Abstract
Aim
Palivizumab has proven effective in reducing hospitalizations, preventing severe illness, improving health outcomes, and reducing healthcare costs for infants at risk of respiratory syncytial virus (RSV) infection. We aim to assess the value of palivizumab in preventing RSV infection in high-risk infants in Colombia, where RSV poses a significant threat, causing severe respiratory illness and hospitalizations.
Methods
We conducted a decision tree analysis to compare five doses of palivizumab with no palivizumab. The study considered three population groups: preterm neonates (≤ 35 weeks gestational age), infants with bronchopulmonary dysplasia (BPD), and infants with hemodynamically significant congenital heart disease (CHD). We obtained clinical efficacy data from IMpact-RSV and Cardiac Synagis trials, while we derived neonatal hospitalization risks from the SENTINEL-1 study. We based hospitalization and recurrent wheezing management costs on Colombian analyses and validated them by experts. We estimated incremental cost-effectiveness ratios and performed 1,000 Monte Carlo simulations for probabilistic sensitivity analyses.
Results
Palivizumab is a dominant strategy for preventing RSV infection in preterm neonates and infants with BPD and CHD. Its high efficacy (78% in preventing RSV in preterm infants), the substantial risk of illness and hospitalization, and the high costs associated with hospitalization, particularly in neonatal intensive care settings, support this finding. The scatter plots and willingness-to-pay curves align with these results.
Conclusion
Palivizumab is a cost-saving strategy in Colombia, effectively preventing RSV infection in preterm neonates and infants with BPD and CHD by reducing hospitalizations and lowering healthcare costs.
Similar content being viewed by others
Introduction
Respiratory syncytial virus (RSV) is a highly contagious respiratory virus that is a common cause of severe lower respiratory tract infections in young children, especially in preterm neonates, infants with bronchopulmonary dysplasia (BPD), and hemodynamically significant congenital heart disease (CHD). RSV is a leading cause of infant hospitalization and can cause severe respiratory illness, including bronchiolitis and pneumonia. Occasionally, RSV can lead to death, particularly in premature infants and those with underlying medical conditions [1]. Palivizumab is a prophylactic measure against severe RSV infection in high-risk children. Palivizumab is a monoclonal antibody administered by injection and works by binding to the RSV virus, preventing it from attaching to and infecting the respiratory tract. Palivizumab can significantly impact the health and well-being of affected children and their families, preventing RSV hospitalizations in preterm neonates and infants with BPD and CHD and recurrent wheezing in childhood [2].
One of the critical clinical impacts of palivizumab is its ability to reduce the number of RSV-related hospitalizations. Several clinical trials have shown that prevention with palivizumab can reduce the risk of hospitalizations by up to 55% in high-risk infants [3]. This reduction in hospitalization is significant in premature infants, who are more susceptible to severe RSV illness and are at increased risk of hospitalization. By reducing the number of RSV-related hospitalizations, palivizumab can lessen the burden on healthcare systems, freeing up hospital beds and other resources for other patients. Moreover, by preventing severe respiratory illness, palivizumab can also reduce the costs associated with hospitalization and other medical interventions [4].
Its ability to reduce the number of RSV-related hospitalizations and prevent severe respiratory illness can improve health outcomes, reduce the burden on healthcare systems, and lower healthcare costs. As such, palivizumab is a crucial component of RSV prevention strategies, particularly in high-risk infants and young children. Therefore, we aim to estimate the value of palivizumab in preventing RSV infection in preterm neonates and infants with BPD and CHD in Colombia in terms of avoided hospitalizations and costs, as well as the health-related quality of life of these patients.
Methods
The population of this economic evaluation is premature neonates with 35 weeks gestational age (wGA) and less and six months of age or younger or 24 months old or younger and a clinical diagnosis of BPD requiring ongoing medical treatment (i.e., supplemental oxygen, steroids, bronchodilators, or diuretics within the past six months) [3]; or ≤ 24 months old with documented hemodynamically significant CHD and an unoperated or partially corrected CHD [5]. The perspective of the analysis is from the third payer, and the annual discount rate is 5% for costs and benefits [6]. The comparators do not use palivizumab versus five doses of palivizumab (the first two 50 mg doses and the remaining 100 mg doses), and the model time horizon is six years.
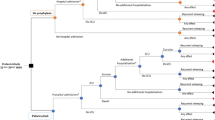
Clinical outcomes are the rate of RSV-related hospitalizations avoided [3, 5] and the rate of relapses avoided for recurrent wheezing. The costs of the model are in US dollars (USD) for the year 2022, the exchange rate is COP 4,800 per USD 1, and the willingness to pay threshold is one gross domestic product (GDP) per capita in Colombia: $ 6,161 [7]. We did a decision tree model structure with two health states: RSV infection or no infection. Patients with RSV infection can be hospitalized or not hospitalized (outpatient management), and all patients with RSV infection, regardless of their hospitalization state, are likely to have recurrent wheezing or not develop it (Fig. 1).
We took the efficacy of palivizumab in preventing RSV-related hospitalizations in preterm neonates ≤ 35 wGA and infants with BPD from the pivotal study of palivizumab, the IMpact-RSV clinical trial [3]. Similarly, we took the drug’s clinical efficacy in preventing RSV-related hospitalizations in infants with CHD from the Cardiac Synagis clinical trial [5]. In addition, we took the probability of RSV-related hospitalization in preterm neonates ≤ 35 wGA without immune-prophylaxis from the SENTINEL1 study [8]. Finally, we took the distribution of hospitalization services in general wards (47.1%) or intensive care units (ICUs) (52.9%) from a prospective multicentric observational study carried out in six Colombian cities for one year, which included more than 700 infants with RSV-related hospitalization [9].
We took the frequency of RSV-related hospitalizations from a study of hospitalization costs of neonates and infants with RSV infection in Colombia [10]. The risk of developing RSV-related wheezing was taken from a six-year follow-up study of infants with RSV infection [11]. We took the healthcare cost of each RSV-related hospitalization in preterm neonates and infants with BPD from a survey of direct medical costs of RSV-related bronchiolitis hospitalizations in Colombia that included more than 80 infants [12].
We adjusted the cost of each RSV-related hospitalization in infants with CHD based on a cost study in this population. The hospital stay is nearly twice that of infants with RSV-related hospitalizations without CHD [13]. We validated this result with pediatric cardiologists in Colombia. In addition, the weighted mean cost of each wheezing exacerbation (weighted from outpatient care, emergency room (ER), or hospitalization) and the annual relapse rate were taken from a cost study in Colombia’s preschool children with viral-triggered wheeze [14]. Finally, we took inpatient service-adjusted Quality Adjusted Life Years (QALYs) from a study that estimated the quality of life lost due to RSV [15].
We did not find data on the disutility generated by wheezing, so we homologated them to those caused by asthma [16] and validated them with a group of neonatologists. We performed the heterogeneity analysis by estimating costs and outcomes independently for preterm neonates, infants with BPD, and infants with CHD. The model assumes that there are no deaths from RSV infection in neonates with RSV-related hospitalizations since we did not find data on the specific mortality rate from RSV in Colombia. In addition, we performed the cost-utility analysis modeling a cohort of 1000 patients with palivizumab vs. 1000 patients without palivizumab in each group in which the heterogeneity analysis was.
We carried out 1000 Monte Carlo simulations to characterize the uncertainty and estimated the maximum net benefit (MNB) through the willingness to pay curves. Neonatologists and pediatric cardiologists from Colombia participated in constructing the model and validating the inputs.
Results
Table 1 shows the epidemiological and clinical efficacy parameters of the economic model. Table 2 shows the healthcare costs of each hospital event for patients with RSV-related hospitalizations and the costs of each exacerbation of wheezing. Table 3 shows the QALYs of neonates and infants with RSV infection adjusted for the level of care and the disutilities generated by wheezing. Finally, Tables 4 and 5, and 6 show the discounted total direct costs and QALYs of palivizumab versus placebo in preterm neonates ≤ 35 wGA, in infants with BPD, and infants with CHD in Colombia, respectively.
The population that receives palivizumab generates lower total costs than those that do not receive it because they have fewer RSV-related hospitalizations due to the drug’s clinical efficacy that prevents them. We observed this result in all population groups: premature neonates and infants with BPD or CHD. Figures 2 and 3, and 4 show the results of the 1,000 Monte Carlo simulations of palivizumab versus placebo in preterm neonates ≤ 35 wGA, in infants with BPD, and infants with CHD in Colombia, respectively. In preterm neonates ≤ 35 wGA (Fig. 2), most simulations are in the southeast quadrant of the cost-effectiveness plane, showing the robustness of the probabilistic results that coincide with the deterministic results.
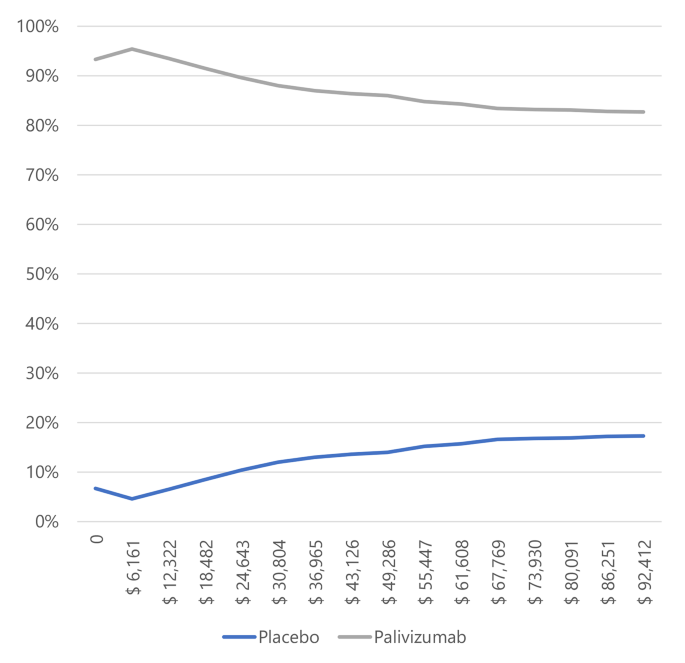
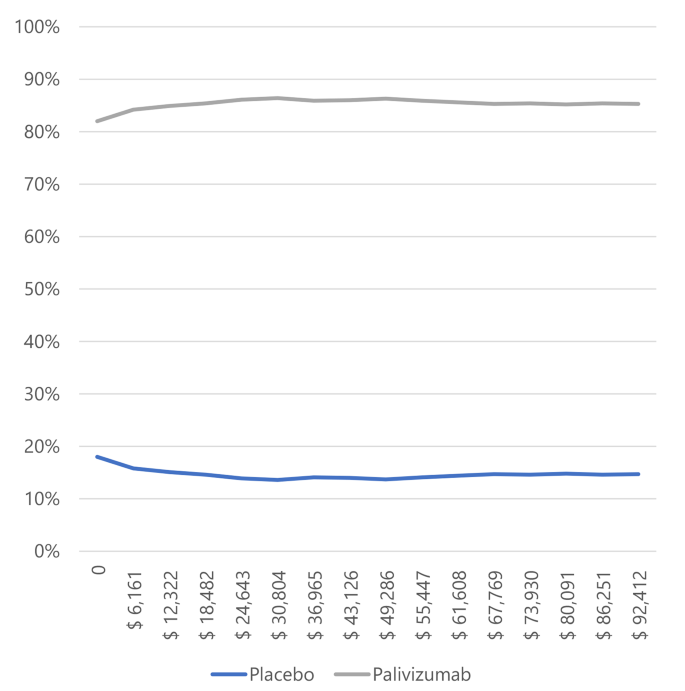
In infants with BPD and CHD (Figs. 3 and 4), most simulations are in the cost-effectiveness plane’s right (east) quadrants. It means that the results are cost-effective or dominant, and we observe the scatterplot grouped with very few outliers, indicating that the results are robust. Figures 5 and 6, and 7 show the results of the willingness to pay curves of palivizumab versus placebo in preterm neonates ≤ 35 wGA, in infants with BPD, and infants with CHD in Colombia, respectively. The probability that palivizumab is an effective therapeutic option to prevent RSV infection compared to placebo with a willingness to pay 1 GDP per capita in Colombia is 95.4% in preterm neonates ≤ 35 wGA, 56.1% in infants with BPD, and 84.2% in infants with CHD.
Discussion
Palivizumab is a cost-saving or dominant strategy to prevent RSV infection in preterm neonates ≤ 35 wGA and infants with BPD or CHD in Colombia. This cost reduction occurs thanks to the clinical efficacy of palivizumab in preventing RSV-related hospitalizations since each hospitalization costs between $ 2,076 and $ 3,772. Similarly, the prevention of wheezing exacerbations improves patients’ quality of life. It saves healthcare costs because the average price of each relapse is $ 174 (a weighted average that includes management in outpatient, ER, or hospitalization).
Since patients have an average of 4.63 (3.70–5.78) relapses per year for at least the first six years of life, this results in savings of $ 4,834 ($ 3,863 - $ 6,034) per patient, maybe $ 174 for a relapse wheezing does not generate a significant economic impact on the Colombian’ health system, but $ 4,834 represents substantial savings, especially in low- and middle-income countries (LMIC). Likewise, the savings generated by avoided RSV-related hospitalizations fluctuate between $176 and $1,522 per patient, results confirmed in the 1,000 Monte Carlo simulations.
For this reason, the willingness to pay curves show that palivizumab has a probability of being an effective option to prevent RSV infection in Colombia, between 56.1% and 94.5%. This variation is because the risk of RSV-related hospitalization in preterm neonates ≤ 35 wGA is higher than in infants with BPD, and the efficacy of palivizumab in preventing RSV infection in preterm neonates is also higher than in infants with BPD.
Although palivizumab generates economic savings and better clinical results than not using it in all populations, the most significant savings are in neonates ≤ 35 wGA. Therefore, it would mean that health systems can offer the most effective clinical benefit and receive the most important economic benefit from applying palivizumab in preterm neonates ≤ 35 wGA. Although the costs of each RSV-related hospitalization of infants with BPD and those of preterm neonates ≤ 35 wGA are similar, the benefit is more significant in preterm neonates. This result is because they have a high risk of hospitalization, and palivizumab efficacy is almost double in preterm neonates than in infants with BPD.
Infants with CHD have a slightly lower risk of RSV-related hospitalization than infants with BPD, and the efficacy of palivizumab is somewhat higher in infants with CHD than in those with BPD (45.3% vs. 38.5%). However, the savings generated to the health system in infants with CHD are more significant than in infants with BPD because the cost of each hospitalization for infants with CHD is higher. It happens because their length of stay (LoS) is usually longer [13], and they typically need more expensive diagnostic tests due to their cardiovascular pathology. As the cost of this population is higher, the savings generated by RSV-related hospitalizations in infants with CHD are also more significant than those generated in infants with BPD.
The conclusions of this economic evaluation are similar to at least 30 other economic evaluations of palivizumab [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] since the fabricant brought it to the market. Similarly, several systematic reviews on the cost-effectiveness of palivizumab versus not using it have similar conclusions to this economic evaluation [4, 50, 51]. Therefore, the amount of evidence with similar findings on the cost-effectiveness of palivizumab in preventing RSV infections increases the validity of this economic evaluation. Furthermore, this monoclonal antibody’s cost-effectiveness is mainly due to its high clinical efficacy. Some authors have pointed it out in their meta-analyses [52, 53] based on the multiple published clinical trials evaluating palivizumab’s effectiveness in preventing RSV infections.
Palivizumab has been on the market for a quarter of a century, long enough to demonstrate its high safety, on which several systematic reviews have been published [54,55,56]. It is essential not only from a clinical point of view but also from an economic point of view since using palivizumab does not generate adverse events that increase healthcare costs. Considering that healthcare costs caused by RSV-related hospitalizations and wheezing relapses are high, it is essential to prevent them from continuing to grow due to adverse events of the medication necessary to prevent it, so this safety contributes to the dominance of palivizumab in Colombia.
This study focuses on preterm neonates with ≤ 35 wGA, like the population in the palivizumab clinical trial (IMpact-RSV) [3]. The largest preterm infant cohort with RSV infection (without immunoprophylaxis) is from the SENTINEL1 study, involving 1,360 infants 29–35 wGA [8]. The neonates were divided into three groups by gestational age: 29–32 wGA, 33–34 wGA, and 35 wGA. They assessed the risk of RSV-related ICU hospitalization and the need for invasive mechanical ventilation (IMV) in each group. The SENTINEL1 study was conducted at 46 sites across the United States [8].
Anderson et al. included 441 neonates at 29–32 wGA, 557 at 33–34 wGA, and 362 at 35 wGA, with ICU admission rates of 48.2%, 45.7%, and 40.0%, respectively. Among those in ICUs, 46.0%, 44.8%, and 61.6% required IMV for 29–32 wGA, 33–34 wGA, and 35 wGA, respectively [8]. These data suggest a similar risk of IMV in RSV-related hospitalizations for neonates aged 29–35 wGA.
The SENTINEL study reported an ICU length of stay (LoS) with a mean of 9 days (SD 9) and an interquartile range (IQR) of 3–13 days for 29–32 wGA, nine days (SD 10) and IQR 3–13 days for 33–34 wGA, and seven days (SD 8) and IQR 3–10 days for 35 wGA. Despite 35-wGA neonates having a 2-day shorter mean LoS, there is overlap in the IQRs, indicating similar ICU stays across these groups.
Concerns regarding antimicrobial resistance (AMR) were raised by a recent Cochrane review on antibiotic use in bronchiolitis [57], and the WHO highlighted AMR’s public health and economic impact [58]. A global AMR study revealed 1.27 million bacterial AMR-associated deaths out of 4.95 million worldwide in 2019 [59]. A UK government review warned that without action, AMR deaths could reach 10 million by 2050, surpassing cancer [60]. The WHO’s global action plan on AMR includes objectives to reduce infection rates and invest in new interventions. As passive immunization, palivizumab lowers RSV-related hospitalizations and antibiotic use, aligning with these objectives and potentially saving costs for the Colombian health system [61].
The main strengths of this economic evaluation are its heterogeneity analysis, which makes it possible to show the results separately for each of the populations of interest, as well as having data on health care costs for this population in Colombia and its distribution in the different care levels. In this way, it was possible to identify that palivizumab generates savings in all interest groups. Although the population with the most savings generated are preterm neonates 29–35 wGA, it is also clear that its use in infants with BPD generates savings for the health system and allows beneficial clinical results.
Although the economic evaluation results are positive, the authors identified some weaknesses that we could resolve in the future with more data to update this study. First, the study assumed no RSV-related mortality because we wanted to focus the analysis on preventing RSV-related hospitalization. However, we must affirm that RSV does cause infant mortality, as evidenced in multiple systematic reviews and disease burden studies [62,63,64]. By not including the RSV mortality rate in infants, the QALYs in the population not receiving palivizumab are higher than they are since more infants would die in the group not receiving palivizumab. Therefore, the ∆ QALYs generated by palivizumab may be greater than those estimated in this study.
This economic evaluation did not calculate the indirect costs due to the lost productivity of these children’s caregivers (usually the mother). Maternity leave in Colombia is 18 weeks (four months) [65], and the time horizon of this economic evaluation is six years. Unfortunately, these children suffer several RSV-related hospitalizations, especially during the first two years of life, and will subsequently be at risk of wheezing relapses, some of which will resolve in the ER. On other occasions, they will require hospitalization. In addition, children need the accompaniment of a caregiver who will incur a series of expenses, including lost productivity due to absenteeism. The model did not consider these costs because the perspective of the analysis is from the third payer.
Finally, the model did not include two types of costs. First, as mentioned before, AMR is a public health problem and economic problem because it leads to more extended stays and higher costs. Since we do not have data from Colombia that indicate the proportion of infants with bronchiolitis who receive antibiotics and the ratio of them who develop AMR, we decided not to include it. In the same way, there are the opportunity costs of not being able to treat infants with other pathologies that are not preventable on time in the ICUs. Second, Colombia is an LMIC; unfortunately, it does not have enough neonatal ICUs to serve the entire population that needs it.
Like any LMIC, Colombia’s health system must prioritize admitting the most severely ill patients to this service. Unfortunately, many patients cannot enter this service, even if needed, because other patients with more severe diseases occupy it. There is not much that the country’s health system can do except to open more neonatal ICUs, but this is not easy due to their high costs. So, a strategy to offer timely health care to the largest possible population is to avoid the admission of these patients by using immunoprophylaxis with palivizumab. In that way, it could reduce the risk of RSV-related hospitalization and thus allow access to other kinds of patients with not immune-preventable diseases, such as RSV.
In conclusion, palivizumab is a cost-saving strategy to prevent RSV-related hospitalizations and relapse wheezing in preterm neonates 29–35 wGA and infants with BPD or CHD in Colombia. Furthermore, its clinical efficacy prevents RSV-related hospitalizations and the treatment of relapse wheezing for six years, which generates high costs for the Colombian health system and significantly improves the health-related quality of life of these children and their families.
Data availability
The authors took the data to build this article from secondary sources referenced in the manuscript.
Data on the risk of RSV hospitalization, risk of developing relapsing wheezing, and efficacy of palivizumab are in references [3, 5, 8, 10, 11, 14, 17]. Data on healthcare costs for these patients are in references [11,12,13,14], and data on QALYs in different health states are in references [15, 16, 18, 19].
References
Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus–a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–79.
Garegnani L, Styrmisdóttir L, Roson Rodriguez P, Escobar Liquitay CM, Esteban I, Franco JV. Palivizumab for preventing severe respiratory syncytial virus (RSV) infection in children. Cochrane Database Syst Rev. 2021;11(11):CD013757.
Palivizumab. A humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102(3 Pt 1):531–7.
Mac S, Sumner A, Duchesne-Belanger S, Stirling R, Tunis M, Sander B. Cost-effectiveness of Palivizumab for respiratory Syncytial Virus: a systematic review. Pediatrics. 2019;143(5):e20184064.
Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Cardiac Synagis Study Group, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143(4):532–40.
IETS. Manual para la solicitud y emisión de conceptos sobre las evaluaciones de tecnologías en salud realizadas por terceros. Bogotá D.C.: Instituto de Evaluación Tecnológica en Salud– IETS; 2021.
Banco de la República– Colombia. Total and per capita GDP at current prices. Available in https://www.banrep.gov.co/en/total-and-capita-gdp Consulted in February 2023.
Anderson EJ, DeVincenzo JP, Simões EAF, Krilov LR, Forbes ML, et al. SENTINEL1: two-season study of respiratory Syncytial Virus hospitalizations among US infants born at 29 to 35 weeks’ gestational age not receiving Immunoprophylaxis. Am J Perinatol. 2020;37(4):421–9.
Piñeros JG, Baquero H, Bastidas J, García J, Ovalle O, Patiño CM, Restrepo JC. Respiratory syncytial virus infection as a cause of hospitalization in population under 1 year in Colombia. J Pediatr (Rio J). 2013 Nov-Dec;89(6):544–8.
Buendía JA, Patiño DG. Costs of respiratory Syncytial Virus hospitalizations in Colombia. Pharmacoecon Open. 2021;5(1):71–6.
Mochizuki H, Kusuda S, Okada K, Yoshihara S, Furuya H, Simões EAF. Scientific Committee for Elucidation of Infantile Asthma. Palivizumab Prophylaxis in Preterm neonates and subsequent recurrent wheezing. Six-year follow-up study. Am J Respir Crit Care Med. 2017;196(1):29–38.
Rodriguez-Martinez CE, Sossa-Briceño MP, Castro-Rodriguez JA. Direct medical costs of RSV-related bronchiolitis hospitalizations in a middle-income tropical country. Allergol Immunopathol (Madr). 2020 Jan-Feb;48(1):56–61.
Meberg A, Bruu AL. Respiratory syncytial virus infections in congenital heart defects–hospitalizations and costs. Acta Paediatr. 2006;95(4):404–6.
Buendía JA, Guerrero Patiño D, Giraldo Ramírez JE. Cost utility of intermittent inhaled corticosteroids in preschoolers with viral-triggered wheeze. Pediatr Allergy Immunol Pulmonol. 2022;35(1):36–42.
Hodgson D, Atkins KE, Baguelin M, Panovska-Griffiths J, Thorrington D, van Hoek AJ, et al. Estimates for quality of life loss due to respiratory Syncytial Virus. Influenza Other Respir Viruses. 2020;14(1):19–27.
Rodriguez-Martinez CE, Nino G, Castro-Rodriguez JA. Cost-utility analysis of daily versus intermittent inhaled corticosteroids in mild-persistent asthma. Pediatr Pulmonol. 2015;50(8):735–46.
Scheltema NM, Nibbelke EE, Pouw J, Blanken MO, Rovers MM, Naaktgeboren CA, et al. Respiratory syncytial virus prevention and asthma in healthy preterm neonates: a randomised controlled trial. Lancet Respir Med. 2018;6(4):257–64.
Greenough A, Alexander J, Burgess S, Bytham J, Chetcuti PA, Hagan J, et al. Health care utilisation of prematurely born, preschool children related to hospitalisation for RSV infection. Arch Dis Child. 2004;89(7):673–8.
Shiri T, Khan K, Keaney K, Mukherjee G, McCarthy ND, Petrou S. Pneumococcal disease: a Systematic Review of Health Utilities, Resource Use, costs, and economic evaluations of interventions. Value Health. 2019;22(11):1329–44.
Wang D, Cummins C, Bayliss S, Sandercock J, Burls A. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assess. 2008;12(36):iii. ix-x.
Banerji A, Panzov V, Young M, Robinson J, Lee B, Moraes T, et al. Hospital admissions for lower respiratory tract infections among infants in the Canadian Arctic: a cohort study. CMAJ Open. 2016;4(4):E615–22.
McGirr AA, Schwartz KL, Allen U, Solomon M, Sander B. The cost-effectiveness of palivizumab in infants with cystic fibrosis in the Canadian setting: a decision analysis model. Hum Vaccin Immunother. 2017;13(3):599–606.
Neovius K, Buesch K, Sandström K, Neovius M. Cost-effectiveness analysis of palivizumab as respiratory syncytial virus prophylaxis in preterm infants in Sweden. Acta Paediatr. 2011;100(10):1306–14.
Narayan O, Bentley A, Mowbray K, Hermansson M, Pivonka D, Kemadjou EN, Belsey J. Updated cost-effectiveness analysis of palivizumab (Synagis) for the prophylaxis of respiratory syncytial virus in infant populations in the UK. J Med Econ. 2020;23(12):1640–52.
Nuijten MJ, Wittenberg W, Lebmeier M. Cost effectiveness of palivizumab for respiratory syncytial virus prophylaxis in high-risk children: a UK analysis. PharmacoEconomics. 2007;25(1):55–71.
Nuijten MJ, Wittenberg W. Cost effectiveness of palivizumab in Spain: an analysis using observational data. Eur J Health Econ. 2010;11(1):105–15.
Nuijten M, Lebmeier M, Wittenberg W. Cost effectiveness of palivizumab for RSV prevention in high-risk children in the Netherlands. J Med Econ. 2009;12(4):291–300.
Nuijten M, Lebmeier M, Wittenberg W. Cost effectiveness of palivizumab in children with congenital heart disease in Germany. J Med Econ. 2009;12(4):301–8.
Resch B, Sommer C, Nuijten MJ, Seidinger S, Walter E, Schoellbauer V, Mueller WD. Cost-effectiveness of palivizumab for respiratory syncytial virus infection in high-risk children, based on long-term epidemiologic data from Austria. Pediatr Infect Dis J. 2012;31(1):e1–8.
Rietveld E, Steyerberg EW, Polder JJ, Veeze HJ, Vergouwe Y, Huysman MW, de Groot R, Moll HA. Passive immunisation against respiratory syncytial virus: a cost-effectiveness analysis. Arch Dis Child. 2010;95(7):493–8.
Roeckl-Wiedmann I, Liese JG, Grill E, Fischer B, Carr D, Belohradsky BH. Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. Eur J Pediatr. 2003;162(4):237–44.
Bentley A, Filipovic I, Gooch K, Büsch K. A cost-effectiveness analysis of respiratory syncytial virus (RSV) prophylaxis in infants in the United Kingdom. Health Econ Rev. 2013;3(1):18.
Salinas-Escudero G, Martínez-Valverde S, Reyes-López A, Garduño-Espinosa J, Muñoz-Hernández O, Granados-García V, Rely K. Cost-effectiveness analysis of the use of palivizumab in the prophylaxis of preterm patients in Mexico. Salud Publica Mex. 2012 Jan-Feb;54(1):47–59.
Sanchez-Luna M, Burgos-Pol R, Oyagüez I, Figueras-Aloy J, Sánchez-Solís M, Martinón-Torres F, Carbonell-Estrany X. Cost-utility analysis of Palivizumab for respiratory syncytial virus infection prophylaxis in preterm infants: update based on the clinical evidence in Spain. BMC Infect Dis. 2017;17(1):687.
Schmidt R, Majer I, García Román N, Rivas Basterra A, Grubb E, Medrano López C. Palivizumab in the prevention of severe respiratory syncytial virus infection in children with congenital heart disease; a novel cost-utility modeling study reflecting evidence-based clinical pathways in Spain. Health Econ Rev. 2017;7(1):47.
Smart KA, Paes BA, Lanctôt KL. Changing costs and the impact on RSV prophylaxis. J Med Econ. 2010;13(4):705–8.
Tam DY, Banerji A, Paes BA, Hui C, Tarride JE, Lanctôt KL. The cost effectiveness of palivizumab in term Inuit infants in the Eastern Canadian Arctic. J Med Econ. 2009;12(4):361–70.
Vogel AM, McKinlay MJ, Ashton T, Lennon DR, Harding JE, Pinnock R, Graham D, Grimwood K, Pattemore PK, Schousboe M. Cost-effectiveness of palivizumab in New Zealand. J Paediatr Child Health. 2002;38(4):352–7.
Weiner LB, Masaquel AS, Polak MJ, Mahadevia PJ. Cost-effectiveness analysis of palivizumab among preterm infant populations covered by Medicaid in the United States. J Med Econ. 2012;15(5):997–1018.
Yount LE, Mahle WT. Economic analysis of palivizumab in infants with congenital heart disease. Pediatrics. 2004;114(6):1606–11.
Elhassan NO, Sorbero ME, Hall CB, Stevens TP, Dick AW. Cost-effectiveness analysis of palivizumab in premature infants without chronic lung disease. Arch Pediatr Adolesc Med. 2006;160(10):1070–6.
Wang D, Bayliss S, Meads C. Palivizumab for Immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses. Health Technol Assess. 2011;15(5):iii–iv.
Lofland JH, O’Connor JP, Chatterton ML, Moxey ED, Paddock LE, Nash DB, Desai SA. Palivizumab for respiratory syncytial virus prophylaxis in high-risk infants: a cost-effectiveness analysis. Clin Ther. 2000;22(11):1357–69.
Blanken MO, Frederix GW, Nibbelke EE, Koffijberg H, Sanders EAM, Dutch RSV Neonatal Network. Cost-effectiveness of rule-based immunoprophylaxis against respiratory syncytial virus infections in preterm infants. Eur J Pediatr. 2018;177(1):133–44. Erratum in: Eur J Pediatr. 2020;179(2):355.
Chirico G, Ravasio R, Sbarigia U. Cost-utility analysis of palivizumab in Italy: results from a simulation model in the prophylaxis of respiratory syncytial virus infection (RSV) among high-risk preterm infants. Ital J Pediatr. 2009;35(1):4.
Hampp C, Kauf TL, Saidi AS, Winterstein AG. Cost-effectiveness of respiratory syncytial virus prophylaxis in various indications. Arch Pediatr Adolesc Med. 2011;165(6):498–505.
Harris KC, Anis AH, Crosby MC, Cender LM, Potts JE, Human DG. Economic evaluation of palivizumab in children with congenital heart disease: a Canadian perspective. Can J Cardiol. 2011 Jul-Aug;27(4):523.e11-5. English, French.
Hascoet JM, Fagnani F, Charlemagne A, Vieux R, Rozé JC, Bendjenana H. Aspects méthodologiques de l’évaluation économique du médicament en pédiatrie: Exemple De La Prophylaxie De L’infection à VRS en France [Methodological aspects of economic evaluation in pediatrics: illustration by RSV infection prophylaxis in the French setting]. Arch Pediatr. 2008;15(12):1739–48. French.
Mahadevia PJ, Masaquel AS, Polak MJ, Weiner LB. Cost utility of palivizumab prophylaxis among preterm infants in the United States: a national policy perspective. J Med Econ. 2012;15(5):987–96.
Smart KA, Lanctôt KL, Paes BA. The cost effectiveness of palivizumab: a systematic review of the evidence. J Med Econ. 2010;13(3):453–63.
Hussman JM, Lanctôt KL, Paes B. The cost effectiveness of palivizumab in congenital heart disease: a review of the current evidence. J Med Econ. 2013;16(1):115–24.
Checchia PA, Nalysnyk L, Fernandes AW, Mahadevia PJ, Xu Y, Fahrbach K, Welliver RC, Sr. Mortality and morbidity among infants at high risk for severe respiratory syncytial virus infection receiving prophylaxis with palivizumab: a systematic literature review and meta-analysis. Pediatr Crit Care Med. 2011;12(5):580–8.
Morris SK, Dzolganovski B, Beyene J, Sung L. A meta-analysis of the effect of antibody therapy for the prevention of severe respiratory syncytial virus infection. BMC Infect Dis. 2009;9:106.
Wegzyn C, Toh LK, Notario G, Biguenet S, Unnebrink K, Park C, Makari D, Norton M. Safety and Effectiveness of Palivizumab in Children at High Risk of Serious Disease due to respiratory syncytial virus infection: a systematic review. Infect Dis Ther. 2014;3(2):133–58.
Wong SK, Li A, Lanctôt KL, Paes B. Adherence and outcomes: a systematic review of palivizumab utilization. Expert Rev Respir Med. 2018;12(1):27–42.
Gonzales T, Bergamasco A, Cristarella T, Goyer C, Wojdyla M, Oladapo A, Sawicky J, Yee J, Moride Y. Effectiveness and Safety of Palivizumab for the Prevention of Serious Lower Respiratory Tract Infection Caused by Respiratory Syncytial Virus: A Systematic Review. Am J Perinatol. 2023 Jan 18.
McCallum GB, Plumb EJ, Morris PS, Chang AB. Antibiotics for persistent cough or wheeze following acute bronchiolitis in children. Cochrane Database Syst Rev. 2017;8(8):CD009834.
Global antimicrobial resistance and use surveillance system (GLASS) report. 2022. Geneva: World Health Organization; 2022. Available in https://www.who.int/publications/i/item/9789240062702 Consulted in February 2023.
Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. Erratum in: Lancet. 2022;400(10358):1102.
Review on Antimicrobial Resistance. Antimicrobial Resistance: tackling a Crisis for the Health and Wealth of nations. London: HM Government; 2014.
World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015 [Internet] Available in https://www.who.int/publications/i/item/9789241509763 Consulted in February 2023.
Stein RT, Bont LJ, Zar H, Polack FP, Park C, Claxton A, Borok G, Butylkova Y, Wegzyn C. Respiratory syncytial virus hospitalization and mortality: systematic review and meta-analysis. Pediatr Pulmonol. 2017;52(4):556–69.
Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, RSV Global Epidemiology Network, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–58.
Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, et al. RESCEU investigators. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64.
Código Sustantivo del Trabajo. Capítulo V (Protección A La Maternidad Y protección de menores). Artículo 236. Descanso remunerado en la época del parto. Ministerio Del Trabajo. Gobierno de Colombia, Bogotá D.C.
Acknowledgements
The authors would like to thank Dr. Ranniery Acuña, pediatric pulmonologist, and Dr. Alejandro Colmenares, neonatologist, for their support and guidance in constructing the economic model, as well as for their active participation in validating the costs included in the model.
Funding
We did this study thanks to the financing of AstraZeneca.
Author information
Authors and Affiliations
Contributions
Jaime Ordóñez wrote the manuscript, created the figures, and contributed to the design of the tables. Víctor Huertas contributed to the creation of the tables. Both authors read and approved the manuscript and its tables and figures.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study is an economic evaluation, and all the data sources were secondary, so we did not require the approval of an Ethics Committee.
No humans participated in this study, and we did not take data from medical records; we took all data from previously published studies. For this reason, we did not request informed consent, nor was the approval of an Ethics Committee requested.
The study meets the publication criteria established in the CHEERS Statement.
Consent for publication
Not Applicable.
This economic evaluation used secondary information sources and did not include human beings.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ordóñez, J.E., Huertas, V.M. Cost-utility analysis of palivizumab for preventing respiratory syncytial virus in preterm neonates and infants in Colombia. BMC Infect Dis 24, 418 (2024). https://doi.org/10.1186/s12879-024-09300-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09300-5