Abstract
Background
Abdominal aorta-duodenal fistulas are rare abnormal communications between the abdominal aorta and duodenum. Secondary abdominal aorta-duodenal fistulas often result from endovascular surgery for aneurysms and can present as severe late complications.
Case presentation
A 50-year-old male patient underwent endovascular reconstruction for an infrarenal abdominal aortic pseudoaneurysm. Prior to the operation, he was diagnosed with Acquired Immune Deficiency Syndrome and Syphilis. Two years later, he was readmitted with lower extremity pain and fever. Blood cultures grew Enterococcus faecium, Salmonella, and Streptococcus anginosus. Sepsis was successfully treated with comprehensive anti-infective therapy. He was readmitted 6 months later, with blood cultures growing Enterococcus faecium and Escherichia coli. Although computed tomography did not show contrast agent leakage, we suspected an abdominal aorta-duodenal fistula. Esophagogastroduodenoscopy confirmed this suspicion. The patient underwent in situ abdominal aortic repair and received long-term antibiotic therapy. He remained symptom-free during a year and a half of follow-up.
Conclusions
This case suggests that recurrent infections with non-typhoidal Salmonella and gut bacteria may be an initial clue to secondary abdominal aorta-duodenal fistula.
Similar content being viewed by others
Background
Secondary ADFs (SADFs) are often caused by the endovascular surgery for aneurysms, presenting as a severe late complication with an incidence ranging from 0.77–1.6% [1]. In this case, we described the diagnosis, treatment and follow-up of an SADF patient who presented with recurrent sepsis and lower extremity abscess (Fig. 1). It was noteworthy that the patient also had Acquired Immune Deficiency Syndrome (AIDS) and syphilis, which may increase the risk of infection and arterial damage. Informed consent was obtained from the patient.
Case presentation
First admission
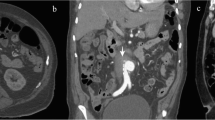
A 50-year-old man presented to the emergency department of our hospital on July 24th, 2020, with a one-week history of lower back and abdominal pain that worsened over the past day. Physical examination was unremarkable except for periumbilical tenderness. Computed Tomography Angiography (CTA) revealed an infrarenal abdominal aortic pseudoaneurysm (1.7 cm × 3.4 cm) at the L2/3 level (Fig. 2). He underwent endovascular reconstruction using two stents (Medtronic ETEW2020C82EE, Diameter: 20 mm, Coating length: 82 mm; Medtronic ETCF2323C49EE, Diameter: 23 mm, Coating length: 49 mm, Bare length: 15 mm). He was diagnosed with AIDS and syphilis before the operation. Postoperative angiography and CTA (Fig. 2) showed no leakage of contrast agent around the stent. He was treated with a combination therapy (tenofovir, lamivudine, efavirenz) for HIV. Between September 2020 to August 2020, he underwent nine treatments for syphilis. He underwent two follow-up CTA scans (August 27ed, 2020 and October 31st, 2021) after endovascular reconstruction, which did not show any leakage (Fig. 3).
The left image depicts an infrarenal abdominal aortic pseudoaneurysm (1.7 cm × 3.4 cm) at the L2/3 level as seen on CTA. The middle image shows the pseudoaneurysm before endovascular treatment via angiography. The right image demonstrates the absence of contrast agent leakage peri-stent after the operation
Second admission
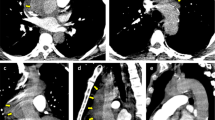
The patient was readmitted on February 21st, 2022, with lower extremity pain and fever for the past ten days. He had a highest recorded temperature of 40℃ accompanied by shivering and chills. Blood cultures grew Enterococcus faecium, Salmonella, and Streptococcus anginosus. CTA revealed intra-stent mural thrombosis and peri-stent soft tissue shadow, with no sign of leakage (Fig. 3). Ultrasonography showed an abscess in the left leg (15 cm×5 cm×6 cm). The culture of the pyogenic fluids grew Salmonella Dublin. He was treated with Meropenem and Vancomycin and underwent incision and drainage of the abscess in the left leg. He discharged after comprehensive therapy.
Third admission
The patient was readmitted on August 15th, 2022, with complains of right low extremity pain, recurrent fever for the past three days, and abdominal pain for one day. Physical examination revealed tenderness, rebound tenderness, and muscular tension throughout the abdomen, with increased local skin temperature in the right lower extremity. Blood cultures grew Enterococcus faecium and Escherichia coli. Sepsis was treated with Meropenem and Vancomycin. CTA showed intra-stent mural thrombosis and small air bubbles, with no sign of leakage. The stent marginbecame adherent to the third portion of the duodenum (Fig. 4). Ultrasonography revealed an abscess in the right planta (4 cm×0.8 cm×1.7 cm). These bacteria were derived from the intestinal tract, suggesting a communication between the intestinal tract and the circulatory system. Esophagogastroduodenoscopy (EGD) revealed a large duodenal ulcer with visualization of graft material in the third portion of the duodenum, indicating an abdominal aorta-duodenal fistula (Fig. 5). According to the MAGIC Classification [2], the diagnosis of aortic graft infection can be made if one major criterion (such as fistula development) and two other criteria from different categories (e.g., suspicious peri-graft findings on radiology, positive blood cultures with no apparent source except aortic graft infection) are met. The patient was transferred to vascular surgery department for in situ abdominal aortic repair, stent fixation, duodenectomy of the third portion, and jejunoduodenostomy. During the operation, the stents and surrounding tissues were soaked in Rifampicin injection for approximately one hour to reduce the risk of post-operation infection. The stents were refastened to the abdominal aorta using vascular sutures and wrapped with an omental pedicle flap. Amoxicillin and Clavulanate Potassium were used for preventing recurrent infection. A year and a half after the operation, the patient was free of infection symptoms, and his infective indices were normal. CTA performed on February 2nd, 2023, showed that the stents was wrapped by soft tissues, with no leakage of contract agent (Fig. 6).
Discussion and conclusions
In this case, the fistula was secondary to endovascular aneurysm repair performed 2 years ago. CTA and EGD were necessary for defining ADF. Signs such as peri-graft or intra-graft gas, effacement of the intervening fat plane, disruption of the aorta, adjacent inflammatory stranding, and intravasation of contrast into the bowel lumen or peri-graft space favor the diagnosis of ADF [3, 4]. Among patients with HIV-associated infective native aortic aneurysm, the most common microbiological pathogens were Treponema pallidum (31%) and Salmonella spp. (26%) [5]. According to a systematic analysis for the global burden of non-typhoidal Salmonella invasive disease, the mean case fatality among those living with HIV was 41.8% compared with 12.0% among those without HIV [6]. CD4 T-cells, which are decreased in patients with HIV, are crucial for immunity to Salmonella infection [7]. The relationship between HIV infection and vascular structure and function was ambiguous. Previous studies have found that HIV infection can aggravate arterial stiffness, atherosclerosis, and endothelial disfunction though HIV-related inflammation and immune activation, increasing reactive oxygen species production and reducing nitric oxide bioavailability [8,9,10,11,12]. However, there was large heterogeneity between HIV and vascular pathologies in previous reviews, and the results were often contradictory [13, 14]. In our case, the comorbidity, AIDS, aggravated the infection and may accelerate vascular damage, expanding the extent of ADF. Moreover, there was a vicious circle between vessel erosion and gut bacteria translocation. Bacterial translocation has been associated with infection of distant tissue and sepsis morbidity [15]. The ability to disseminate to and infect distal organs was a characteristic property of Salmonella infection [16]. At the second admission, blood cultures grew Enterococcus faecium, Salmonella, and Streptococcus anginosus and the culture of pyogenic fluids grew Salmonella Dublin. These reasons might partly explain the recurrent sepsis and lower extremity abscess in this case. Salmonella has been confirmed to cause vascular wall damage, aortic grafts infection, and ADFs [17,18,19,20,21]. Thus, we suspected that the Salmonella sepsis facilitated the formation of SADF in this patient.
For patients with aortic endograft infections, surgical treatment was a better option compared with conservative management [22]. After considering the high risk of hemorrhage and the significant incision required for axillary-bifemoral bypass surgery, we decided, following discussions with the patient and his family members, to opt for in situ abdominal aortic repair. According to previous studies [23,24,25], soaking prosthetic grafts with rifampicin might inhibit the growth of microorganisms, and reduce the recurrence of infection. Moreover, the course of antibiotic treatment was ambiguous among patients with evident infection, and some researchers advocated that the course between six weeks to a lifetime [18, 26, 27]. Considering the recurrent pre-operative infection and the comorbidity (AIDS), this patient was concepted for long-term antibiotic treatment.
In summary, the initial clue of SADFs could be recurrent infections of non-typhoidal Salmonella and gut bacteria, even in the absence of apparent gastrointestinal bleeding. Patients with immune deficiencies are susceptible to Treponema pallidum and Salmonella infections, which could exacerbate the development of SADFs.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- SADFs:
-
Secondary Abdominal Aorta-Duodenal Fistulas
- AIDS:
-
Acquired Immune Deficiency Syndrome
- CTA:
-
Computed Tomography Angiography
- EGD:
-
Esophagogastroduodenoscopy
References
Spanos K, Kouvelos G, Karathanos C, Matsagkas M, Giannoukas AD. Current status of endovascular treatment of aortoenteric fistula. Semin Vasc Surg. 2017;30(2–3):80–4.
Lyons OT, Baguneid M, Barwick TD, Bell RE, Foster N, Homer-Vanniasinkam S, Hopkins S, Hussain A, Katsanos K, Modarai B, et al. Diagnosis of aortic graft infection: a Case Definition by the management of Aortic Graft Infection Collaboration (MAGIC). Eur J Vascular Endovascular Surgery: Official J Eur Soc Vascular Surg. 2016;52(6):758–63.
Zhou JC, Xu QP, Shen LG, Pan KH, Mou YP. Aortoduodenal fistula following aortic reconstruction of a pseudoaneurysm caused by stab wound 12 years ago. J Zhejiang Univ Sci B. 2009;10(5):400–3.
Curtis W, Yano M. Acute non-traumatic disease of the abdominal aorta. Abdom Radiol (New York). 2018;43(5):1067–83.
Jönsson A, Ljungquist O, Sörelius K. HIV-associated infective native aortic aneurysms. APMIS: Acta Pathologica Microbiol et Immunol Scand. 2023;131(1):3–12.
Collaborators GN-TSID. The global burden of non-typhoidal salmonella invasive disease: a systematic analysis for the global burden of Disease Study 2017. Lancet Infect Dis. 2019;19(12):1312–24.
Loomis WP, Delaney MA, Johnson ML, Cookson BT. Failure of CD4 T cell-deficient hosts to Control Chronic Nontyphoidal Salmonella Infection Leads to exacerbated inflammation, chronic Anemia, and altered myelopoiesis. Infect Immun 2020, 89(1).
Kuate Defo A, Chalati MD, Labos C, Fellows LK, Mayo NE, Daskalopoulou SS. Association of HIV infection and antiretroviral therapy with arterial stiffness: a systematic review and Meta-analysis. Hypertens (Dallas Tex: 1979). 2021;78(2):320–32.
Kovacs L, Kress TC, Belin de Chantemèle EJ. HIV, Combination Antiretroviral Therapy, and Vascular Diseases in Men and Women. JACC Basic to translational science. 2022, 7(4):410–421.
Stein JH, Kime N, Korcarz CE, Ribaudo H, Currier JS, Delaney JC. Effects of HIV infection on arterial endothelial function: results from a large pooled cohort analysis. Arterioscler Thromb Vasc Biol. 2021;41(1):512–22.
Marincowitz C, Genis A, Goswami N, De Boever P, Nawrot TS, Strijdom H. Vascular endothelial dysfunction in the wake of HIV and ART. FEBS J. 2019;286(7):1256–70.
Jaworowski A, Hearps AC, Angelovich TA, Hoy JF. How Monocytes Contribute to increased risk of atherosclerosis in Virologically-suppressed HIV-Positive individuals receiving combination antiretroviral therapy. Front Immunol. 2019;10:1378.
Majonga ED, Ferrand RA, Deanfield JE, Chiesa ST. The effect of perinatal HIV and antiretroviral therapy on vascular structure and function in young people: a systematic review and meta-analysis. Atherosclerosis. 2022;352:53–61.
Hudson JA, Majonga ED, Ferrand RA, Perel P, Alam SR, Shah ASV. Association of HIV infection with Cardiovascular Pathology based on Advanced Cardiovascular Imaging: a systematic review. JAMA. 2022;328(10):951–62.
Woodcock NP, Sudheer V, El-Barghouti N, Perry EP, MacFie J. Bacterial translocation in patients undergoing abdominal aortic aneurysm repair. Br J Surg. 2000;87(4):439–42.
Ye C, Li Q, Li X, Park CG, He Y, Zhang Y, Wu B, Xue Y, Yang K, Lv Y et al. Salmonella enterica Serovar Typhimurium interacts with CD209 receptors to promote host dissemination and infection. Infect Immun 2019, 87(8).
McIntyre KE Jr., Malone JM, Richards E, Axline SG. Mycotic aortic pseudoaneurysm with aortoenteric fistula caused by Arizona hinshawii. Surgery. 1982;91(2):173–7.
Adeeb SJ, Yusha AW, Samad SA. Primary repair with in-situ interposition graft for infrarenal mycotic aortic pseudoaneurysm. Med J Malay. 1997;52(2):178–80.
Tozzi FL, da Silva ES, Campos F, Fagundes Neto HO, Lucon M, Lupinacci RM. Primary aortoenteric fistula related to septic aortitis. Sao Paulo Med J = Revista paulista de Med. 2001;119(4):150–3.
Skourtis G, Papacharalambous G, Makris S, Kasfikis F, Kastrisios G, Goulas S, Antoniou I, Giannakakis S, Maltezos C. Primary aortoenteric fistula due to septic aortitis. Ann Vasc Surg. 2010;24(6):e825827–811.
Chen YW, Tang HJ, Tsai YS, Lee NY, Hung YP, Huang CF, Lee CC, Li CW, Li MC, Syue LS et al. Risk of non-typhoidal Salmonella vascular infections is increased with degree of atherosclerosis and inflammation: A multicenter study in southern Taiwan. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2022, 55(3):474–481.
Li HL, Chan YC, Cheng SW. Current evidence on management of aortic stent-graft infection: a systematic review and Meta-analysis. Ann Vasc Surg. 2018;51:306–13.
Juszczak M, Mann H, Riste M, Woodhouse A, Sörelius K, Claridge M, Adam DJ. Complex Endovascular Repair of Paravisceral Infective native aortic aneurysms. J Endovascular Therapy: Official J Int Soc Endovascular Spec. 2024;31(2):223–31.
Escobar GA, Eliason JL, Hurie J, Arya S, Rectenwald JE, Coleman DM. Rifampin soaking dacron-based endografts for implantation in infected aortic aneurysms–new application of a time-tested principle. Ann Vasc Surg. 2014;28(3):744–8.
Schaefers JF, Donas KP, Panuccio G, Kasprzak B, Heine B, Torsello GB, Osada N, Usai MV. Outcomes of Surgical Explantation of infected aortic grafts after endovascular and open abdominal aneurysm repair. Eur J Vascular Endovascular Surgery: Official J Eur Soc Vascular Surg. 2019;57(1):130–6.
Gaines S, Babrowski TA, Skelly C, Milner R. Unique endovascular repair of an aortic pseudoaneurysm after staged approach for an aortoduodenal fistula. J Vascular Surg Cases Innovative Techniques. 2020;6(4):612–3.
Georgeades C, Zarb R, Lake Z, Wood J, Lewis B. Primary Aortoduodenal Fistula: A Case Report and Current Literature Review. Ann Vasc Surg. 2021, 74:518.e513-518.e523.
Acknowledgements
The authors are grateful to the patient for participating in this study.
Funding
This case report was supported by the Science and Technology Project of the Health Commission of Sichuan Province in China (Grant No. 23009). The funding body had no role in the collection, analysis, and interpretation of this case, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
LBY and XJH collected and analyzed the patient data in this study. XJH contributed to the drafting of the manuscript. LBY contributed to critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethical approval was waived by the research ethics committee of West China Hospital because of the retrospective and anonymous nature of the study. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Informed consent from all subjects for both study participation and publication of identifying information/images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, X., Yan, L. A secondary abdominal aorta-duodenal fistula accompanied with acquired Immune Deficiency Syndrome presented with recurrent sepsis: a case report. BMC Infect Dis 24, 669 (2024). https://doi.org/10.1186/s12879-024-09559-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09559-8