Abstract
Background
Tubal factor infertility (TFI) is common in sub-Saharan Africa and often secondary to pelvic inflammatory disease (PID). Anaerobes associated with bacterial vaginosis (BV) are also found in PIDs widely dominated by Chlamydia trachomatis (C. trachomatis), whose role in TFI is better demonstrated than that of BV.
Objectives
To determine the prevalence of BV and C. trachomatis and to investigate the association between BV, C. trachomatis and TFI.
Methods
We included 137 patients treated for infertility between January 2020 and November 2021. Cases were defined as women with infertility aged 18-45 years presenting with TFI (n = 52), and controls as infertile women in the same age groups without TFI (n = 85). Data on social habits, life style and infertility parameters were collected, and we performed screening for BV and C. trachomatis. Multiple regression was used to measure associations.
Results
The prevalence of BV and C. trachomatis was 42.3% (58/137) and 23.4% (32/137), respectively. BV (61.5% vs 30.6%, p<0.001) and C. trachomatis (48.1 vs 8.2%, p<0.001) were more frequent in cases of TFI.
BV and C. trachomatis increased the risk of TFI approximately 4-fold [aOR: 3.77 (1.61-8.83), p=0.002] and 14-fold [aOR: 13.77 (4.59-41.27), p<0.001], respectively.
Conclusion
BV and C. trachomatis infection are strongly associated with TFI in Bukavu. Prevention and screening should be implemented to reduce the risk of TFI.
Similar content being viewed by others
Introduction
Infertility, defined as the inability to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse [1] and concerns between 9% and 30% of couples [2]. Infertility occurs with an alteration in female and male infertility factors, which may be associated with varying degrees [2].
Previous studies have reported that tubal factors are the main cause of infertility in Africa [3, 4], up to 30% of women consult for infertility [5]. This is essentially related to the high prevalence of pelvic inflammatory disease (PID), a major cause of tubal pathologies related to infertility [6]. PID is caused by the ascent of microorganisms from the lower to the upper genital tract. A variety of organisms are implicated in the etiology of PID, including Chlamydia trachomatis (C. trachomatis), Neisseria gonorrhoeae (N. gonorrhoeae), Mycoplasma genitalium, and the anaerobic and aerobic bacteria commonly associated with bacterial vaginosis (BV) [7].
BV is a dysbiosis characterized by an imbalance in vaginal flora, with an increase in anaerobic bacteria and a simultaneous disappearance of protective lactobacilli [8]. It is associated with a heterogeneous group of pathogens rather than a single etiological agent and includes Gardnerella vaginalis, Atobopium vaginae, M. hominis, and various species of Prevotella, Porphyromonas, Mobiluncus, Sneathia, Peptoniphilus, etc. [9].
Several women with BV are asymptomatic [10, 11]. Although the prevalence of BV varies greatly from one region to another, depending on the population study, there is a general agreement that BV is more common in black and hispanic women, women who smoke, sexually active women compared to virgin women, lesbians and those with vaginal douching [12, 13].
Well known for its association with adverse pregnancy outcomes [14, 15], the involvement of BV in infertility remains controversial, unlike that of C. trachomatis infection [16]. Indeed, although some studies have shown an association between BV and PID and hence tubal factor infertility [17,18,19], other studies have found no such relationship. In addition, some studies have reported a high prevalence of BV among patients with nontubal infertility and unexplained infertility [20, 21]. To the best of our knowledge, there are no data about the association between VB, C. trachomatis infection and infertility in our area, especially in Bukavu.
Therefore, the aim of this study was to determine the prevalence of BV and C. trachomatis infection among patients with infertility and to investigate the association between BV, C. trachomatis infection and tubal factor infertility.
Materials and methods
Study design
This is an unmatched case-control study involving women who consulted HPGRB’s Department of Obstetrics and Gynecology for infertility treatment between January 2020 and November 2021.
Sample size and statistical power
The minimum sample size was calculated in OpenEpi online software using Kelsey’s formula [22]: 83 patients (28 cases and 55 controls) based on a prevalence of BV of 40% among women without tubal obstruction in a study carried out in Rwanda [23].
Inclusion criteria
Women between 18 and 45 years old and married.
Women who agree to participate in the study and provide complete information.
Women who have undergone investigation of infertility factors [(ovulatory, hormonal, mechanical and andrological (semen analysis)].
Women who had a vaginal swab for screening for BV and C. trachomatis infection.
According to assessment of tubes, Cases were defined as women in infertile couple aged 18-45 years with TFI (n = 52).
For controls group, eighty five (n= 85) infertile women were recruited at the same period with the same inclusion criteria but without TFI.
Exclusion criteria
Women who received treatment with clindamycin and/or metronidazole within the last 90 days for any indication.
Women with a history of documented pelvic endometriosis, tubal obstructions following one or more abdominal surgeries or genital tuberculosis.
Isolated male infertility.
Infertility following chemical, surgical or radiation castration.
Data collection and analysis
Data on anthropometric parameters, sociodemographic characteristics, medical, surgical, gynecological and obstetrical history, habits and lifestyle were collected.
Clinical and paraclinical examinations were performed to investigate infertility factors:
Ovulatory and hormonal factors: Apart from age and cycle length, a blood sample was taken between day 2 and day 5 of the cycle to measure FSH (to assess ovarian reserve). At the same time, transvaginal ultrasound was performed to count the number of antral follicles by summing the number of 2 to 9 mm follicles on both ovaries.
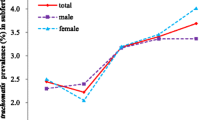
Mechanical factors: ultrasound, hysterosalpingography, or laparoscopy with chromotubation combined with diagnostic hysteroscopy.
The andrological factor: semen analysis.
Screening for BV and C. trachomatis was performed during the examination: the patient was in the gynaecological position, then a sterile, nonlubricated Cusco speculum was inserted into the vagina. An intracervical swab was taken to test for C. trachomatis antigen using the One Step Chlamydia trachomatis Antigen Rapid Test (Colloidal Gold) [24]. A second sample was taken from the posterior fornix using a wooden spatula. The sample was immediately spread onto a slide that was fixed and brought in the AVEONS (Angamiza Vizuri Early Onset Neonatal Sepsis) laboratory, where Gram staining was performed. All Gram-stained slides were read according to Nugent’s morphotype analysis [25] for laboratory diagnosis of BV. Five microscopic fields were read at 100x magnification (with immersion oil) for the presence and quantity of Lactobacillus (gram-positive rods), Gardnerella vaginalis/Bacteroides (gram-variable coccobacilli) and Mobiluncus (gram-negative curved rods).
All slides were scored by two independent readers. In case of discrepancy in results, the criteria were discussed to obtain a consensus on the assessment. If there was no consensus, a third experienced person assessed the slide to make the final categorization.
Operational definitions
The smear is classified as having either healthy vaginal flora (Nugent score: 0-3), intermediate vaginal flora (Nugent score: 4 to 6) or BV (Nugent score: 7 to 10) [26].
Tubal factor infertility (TFI): radiological or laparoscopic signs of tubal obstruction and/or peri-tubal adhesions altering the normal functional anatomy of the tube [1].
Polycystic ovary syndrome (PCOS): according to the 2003 Rotterdam consensus [27], the presence of two of the following three criteria: oligo/anovulation; clinical or biological hyperandrogenism; polycystic ovaries on ultrasound.
Primary infertility: infertility with no history of clinical pregnancy [28].
Secondary infertility: infertility with a history of clinical pregnancy regardless of outcome [28].
Diminished ovarian reserve (DOR): FSH (follicle stimulating hormone)>10 mUI/ml and/or AFC (antral follicle count) <10 small follicles [29].
Regular cycles: cycle of 24-38 days [30].
Data management and statistical analysis
Data were recorded in the Excel 2016 database and analysed using STATA 14. Data were summarized into frequencies for categorical variables and into mean± standard deviation (SD) for continuous variables with normal distribution.
We determined the prevalence of BV (Nugent score: 7-10) and C. trachomatis infection in the study population and in subgroups. This prevalence was reported with a 95% confidence interval (CI).
Association between dependent variable (TFI) and independent variables were determining using the chi-square test (for BV, C. trachomatis infection, type of infertility, occupation); and chi-square of linear trend (for education level). Independent variables with p<0.10 in the univariate analysis were included in the logistic regression model. In this model, independent variables with a value of p<0.05 are significantly associated with TFI.
Ethics approval and consent to participate
An informed consent was obtained from all subjects in this study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics committee of the Catholic University of Bukavu and the Ministry of Public Health of DRC (reference number 062/CD/DPS/SK/2017) within the framework of the AVEONS project.
Results
Sociodemographic and clinical description of the cases and controls
This study included 137 women with a mean age of 32.9± 5.6 years, ranging from 23 to 45 years. Most of them had higher education, were unemployed and had a monthly household income of over $500 (Table 1). Secondary infertility was found in 67.1% (92/137), with the majority lasting more than 2 years (Table 2).
In the hormonal profile study, we found 20.4%(28/137) irregular cycles, 17.5% (24/137) DOR and 24.3% (33/137) PCOS. TFI was the most common infertility factor (37.9%) (Table 2). In the FTI group (cases group), BV was more frequent among women with a secondary education level (77.8% vs. 22.2%, p=0.013), those with no occupation and students (83.3% vs. 16.7%, p=0.005) (Table 1).
BV was less frequent among women with secondary infertility when they had no TFI (controls group) (23.7% vs 76.3%, p=0.039) (Table 2).
Prevalence of BV and C. trachomatis infection
The prevalence of BV and C. trachomatis infection in the study population was 42.3%(58/137) (95% CI, 33.9%-51.1%) and 23.4%(32/137) (95% CI, 16.6%-31.3%), respectively.
BV was more frequent among patients with TFI (61.5% vs. 30.6%, p<0.001). The same applies to C. trachomatis infection (48.1 vs 8.2%, p<0.001) (Table 2).
Association of VB, C. trachomatis infection and TFI
BV and C. trachomatis infection increased the risk of tubal damage by 4-fold [aOR 3.77 (1.61-8.83), p=0.002] and 14-fold [aOR 13.77 (4.59-41.27), p<0.001], respectively, regardless of each other and regardless of infertility type, education level and occupation (Table 3).
Discussion
General and clinical characteristics of study population
Secondary infertility was found in 67.1% of cases, and tubal factors were the most common factor (37.9%).
In sub-Saharan Africa, secondary infertility is more common than primary infertility [31]. The high rate of secondary infertility in sub-Saharan Africa is thought to be related to the high prevalence of pelvic inflammatory disease secondary to sexually transmitted infections (STIs) and medical interventions in unsanitary conditions: during childbirth or abortion, and especially unsafe abortion [28, 32]. In the meta-analysis by Abebe et al., secondary infertility is predominant in sub-Saharan Africa, and its prevalence varies between 31 and 85% [33].
These results were close to those of several African studies, notably in Nigeria (62-71%), Gambia (59%) and Tanzania (63%) [33], and confirm the WHO report, which mentioned that in sub-Saharan Africa, most couples (52%) suffer from secondary infertility. High rates of secondary infertility (40%) are also observed in Latin America, while in Asia, only 23% of infertile couples suffer from secondary infertility [33].
Regarding infertility factors, tubal alterations are predominant. Indeed, according to the meta-analysis by Abebe et al. [33] on primary and secondary infertility in Africa, the main factor of infertility is tubal factors in developed countries, whereas ovulation abnormalities are the main factor [34].
Prevalence of BV and C. trachomatis infection
The prevalence of BV was 42.3%. Table 4 shows the prevalence of BV among women with infertility, as reported in several studies worldwide. Prevalence varies from one study to another. In sub-Saharan Africa, it is relatively high and close to our results when compared with other continents [35,36,37]. This can be explained by variations in diagnosis (Amsel criteria, Nugent score, Hay Ison criteria, qPCR, 16S rRNA, IS-proTM, etc. ), differences in clinical profiles and ethnic differences in the study populations. Indeed, studies in the general population report that the prevalence of BV is higher in black women than in Hispanic and Caucasian women [13]. The frequency of practices such as douching, which is associated with BV, may explain this higher prevalence.
The prevalence of C. trachomatis infection was 23.4% in the study population. Our result was higher than the prevalence of 8.7% and 18.2% found in two African studies using the rapid antigenic test [38, 39]. A recent meta-analysis of C. trachomatis infection in North Africa and the Near East reported a prevalence of 12.4% in women with infertility [40].
Association between BV, C. trachomatis and TFI
A high prevalence of BV was found among patients with TFI compared to those without TFI (61.5% vs. 30.6%, p<0.001). BV was therefore strongly associated with a 4-fold increased TFI risk, regardless of C. trachomatis infection.
Very few studies have investigated this association: in three British studies involving patients undergoing in vitro fertilization for infertility, Gaudoin et al. [18]; Liversedge et al. [53]; Wilson JD et al. [49] reported that patients with tubal infertility were 6-, 2- and 3-fold more likely to have BV than those without tubal infertility, with prevalences of 87.5%, 31.5% and 36.4%, respectively.
In Africa, Dhont et al. [37], in a study of predictors of infertility in Rwanda, reported that BV was 2-fold more likely to have tubal infertility, with a prevalence of 25% according to the Amsel criteria and 56% according to the Nugent score. Innoncent Durugbo et al. [4] compared BV between women with tubal infertility and women without infertility in Nigeria and found a higher prevalence (28.1%) among women with tubal infertility.
All these results support the existence of an association between BV and TFI through PID, even though they do not establish a cause-and-effect relationship.
However, in an American study of women undergoing in vitro fertilization, patients with idiopathic infertility were more likely to have BV than women with other causes of infertility; highlighting the potential role of pro-inflammatory cytokines such as IL-1beta and IL-8 at the cervical level in women with altered vaginal flora [21].
Women with TFI had a higher prevalence of C. trachomatis than those without tubal alterations (48.1% vs. 8.2%, p<0.001). Analyses adjusted for BV showed that C. trachomatis infection is strongly associated with tubal alterations (with 14-fold increased risk).
C. trachomatis infection can induce PID, which can lead to tubal infertility, ectopic pregnancy and chronic pelvic pain [16].
Although C. trachomatis is not the only germ incriminated [16], several studies have also found a strong association between C. trachomatis infection and tubal damage [59,60,61,62,63,64].
The study main limitations were as follows:
The sample size does not allow us to generalize our conclusions to the entire population.
This study does not establish a causal link between BV, C. trachomatis infection and TFI.
Using the rapid antigenic test may underestimate certain results compared with most studies using serological tests.
Using the Nugent score, we endorse the limit of interpretation of intermediate flora that can be better categorized by genome sequencing and qPCR methods.
In conclusion, despite the limitations, these data highlight the high prevalence of bacterial vaginosis (42.3%) and C. trachomatis infection (23,4%) among women with infertility and their strong association with tubal factor infertility, which is the major cause of infertility in our environment. Prevention and screening should be implemented to reduce the risk of infertility.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 16S rRNA:
-
16S ribosomal ribonucleic acid
- AFC:
-
Antral follicle count
- AVEONS:
-
Angamiza Vizuri Early Onset Neonal Sepsis
- BV:
-
Bacterial vaginosis
- DOR:
-
Diminished ovarian reserve
- FSH:
-
Follicle Stimulating Hormon
- HPGRB:
-
Hôpital provincial général de référence de Bukavu
- IL:
-
Interleukin
- IS-proTM :
-
Interspace profiling
- PCOS:
-
Polycystic ovary syndrome
- PID:
-
Pelvic inflammatory disease
- qPCR:
-
Quantitative polymerase chain reaction
- TFI:
-
Tubal factor infertility
- UCB:
-
Université Catholique de Bukavu
References
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406. Available from: https://doi.org/10.1016/j.fertnstert.2017.06.005.
Inhorn MC, Patrizio P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2014;21(4):411–26.
Ibekwe PC, Udensi AM, Imo AO. Hysterosalpingographic findings in patients with infertility in South eastern Nigeria. Niger J Med. 2010;19(2):165–7.
Durugbo II, Nyengidiki TK, Bassey G, Wariso KT. Bacterial vaginosis among women with tubal factor infertility in Nigeria. Int J Gynecol Obstet. 2015;131(2):133–6. Available from: https://doi.org/10.1016/j.ijgo.2015.05.031.
Evers JLH. Female subfertility. Lancet. 2002;360(9327):151–9.
Tao X, Ge SQ, Chen L, Cai LS, Hwang MF, Wang CL. Relationships between female infertility and female genital infections and pelvic inflammatory disease: A population-based nested controlled study. Clinics. 2018;73:1–6.
Haggerty CL, Totten PA, Tang G, Astete SG, Ferris MJ, Norori J, et al. Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm Infect. 2016;92(6):441–6.
Quentin R, Verdon R. Les infections génitales hautes: bases microbiologiques du diagnostic et du traitement. J Gynecol Obstet Biol la Reprod. 2012;41(8):850–63. Available from: https://doi.org/10.1016/j.jgyn.2012.09.015.
Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin Microbiol Rev. 2016;29(2):223–38.
Yen S, Shafer MA, Moncada J, Campbell CJ, Flinn SD, Boyer CB. Bacterial vaginosis in sexually experienced and non-sexually experienced young women entering the military. Obstet Gynecol. 2003;102(5):927–33.
Klebanoff MA, Schwebke JR, Zhang J, Nansel TR, Yu KF, Andrews WW. Vulvovaginal symptoms in women with bacterial vaginosis. Obstet Gynecol. 2004;104(2):267–72.
Vodstrcil LA, Walker SM, Hocking JS, Law M, Forcey DS, Fehler G, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. 2015;60(7):1042–53.
Allsworth JE, Peipert JF. Prevalence of bacterial vaginosis. Obstet Gynecol. 2007;109(1):114–20.
García-Velasco JA, Budding D, Campe H, Malfertheiner SF, Hamamah S, Santjohanser C, et al. The reproductive microbiome – clinical practice recommendations for fertility specialists. Reprod Biomed Online. 2020;41(3):443–53. Available from: https://doi.org/10.1016/j.rbmo.2020.06.014.
Mulinganya G, de Vulder A, Bisimwa G, Boelens J, Claeys G, de Keyser K, et al. Prevalence, risk factors and adverse pregnancy outcomes of second trimester bacterial vaginosis among pregnant women in Bukavu, Democratic Republic of the Congo. PLoS One. 2021;16(10 October):1–17. Available from: https://doi.org/10.1371/journal.pone.0257939.
Price MJ, Ades AE, Soldan K, Welton NJ, Macleod J, Simms I, et al. The natural history of chlamydia trachomatis infection in women: A multi-parameter evidence synthesis. Health Technol Assess (Rockv). 2016;20(22):1–250.
Ross JDC. Is Mycoplasma genitalium a cause of pelvic inflammatory disease? Infect Dis Clin North Am. 2005;19(2 SPEC. ISS.):407–13.
Gaudoin M, Rekha P, Morris A, Lynch J, Acharya U. Bacterial vaginosis and past chlamydial infection are strongly and independently associated with tubal infertility but do not affect in vitro fertilization success rates. Fertil Steril. 1999;72(4):730–2.
Ness RB, Kip KE, Hillier SL, Soper DE, Stamm CA, Sweet RL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162(6):585–90.
Mania-Pramanik J, Kerkar SC, Salvi VS. Bacterial vaginosis: A cause of infertility? Int J STD AIDS. 2009;20(11):778–81.
Spandorfer SD, Neuer A, Giraldo PC, Rosenwaks Z, Witkin SS. Relationship of abnormal vaginal flora, proinflammatory cytokines and idiopathic infertility in women undergoing IVF. J Reprod Med. 2001;46(9):806–10.
OpenEpi - Sample size for unmatched case-control studies. Available from: https://www.openepi.com/SampleSize/SSCC.htm. Cited 2022 Jan 28.
Dhont N, Luchters S, Muvunyi C, Vyankandondera J, De Naeyer L, Temmerman M, et al. The risk factor profile of women with secondary infertility: An unmatched case-control study in Kigali, Rwanda. BMC Womens Health. 2011;11(1):32. Available from: http://www.biomedcentral.com/1472-6874/11/32.
Stephen S, Muchaneta-Kubara CGE, Munjoma MW, Mandozana G. Evaluation of cortez onestep Chlamydia rapicardTM insta test for the detection of chlamydia trachomatis in pregnant women at mbare polyclinic in Harare, Zimbabwe. Int J MCH AIDS. 2016;6(1):19.
Forsum U, Halle A. Bacterial vaginosis – a laboratory and. Apmis. 2005;113(5):153–61.
Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301.
Fauser BCJM. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25.
Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018;62(February):2–10.
Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder—a plea for universal definitions. J Assist Reprod Genet. 2015;32(12):1709–12.
Fraser IS, Critchley HOD, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):383–90.
Lunenfeld B, Van Steirteghem A. Infertility in the third millenium: Implications for the individual, family and society: Condensed meeting report from the Bertarelli Foundation’s Second Global Conference. Hum Reprod Update. 2004;10(4):317–26.
Dhont N, Muvunyi C, Luchters S, Vyankandondera J, De Naeyer L, Temmerman M, et al. HIV infection and sexual behaviour in primary and secondary infertile relationships: a case - control study in Kigali. Rwanda Sex Transm Infect. 2011;87(1):28–34.
Abebe MS, Afework M, Abaynew Y. Primary and secondary infertility in Africa: systematic review with meta-analysis. Fertil Res Pract. 2020;6(1):1–11.
Benbella A, Aboulmakarim S, Hardizi H, Zaidouni A, Bezad R. Infertility in the Moroccan population: an etiological study in the reproductive health centre in Rabat. Pan Afr Med J. 2018;30:1–7.
Selim SA, El Alfy SM, Aziz MHA, Mohamed HM, Alasbahi AA. Effective of metronidazole to bacterial flora in vagina and the impact of microbes on live birth rate during intracytoplasmic sperm injection (ICSI). Arch Gynecol Obstet. 2011;284(6):1449–53.
Salah RM, Allam AM, Magdy AM, Mohamed AS. Bacterial vaginosis and infertility: cause or association? Eur J Obstet Gynecol Reprod Biol. 2013;167(1):59–63. Available from: https://doi.org/10.1016/j.ejogrb.2012.10.031.
Dhont N, Van De Wijgert J, Luchters S, Muvunyi C, Vyankandondera J, Temmerman M. Sexual violence, HSV-2 and HIV are important predictors for infertility in Rwanda. Hum Reprod. 2010;25(10):2507–15.
Nwankwo EO, Sadiq MN. Prevalence of chlamydia trachomatis infection among patients attending infertility and sexually transmitted diseases clinic (STD) in Kano, north western Nigeria. Afr Health Sci. 2014;14(1):672–8.
Oloyede OAO, Fakoya TA, Oloyede AA, Alayo AM. Prevalence and awareness about chlamydial infection in women undergoing infertility evaluation in Lagos. Nigeria Int J Heal Res. 2009;2(2):157–62.
Smolak A, Chemaitelly H, Hermez JG, Low N, Abu-Raddad LJ. Epidemiology of Chlamydia trachomatis in the Middle East and north Africa: a systematic review, meta-analysis, and meta-regression. Lancet Glob Heal [Internet]. 2019;7(9):e1197–225. Available from: https://doi.org/10.1016/S2214-109X(19)30279-7.
AboulEnien WM, EMH. Association of abnormal vaginal flora with increased cervical tumour necrosis factor–alpha and interferon–gamma levels in idiopathic infertility. Egypt J Immunol. 2005;12(2):53–9 PMID: 17977210. Egypt J Immunol. 2005;12(2)(53):9.
Haahr T, Jensen JS, Thomsen L, Duus L, Rygaard K, Humaidan P. Abnormal vaginal microbiota may be associated with poor reproductive outcomes: A prospective study in IVF patients. Hum Reprod. 2016;31(4):795–803.
Vergaro P, Tiscornia G, Barragán M, García D, Rodriguez A, Santaló J, et al. Vaginal microbiota profile at the time of embryo transfer does not affect live birth rate in IVF cycles with donated oocytes. Reprod Biomed Online [Internet]. 2019;38(6):883–91. Available from: https://doi.org/10.1016/j.rbmo.2018.12.019.
Bernabeu A, Lledo B, Díaz MC, Lozano FM, Ruiz V, Fuentes A, et al. Effect of the vaginal microbiome on the pregnancy rate in women receiving assisted reproductive treatment. J Assist Reprod Genet. 2019;36(10):2111–9.
Mangot-Bertrand J, Fenollar F, Bretelle F, Gamerre M, Raoult D, Courbiere B. Molecular diagnosis of bacterial vaginosis: Impact on IVF outcome. Eur J Clin Microbiol Infect Dis. 2013;32(4):535–41.
Moragianni D, Dryllis G, Andromidas P, Kapeta-Korkouli R, Kouskouni E, Pessach I, et al. Genital tract infection and associated factors affect the reproductive outcome in fertile females and females undergoing in vitro fertilization. Biomed Reports. 2019;10(4):231–7.
Boomsma CM, Kavelaars A, Bozkurt N, Eijkemans MJC, Fauser BCJM, Heijnen CJ, et al. Is bacterial vaginosis associated with a pro-inflammatory cytokine profile in endometrial secretions of women undergoing IVF? Reprod Biomed Online. 2010;21(1):133–41. Available from: https://doi.org/10.1016/j.rbmo.2010.03.022.
Koedooder R, Singer M, Schoenmakers S, Savelkoul PHM, Morré SA, De Jonge JD, et al. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: A prospective study. Hum Reprod. 2019;34(6):1042–54.
Wilson JD, Ralph SG, Rutherford AJ. Rates of bacterial vaginosis in women undergoing in vitro fertilisation for different types of infertility. BJOG An Int J Obstet Gynaecol. 2002;109(6):714–7.
McCaffrey M, Cottell E, Keane D, Mallon E, Walsh T, McMorrow J, et al. Bacterial vaginosis and infertility. Int J STD AIDS. 1997;8(SUPPL.1):25.
Morgan DJ, Wong SJ, Trueman G, Priday A, Lamont RF, Kapembwa MS, et al. Can bacterial vaginosis influence fertility? Its increased prevalence in a subfertile population. Int J STD AIDS. 1997;8(SUPPL.1):19–20.
Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: Cohort study. Br Med J. 1999;319(7204):220–3.
Liversedge NH, Turner A, Horner PJ, Keay SD, Jenkins JM, Hull MGR. The influence of bacterial vaginosis on in-vitro fertilization and embryo implantation during assisted reproduction treatment. Hum Reprod. 1999;14(9):2411–5.
Moini A, Mohammadi Yeganeh L, Shiva M, Ahmadieh M, Salman Yazdi R, Hasani F, et al. Bacterial vaginosis and the risk of early miscarriage in women undergoing intracytoplasmic sperm injection cycles: a prospective cohort study. Hum Fertil. 2018;21(4):263–8. Available from: https://doi.org/10.1080/14647273.2017.1353709.
Kyono K, Hashimoto T, Nagai Y, Sakuraba Y. Analysis of endometrial microbiota by 16S ribosomal RNA gene sequencing among infertile patients: a single-center pilot study. Reprod Med Biol. 2018;17(3):297–306.
Eldivan Ö, Evliyaoğlu Ö, Ersoy E, Aksu G, Dilbaz S, Göktolga Ü. Does screening for vaginal infection have an impact on pregnancy rates in intracytoplasmic sperm injection cycles? Turkish J Obstet Gynecol. 2016;13(1):11–5.
Eckert LO, Moore DE, Patton DL, Agnew KJ, Eschenbach DA. Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in-vitro fertilization. Infect Dis Obstet Gynecol. 2003;11(1):11–7.
Moore DE, Soules MR, Klein NA, Fujimoto VY, Agnew KJ, Eschenbach DA. Bacteria in the transfer catheter tip influence the live-birth rate after in vitro fertilization. Fertil Steril. 2000;74(6):1118–24.
Tukur J, Shittu SO, Abdul AM. A case control study of active genital Chlamydia trachomatis infection among patients with tubal infertility in northern Nigeria. Trop Doct. 2006;36(1):14–6.
Omo-Aghoja LO, Okonofua FE, Onemu SO, Larsen U, Bergstrom S. Association of Chlamydia trachomatis serology with tubal infertility in Nigerian women. J Obstet Gynaecol Res. 2007;33(5):688–95.
Siemer J, Theile O, Larbi Y, Fasching PA, Danso KA, Kreienberg R, et al. Chlamydia trachomatis infection as a risk factor for infertility among women in Ghana, West Africa. Am J Trop Med Hyg. 2008;78(2):323–7.
El Qouqa IA, Shubair ME, Al Jarousha AM, Sharif FA. Prevalence of Chlamydia trachomatis among women attending gynecology and infertility clinics in Gaza. Palestine Int J Infect Dis. 2009;13(3):334–41.
Akande VA, Hunt LP, Cahill DJ, Caul EO, Ford WCL, Jenkins JM. Tubal damage in infertile women: Prediction using chlamydia serology. Hum Reprod. 2003;18(9):1841–7.
Machado ACS, Guimarães EMB, Sakurai E, Fioravante FCR, Amaral WN, Alves MFC. High titers of Chlamydia trachomatis antibodies in Brazilian women with tubal occlusion or previous ectopic pregnancy. Infect Dis Obstet Gynecol. 2007;2007:10–5.
Acknowledgments
We thank all members of AVEONS laboratory for their helpful contribution to this study. We also thank all other colleagues in the Department of Obstetrics and Gynecology for their collaboration during data collection.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Conception and design of work: JM,GM,EK,DS. Acquisition: JM,EH,EK,FK,SC,CK,IB,YK,JB,BK,GM. Analysis and interpretation: JM, GM. Draft the work and revision: JM,EK,DS,GM.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics committee of the Catholic University of Bukavu, DR Congo.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mongane, J., Hendwa, E., Sengeyi, D. et al. Association between bacterial vaginosis, Chlamydia trachomatis infection and tubal factor infertility in Bukavu, Democratic Republic of Congo. BMC Infect Dis 24, 480 (2024). https://doi.org/10.1186/s12879-024-09379-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09379-w