >/= 65 (CURB-65) score among patients with COVID-19 pneumonia in an emergency department triage setting: a retrospective study. Sao Paulo Med J. 2021;139(2):170–7. https://doi.org/10.1590/1516-3180.2020.0649.R1.10122020.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. https://doi.org/10.1016/0021-9681(87)90171-8.
Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM, Dunning J, Fairfield CJ, Gamble C, Green CA, Gupta R, Halpin S, Hardwick HE, Holden KA, Horby PW, Jackson C, McLean KA, Merson L, Nguyen-Van-Tam JS, Norman L, Noursadeghi M, Olliaro PL, Pritchard MG, Russell CD, Shaw CA, Sheikh A, Solomon T, Sudlow C, Swann OV, Turtle LC, Openshaw PJ, Baillie JK, Semple MG, Docherty AB, Harrison EM, investigators IC. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. https://doi.org/10.1136/bmj.m3339.
Bahrs C, Weis S, Kesselmeier M, Ankert J, Hagel S, Beier S, Maschmann J, Stallmach A, Steiner A, Bauer M, Behringer W, Baier M, Richert C, Zepf F, Walter M, Scherag A, Kiehntopf M, Loffler B, Pletz MW. Non-patient-related SARS-CoV-2 exposure from colleagues and household members poses the highest infection risk for hospital employees in a German university hospital: follow-up of the prospective Co-HCW seroprevalence study. Infection. 2023;51(4):1051–9. https://doi.org/10.1007/s15010-023-01995-z.
Elfstrom KM, Blomqvist J, Nilsson P, Hober S, Pin E, Manberg A, Pimenoff VN, Arroyo Muhr LS, Lundgren KC, Dillner J. Differences in risk for SARS-CoV-2 infection among healthcare workers. Prev Med Rep. 2021;24: 101518. https://doi.org/10.1016/j.pmedr.2021.101518.
Hildebrandt A, Hokelekli O, Uflacker L, Rudolf H, Paulussen M, Gatermann SG. Seroprevalence of SARS-CoV-2 Antibodies in Employees of Three Hospitals of a Secondary Care Hospital Network in Germany and an Associated Fire Brigade: Results of a Repeated Cross-Sectional Surveillance Study Over 1 Year. Int J Environ Res Public Health. 2022;19(4). https://doi.org/10.3390/ijerph19042402.
Wachtler B, Neuhauser H, Haller S, Grabka MM, Zinn S, Schaade L, Hövener C, Hoebel J. The risk of infection with SARS-CoV-2 among healthcare workers during the pandemic - Findings of a nationwide sero-epidemiological study in Germany. Dtsch Arztebl Int. 2021;118:842–3. https://doi.org/10.3238/arztebl.m2021.0376.
Acknowledgements
We thank Wolfgang Mueller for the possibility and support to perform this study.
Author declarations
All named authors have seen and agreed to the submitted version of the paper “SARS-CoV-2 infections in patients, hospital employees and nosocomial transmissions during the first 3 waves of the pandemic: a retrospective analysis in a secondary care hospital network in Germany”; that all who are included in the acknowledgements section, or as providers of personal communications, have agreed to those inclusions; and that the material is original, unpublished and has not been submitted elsewhere. There are no previous or pending publications of the material in conference proceedings, letters to journals and brief communications etc.
Informed consent statement
Not applicable.
Funding
The study was financed by internal funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.H. and K.D.; Methodology, A.H. and K.D.; Validation, A.H., K.D. and L.U.; Formal analysis, A.H. and S.G.; Investigation, K.D.; Resources, L.U.; Data curation, A.H. and K.D.; Writing—original draft preparation, A.H.; Writing—review and editing, A.H., K.D., L.U. and S.G. All authors have read and agreed to the submitted version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was conducted in accordance with the Declaration of Helsinki and national standards. Due to the retrospective nature of the study and the anonymisation, the need for informed consent was waived by the relevant ethics committee of the Chamber of Physicians Westphalia-Lippe and University of Münster (approval no. 2021–475-f-S). The study was registered at the German Clinical Trials Register (DRKS00025865).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hildebrandt, A., Dolega, K., Uflacker, L. et al. SARS-CoV-2 infections in patients, health care workers and hospital outbreaks during the first 3 waves of the pandemic: a retrospective analysis in a secondary care hospital network in Germany. BMC Infect Dis 24, 859 (2024). https://doi.org/10.1186/s12879-024-09641-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09641-1