Abstract
Background
Research on the advantages of probiotics has attracted increasing interest based on the number of publications, products, and public awareness of their benefits. This review evaluated the role of probiotics (single and multiple regimens) as an additional regimen to treat common infectious diseases, including Helicobacter. pylori, diarrheal infections, urinary tract infections (UTIs), upper respiratory tract infections (URTIs), and HIV infections.
Methods
We searched randomized controlled trials from PubMed, Scopus, Embase, and Cochrane and identified 6,950 studies. Duplicates were removed, and titles and abstracts were filtered. Bias was evaluated using the Cochrane Risk of Bias Tool for Randomized Trials (ROB 1.0 and 2.0). The certainty of the evidence was evaluated using GRADE. Data were extracted and meta-analysis was performed using RevMan.
Results
A total of 32 studies were included in this study (22 H. pylori studies, 2 diarrheal infection studies, 6 UTI studies, and 2 HIV infection studies). There was no study on URTI. Probiotics, in addition to primary treatment, could improve the eradication of H. pylori versus the control (RR: 1.09; 95% CI:1.04 − 1.13, p value = 0.001) and achieve a cure range of Nugent score in UTI patients (RR 1.38; 95% CI: 1.01 − 1.89, p value = 0.04). For eradicating H. pylori infection, subgroup analysis based on the therapy regimen showed that standard triple therapy was slightly superior compared to quadruple therapy in eradicating H. pylori (RR: 1.14 vs. 1.01, respectively). Single strain probiotics showed a similar effect to multiple strain probiotic regimens (both had an RR of 1.09). The effect estimates of the use of single strain probiotics as adjuvant therapy in eradicating H. pylori and the use of probiotics in UTI had a high certainty of evidence. Meta-analysis was not performed for infectious diarrheal because there were only two eligible studies with different probiotic supplementations and outcome parameters. Nonetheless, they showed that the diarrheal incidence was lower and complete remission of diarrheal was higher after the regimen of probiotics. Similarly, a meta-analysis was not performed for HIV infection because the two eligible studies used different designs and comparators with contradicting findings.
Conclusion
This meta-analysis showed beneficial use of single strain probiotics as adjuvant therapy in eradicating H. pylori and the use of probiotics in UTI. Probiotic supplementation might not be beneficial for patients given a quadruple therapy. Single-strain and multi-strain probiotic regimens had similar effects in increasing the eradication rate of H. pylori. Our study also suggested that the benefits of probiotics as an additional regimen in infectious diarrheal and HIV infections remain unclear; more studies are needed to confirm the benefits.
Similar content being viewed by others
Introductions
Until the twentieth century, the largest global burden of premature death and disability was mostly caused by infectious diseases [16]. Heretofore, vaccines, and curative treatments have become the ultimate approaches to preventing and treating infections. Although these approaches against infectious diseases are effective, other emerging pandemic infections remain a constant threat. For the past few years, probiotics have received much attention from studies demonstrating their ability to treat human diseases [32]. Probiotics are assumed to have a positive impact on human health by stimulating the immune system and inhibiting pathogens [61].
According to the Food and Agriculture Organization of the United Nations (FAO) and WHO, probiotics are consumable living organisms capable of inducing beneficial effects on human health [38]. Recent studies have demonstrated the ability of probiotics to boost human immunity, hence preventing the colonization of pathogens and reducing the number and severity of infections. Nevertheless, the underlying methods of probiotic mechanisms against infecting pathogens are largely unknown.
To date, studies have theorized that probiotics are involved in maintaining the balance and the stability of the gut microbiota by regulating the composition of the intestinal flora, maintaining the epithelial barrier, inhibiting pathogens from adhering to the intestinal surface, and modulating and properly maturing the immune system [59]. In the immune system, probiotics strengthen both innate and adaptive immune responses through bacterial-epithelial-immune cell crosstalk by acting as Toll-like receptors (TLRs) and modulating dendritic cells (DCs) [39].
Previous studies have proven probiotics' ability to reduce the risk of infectious diseases and the use of antibiotics as one of their broad functions [34]. For instance, the regimen of probiotics with antibiotics reduces the risk of AAD in adults by 37%, according to a study in Australia. In subgroup analyses, a high dose compared with a low dose of the same probiotic demonstrated positive protection [18]. Another study that included children, adults, and elderly individuals to assess probiotic effectiveness and safety in the prevention of acute URTIs showed that probiotic consumption is likely to reduce the number of participants diagnosed with URTIs, the incidence rate of URTIs, the mean duration of an episode of acute URTIs, and the number of participants who used prescribed antibiotics for acute URTIs [65]. The effect of probiotics in treating human immunodeficiency virus (HIV) infections benefits the CD4 count and may reduce immune activation and bacterial translocation thus reducing the acquisition or transmission of infections [5]. Furthermore, probiotics also improved the eradication rate and reduced side effects when added to the treatments designed to eradicate H. pylori [24].
The consumption of probiotics conceivably can improve immune function and prevent infectious diseases. However, more evidence is needed to investigate the effectiveness of probiotics as an additional regimen in treating infectious diseases. In this study, we analysed probiotic function as an adjuvant therapy in treating common infectious diseases including H. pylori, infectious diarrheal, urinary tract infections (UTIs), upper respiratory tract infection (URTI), and human immunodeficiency virus (HIV) infections.
Methods
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2020 guidelines, which can be accessed through http://www.prismastatement.org/. The study protocol of this study was registered on the International Prospective Register of Systematic Reviews PROSPERO (CRD42022345021). No amendments to the protocol were needed.
Information sources and search strategy
Four authors (AH, SMY, RKL, and AGIK) systematically searched the PubMed, Scopus, Embase, and Cochrane databases using the keywords (“probiotics” and “H. pylori” or “Helicobacter pylori”); (“probiotics” and “ID” or “Infectious-diarrhea”); (“probiotics” and “URTI” or “Upper Respiratory Tract Infection”); (“probiotics” and “UTI” or “Urinary Tract Infection”); and (“probiotics” and “HIV” or “Human Immunodeficiency Virus”) from January 2012 until 25th January 2024. The search was also conducted for unpublished trials through ClinicalTrials.gov. The reference lists of eligible articles were searched manually to identify additional literature. Supplementary Data 1 (a) displays a table of the source database and (b) table of the search strategy of every database, including detailed keywords used.
Study eligibility criteria and determination of main outcome indicators
Following the literature search, studies were further screened using predetermined inclusion and exclusion criteria. All studies published in English in the last ten years assessing the role of probiotics in treating infectious diseases were included. The inclusion criteria used in this study were (1) RCT; (2) adults with infections defined as H. pylori infection, ID, UTIs, RTIs, or HIV infection, without a prior history of having the disease to adjust for confounding factors; (3) giving probiotics in addition to standard therapy, defined as triple or quadruple antibiotics or Proton Pump Inhibitor (PPI) for H. pylori infection; antiretroviral therapy (ART) for HIV; and antibiotic for ID, UTIs, URTIs, as their intervention; (4) placebo or conservative treatment only as their control; (5) cure or clinical improvement parameters as their outcome, defined as H. pylori eradication rates, achieved bristol stool scale for ID, Nugent score for UTI, improvement of CD4+ for HIV. The diseases chosen were the five common infectious diseases in Indonesia. This study excluded (1) cadaveric or animal studies; (2) studies with no follow–up; (3) studies in infants, children, or young adults; (4) studies with mixed subject ages; and (5) probiotic prevention studies. The outcomes of this study are defined further in Table 1.
Study selection
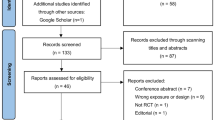
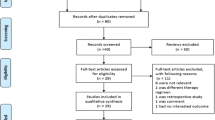
Duplicates were removed prior to title and abstract screening using EndNote X9 Software and Mendeley Desktop Software. Furthermore, title and abstract screening of the included studies was performed according to study eligibility criteria by four independent reviewers (AH, SMY, AGIK, and RKL). Disagreements were then discussed further until a consensus was reached. A detailed planned literature search procedure is illustrated in Fig. 1.
Data extraction
Four reviewers (AH, SMY, AGIK, and RKL) independently extracted data, which were then discussed to reach a consensus. Data extracted included: author and publication year; study design; study location; subject characteristics; follow-up durations; interventions (including the types of probiotics); and outcomes per disease, which were stated according to the disease cure or clinical improvement parameters. Studies were grouped according to the diseases assessed.
Quality assessment
The included studies were also assessed in terms of their quality using the Cochrane Risk of Bias Tool for Randomized Trials (RoB 1.0 and ROB 2.0) (Supplementary Data 2). Results of RoB 1.0 and RoB 2.0 were then compared to ensure the quality of the studies assessed. The quality assessment was performed by four reviewers (AH, SMY, RKL, and AGIK) with each other blinded to each other's scoring and then discussed until consensus was reached. A funnel plot was also used to determine publication bias if the study included for each group was more than 10, as recommended by the Cochrane Handbook. GRADE Assessment (Supplementary Data 3) was also done to assess the quality of evidence among included studies. A completed PRISMA checklist is displayed in Supplementary Data 4.
Data synthesis
We analysed the data using Review Manager software (RevMan v5.4). We calculated the pooled estimates as the risk ratios (RRs), both with the corresponding 95% confidence intervals (CIs). Statistical heterogeneity among studies was evaluated by I2 with values of 0–40%, suggesting a low heterogeneity. We utilized fixed effect models for the meta-analysis of trials with low heterogeneity and random effect models for trials with high heterogeneity. Subgroup analysis was performed for therapy (triple vs. quadruple) and probiotic regimens (single vs. multiple strains) based on risk ratios. Furthermore, sensitivity analyses were performed using Duval and Tweedie’s trim-and-fill analysis.
Results
Search results and study selection
The initial literature search yielded a total of 6,950 studies, detailing 1,818 from PubMed, 4,150 from Scopus, 300 from Cochrane, and 682 from EMBASE. After the deletion of duplicates, titles, and abstracts were screened, and a total of 128 studies were obtained to be evaluated for eligibility evaluation. Due to irrelevant clinical data, 35 studies were subsequently excluded. Furthermore, 15 were non-placebo studies, 17 were non-RCT studies, 18 were studies with irrelevant results, and full texts were not available for 11 studies. All rejected articles and the reason for rejection are provided in Supplementary Data 4. As a result, we reviewed 32 studies, detailing 22 H. pylori studies, 2 diarrheal infection studies, 6 UTI studies, and 2 HIV studies (Fig. 1).
Study characteristics and findings
Overall, this review included a total of 6,509 patients (Table 2), detailing 4721 patients from 22 H. pylori studies, 1194 patients from 2 ID studies, 552 patients from 6 UTI studies, and 42 patients from 2 HIV studies. The study locations were spread across Asia, America, and Europe. The outcomes of the study were defined as the cures described in our methods. The study characteristics and findings of the included studies are displayed in Table 2.
Risk of bias and certainty of evidence
Upon RoB 1.0 analysis, one study had a moderate risk of bias (Happel) and five studies (Grgov, Srinarong, Tang, Tongtawee 2015a, and Tongtawee 2015b) had a high risk of bias. Similarly, RoB 2.0 analysis showed that only one study had a moderate risk (Dajani) and only two studies had a high risk of bias (Grgov, Srinarong). Details of the bias of the studies are presented in Supplementary Data 2. GRADE Assessment (Supplementary Data 3) indicated that the effect estimates of the use of single strain probiotics as adjuvant therapy in eradicating H. pylori and the use of probiotics in UTI had a high certainty of evidence. The effect estimates in other subgroups: the use of probiotics as adjuvant to standard triple or quadruple therapy as well as the use of multiple strain probiotics as adjuvant therapy in eradicating H. pylori had a moderate certainty of evidence.
Probiotic and infectious diarrhea
A meta-analysis was not performed for infectious diarrhea because the two eligible studies used different probiotic supplementations and outcome parameters. Among acute diarrhea patients, Greuter et al. found that the diarrheal incidence after a regimen of a probiotic (E. faecium) three times a day for a week was lower (8.6%) than that after a regimen of a placebo (16.2%) (p-value < 0.001) [20]. Meity et al. showed that by giving probiotics (B. coagulans) with the same time and duration of regimen (three times a day for a week), the complete remission of diarrhea was 100% on day 5 of the probiotic regimen, while in the placebo group, it was only 26.7% (p-value < 0.001) [37].
Probiotic and HIV
A meta-analysis was not performed for HIV infection because the two eligible studies used different designs and comparators. Hemsworth et al., used a crossover design to evaluate micronutrients and probiotics (A), micronutrients alone (B), and probiotics alone (C). The highest mean increase in CD4 was obtained with micronutrients alone (41 cells/µL, SD 221). After a washout period and given a probiotic regimen alone, the mean CD4 level declined (-7 cells/µL, SD 154). Yang et al. performed a two-arm RCT with more promising results [62]. They found that the percentage of blood CD4( +) T cells in the probiotics group was higher than that in the placebo group (+ 2.8% versus -1.8%, p = 0.018).
Meta-analysis: probiotic and helicobacter pylori infection
Overall, the included studies showed a low risk of bias and were relatively good studies. We found 22 studies that met the PICO criteria that involved 4,721 patients (Table 2). We divided the H. pylori analysis into two groups based on the standard therapy regimen (triple and quadruple) (Fig. 2) and the probiotic regimen (single or multiple strains) (Fig. 3a and b). Eighteen of twenty-two (82%) studies showed that regimen of probiotics is superior (RR ≥ 1.00) in achieving H. pylori eradication compared to the control group (Fig. 2). Nine out of twenty-two (41%) studies showed that regimen of probiotics could significantly eradicate H. pylori and was superior in achieving H. pylori eradication compared to the control group, however, the heterogeneity was high (RR 1.09, 95% CI 1.04–1.13, p value: 0.001, I2 = 52%) (Fig. 2).
Types of regimen subgroup analysis
Probiotics significantly improved H pylori eradication compared to placebo in the standard triple therapy group (RR 1.14, 95% CI 1.10–1.18, p value: < 0.001, I2 = 24%), but not in the quadruple therapy group (RR 1.01, 95% CI 0.96–1.06, p value: 0.62, I2 = 30%). Low heterogeneity was found in both standard triple and quadruple therapy or single and multiple probiotics. The funnel plot shows a symmetrical plot, which shows that studies included a low risk of bias (Supplementary Data 6).
Number of administered probiotics subgroup analysis
Subgroup analysis based on the number of administered probiotics showed that single probiotics had a same effect (RR 1.09 95% CI 1.05–1.13, p value: < 0.0001, I2 = 32%) than multiple probiotic regimens (RR 1.09 95% CI 1.05–1.13, p value: < 0.0001, I2 = 43%) as shown in Fig. 3a. Sensitivity analysis was performed by excluding the study by Hauser et al., which was performed in a younger population and involved more males than other studies, and resulted in a pooled RR of 1.07 (95% CI 1.04–1.10, p: < 0.0001), with low heterogeneity (I2 = 20%) (Fig. 3b).
Single probiotics subgroup analysis
Another subgroup analysis was performed by the type of probiotics used. We identified the single probiotic regimen used as members of the Bifidobacterium, Lactobacillus, Saccharomyces, and Clostridium families. The pooled RR for Bifidobacterium was 1.23 (95% CI 1.10–1.37, p value: 0.0003, I2 = 0%), for Lactobacillus 1.18 (95% CI 1.07–1.31, p: 0.001, I2 = 0%), and Saccharomyces 1.07 (95% CI 1.01–1.13, p: 0.03; I2 = 0%) (Fig. 4a). There was only one trial using Clostridium with an RR of 1.00 (95% CI 0,91–1.09, p: 0.98). Single probiotic regimen of Bifidobacterium appeared to have the highest curing success status.
Subgroup analysis was further performed to suggest which single probiotic has the highest efficacy. Our forest plot shows that groups that are given single Bifidobacterium probiotics produce the most superior effects compared to other single probiotics significantly, followed by Lactobacillus, Saccharomyces, and Clostridium single probiotics (1.23 vs 1.18; 1.07; 1.00) (Fig. 4a). Sensitivity analysis was then performed due to heterogeneity (I2 = 45%) (Fig. 4a), by excluding the study by Chen et al., which is the only Clostridium studies, and resulted in a pooled RR of 1.14 (95% CI 1.08–1.19, p: < 0.0001), with low heterogeneity (I2 = 33%) (Fig. 4b).
Probiotic and UTI
Our forest plot shows that the groups that were given probiotics had a better cure range (Nugent score ≤ 3) than the placebo group (RR 1.38: 95% CI 1.01–1.89, p: 0.04), although the heterogeneity was high (I2 = 72%), as shown in Fig. 5a. This has shown the potential of probiotics as a treatment for UTIs.
A sensitivity analysis by excluding the study by Happel [23], which was the only study that took place in Africa whereas others in America and European continents. It resulted in a higher pooled estimate with slightly lower heterogeneity (RR 1.52,95% CI 1.15–2.011.16, p: 0. 003; I2 = 66%) (Fig. 5b).
Discussions
To our knowledge, this systematic review and meta-analysis is the first to summarize available evidence on the role of probiotics in treating common infectious diseases i.e., H. pylori infections, infectious diarrhea, urinary tract infections, and HIV infections.
Probiotic and helicobacter pylori infection
In this study, the data generated from 23 heterogeneous studies demonstrated that the regimen of probiotics increased H. pylori eradication by 8% compared to the control group. Our findings suggest that probiotic supplementation might be used as an adjunctive therapy to improve the effectiveness of antibiotics. Several mechanisms are postulated to explain this finding. In a series of in vitro and in vivo studies, L. reuteri DSM 17648 has been shown to specifically bind to H. pylori in the gastric environment to form copolymers that interfere with its adhesion to the gastric mucosa and facilitate its elimination, thereby reducing the H. pylori load in the stomach [31, 35, 42]. Probiotics also aid in increasing the barrier effect of the stomach, which is the first line of defence against pathogenic bacteria [55]. Some probiotics can upregulate tight junction protein expression, promote mucin and mucus secretion and thus mucus secretion, and enhance the barrier effect of the gastric mucosa. Moreover, some probiotics can secrete antimicrobial substances, such as lactic acid, short-chain fatty acids (SCFAs), hydrogen peroxide, and bacteriocins. Organic acids can cause damage to H. pylori and inhibit its urease activity. Meanwhile, hydrogen peroxide and bacteriocins have direct antibacterial effects [26]. Probiotics are also able to interfere with the colonization of H. pylori in gastric mucosal epithelial cells by competing for adhesion sites, interfering with the adhesion process, and binding to H. pylori to form copolymers to facilitate its excretion ([30]). In terms of immune effects, probiotics may reduce the host inflammatory response by inhibiting the expression of proinflammatory factors [46]. We also conducted sensitivity analysis by excluding studies with the heaviest weights due to the high heterogeneity, which generated similar results (an 8% increase in the eradication rate).
Subgroup analysis based on therapy regimen showed that probiotics had better adjunctive effects in the standard triple therapy group than in the quadruple therapy group (10% vs 1% increase in the eradication rate). Importantly, our analysis also revealed that the increase in the eradication rate in quadruple therapy was not significant. This finding indicates that probiotic supplementation might offer less adjunctive effect in patients who have already been treated with quadruple therapy. The quadruple therapy is preferred as a first-line treatment in areas with a high incidence of clarithromycin resistance and as a second-line therapy after failure of the classical triple therapy. The finding in our analysis might be explained by the already higher cure rate with the use of quadruple therapy in several randomized controlled trials (RCTs) and a meta-analysis. In a multicenter RCT, the curing rate of bismuth quadruple therapy was significantly higher than that of standard triple therapy (90.4% vs 83.7%) for 14 days [36]. In a meta-analysis of Twenty-two randomized controlled trials (RCTs), diverse perspectives emerged. The eradication rate associated with triple therapy supplemented with probiotics exhibited a higher efficacy, in contrast to quadruple therapy, which did not demonstrate a uniform effect, aligning with the findings of our own studies ([63]). Notably, variations were observed in the geographical distribution of patients receiving quadruple therapy. As previously elucidated, quadruple therapy is recommended as the primary treatment in regions with elevated clarithromycin or metronidazole resistance. The meta-analysis encompassed diverse locations with varying resistance profiles, including those with high resistance, potentially influencing eradication rates. The consideration of various combinations of standard quadruple therapy in this meta-analysis further introduces potential variability in eradication rates across different locations. Despite the highly potent effects of H. pylori quadruple therapy, the addition of it may render its effects imperceptible. Consequently, the overall cure rates are anticipated to be influenced by participant demographics, the prevalence of susceptible infections, probiotics dosage and species, and the geographic variations in resistance patterns.
Another subgroup analysis compared single-strain probiotics to multi-strain probiotic regimens and showed that both had similar effects in increasing the eradication rate of H. pylori. Our finding is consistent with a previous systematic review and meta-analysis involving various types of infections. The study also demonstrated that the efficacy of multiple strains and single-strain probiotics were similar in their effectiveness [40]. The different efficacies of probiotic strains may be due to varying mechanisms of action possessed by different strains and if they are given singly or in combination with other strains. A clear advantage of a single strain has only been proven in necrotizing enterocolitis patients who receive Lactobacillus rhamnosus GG [43]. On the other hand, the efficacies of multi-strain probiotics might be enhanced if the mixture possesses synergistic effects, but vice versa if the effects are antagonistic. Eventually, the dynamic interactions between different strains in a mixture make the efficacies of multi-strain probiotics unpredictable. Therefore, the choice of an appropriate probiotic product for each specific disease will continue to be a clinical challenge and for cost-effectiveness, the decision must be based on available scientific evidence.
We also conducted a subgroup analysis comparing the single probiotic regimen by its families of bacteria (Bifidobacterium, Lactobacillus, Saccharomyces, and Clostridium). In our analysis, single probiotic regimen of Bifidobacterium appeared to deliver the highest increase in curing rate (23%). Bacteria belonging to the genus Bifidobacterium are among the first colonizers in the human gut after birth. Although the exact mechanism is not fully elucidated yet, numerous have been proposed mechanisms that account for this phenomenon, such as modulation of NFkB signaling and synthesis of antimicrobial peptides by Bifidobacterium [53]. Another important previous finding is the association between a low abundance of Bifidobacterium in the lower gut microbiota of H. pylori-infected patients [13]. Our findings support the use of Bifidobacterium as a probiotic supplement in H. pylori infection.
Probiotic and urinary tract infection
Our analysis revealed that probiotics were superior (38% more decrease) in achieving a cure of UTI, indicated by a Nugent score of ≤ 3, compared to placebo as an adjunctive treatment to antibiotics. This effect might be accounted for by several mechanisms. Probiotics assist the work of antibiotics in treating UTI by binding to uroepithelial cells and inhibiting pathogenic growth and biosurfactant secretion. Oral Lactobacillus therapy can colonize these bacteria in the urinary tract following intestinal colonization [68]. The inhibition exerted by Lactobacillus sp. is mainly due to the release of lactic acid resulting from the metabolism of carbohydrates, which leads to a decrease in pH, making the environment hostile to most pathogens. The antagonistic activity of lactic acid seems to act synergistically with H2O2, which is also released by several Lactobacillus species in an aerobic environment [7]. The idea of oral probiotic application is based on the knowledge that pathogens that cause most urogenital infections progress from the rectum to the perineal region and then to the vagina and the mesentery [2]. In several studies, the antimicrobial activity of probiotics was tested by the agar diffusion method against reference strains or clinical isolates of urinary tract pathogens, mainly including enterobacteria, such as E. coli, K. pneumoniae, and P. mirabilis, and other bacteria, including P. aeruginosa, E. faecalis, and S. saprophyticus [52].
Sensitivity analysis was conducted by excluding the study with the lowest weight i.e., [23]. The result did not appear to favour probiotics to be utilized as an adjunctive therapy in the treatment of UTI. This indicated that the primary analysis result was greatly influenced by this one study due to the small number of participants. With this finding, future multi-center RCTs with a considerable number of participants are needed to confirm the effectiveness of probiotic supplementation as an adjunctive therapy for UTIs.
Probiotic and infectious diarrhea
A meta-analysis was not performed for infectious diarrhea because there were only two eligible studies with different probiotic supplementations and outcome parameters. Nonetheless, they showed that diarrheal incidence was lower after regimen of a probiotic (E. faecium SF68) (T [20]) and complete remission of diarrheal was higher after the regimen of B. coagulans [37]. Probiotics used for diarrheal treatment mainly belong to the genera Bacillus, Saccharomyces, Streptococcus, Lactobacillus, and Bifidobacterium. The potential mechanisms by which probiotics fight infectious diarrhea include the exclusion of pathogens by means of competition for binding sites and available substrates, lowering of luminal pH and production of bacteriocins, and promotion of mucus production. Specific probiotic strains have been shown to normalize increased intestinal permeability and altered gut microecology, to promote intestinal barrier functions, and to alleviate the intestinal inflammatory response [29]. Further studies are needed to conduct a meta-analysis on the impact of probiotics on infectious diarrhea.
Probiotic and HIV infection
A meta-analysis was not performed for HIV infection because the two eligible studies used different designs and comparators with contradicting findings. A crossover trial by Hemsworth et al. showed that CD4 declined after treatment with probiotics alone compared to micronutrients alone. In contrast, a two-arm RCT by Yang et al. yielded a higher percentage of blood CD4( +) T cells in the probiotics group than in the placebo group. HIV infection alters gut microbial ecology. HIV enteropathy includes pronounced gut-associated CD4+ T-cell loss and an impaired gastrointestinal (GI) epithelial barrier [45]. These detrimental changes presumably result in microbial translocation and a loss of gut homeostasis, which in turn leads to chronic immune activation and disease progression [19]. Hypothetically, probiotics oppose this effect by secreting polymeric IgA, avoiding the overgrowth and translocation of bacteria, and promoting the development of regulatory T cells through the production of anti-inflammatory cytokines [49]. Further studies are necessary to confirm the impact of probiotics on CD4+ cell count in HIV infection.
Limitation
There are some limitations to our review. Except for the H. pylori study, our sample size was rather small for a meta-analysis of a few studies. We could not proceed with a meta-analysis for infectious diarrhea and HIV infection. The elevated heterogeneity observed in the study may be attributed to variations in data or design elements. These distinctions encompass differences in study target populations, targeted effects, methods of survey recruitment and administration, measurement instruments, intervention doses, timing of outcome measurements, analytical methods, and potential sources of bias, including adjustments for covariates [27]. Upon reviewing bias assessments utilizing both RoB 1 and RoB, it was observed that both assessments yielded similar conclusion regarding the presence of a high risk of bias. The primary distinction between these tools pertains to subjective outcomes in open-label studies, where RoB 1 tends to impose sanctions more frequently than RoB 2. Furthermore, RoB 1 is more prone to generating a heightened risk of biased judgments due to limited options, whereas RoB 2, with its integrated ratings, algorithms, signal questions, and guidance, facilitates a more straightforward assessment of complexity and context. Nonetheless, booth tools consistently showed that the majority of the studies had a low risk of bias. GRADE assessment indicated that the effect estimates of the use of single strain probiotics as adjuvant therapy in eradicating H. pylori and the use of probiotics in UTI had a high certainty of evidence. The effect estimates in other subgroups had a moderate certainty of evidence because some studies had a high risk of performance bias and/or conflicting interest with source of funding.
Conclusion
In conclusion, this meta-analysis showed beneficial use of single strain probiotics as adjuvant therapy in eradicating H. pylori and the use of probiotics in UTI. Probiotic supplementation might not be beneficial for patients given a quadruple regimen. Single-strain and multi-strain probiotic regimens had similar effects in increasing the eradication rate of H. pylori. The benefits of probiotics as an additional regimen in infectious diarrhea and HIV infections remain unclear. Therefore more studies with more samples and effect sizes are still needed to confirm the benefits. Further studies are also needed to explore the potency of probiotics in another infection.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the Zenodo repository, https://zenodo.org/doi/https://doi.org/10.5281/zenodo.10666345.
Abbreviations
- AAD:
-
Antibiotic-Associated Diarrhea
- AIDS:
-
Acquired Immunodeficiency Syndrome
- AH:
-
Allerma Herdiman
- ART:
-
Antiretroviral Therapy
- AGIK:
-
Ayers Gilberth Ivano Kalaij
- BV:
-
Bacterial Vaginosis
- BQT:
-
Bismuth-containing Quadruple Therapy,
- C:
-
Control, CD4+: Cluster Differentiation 4
- CFU:
-
Colony-Forming Units
- CI:
-
Confidance Interval
- DCs:
-
Dendritic cells
- ERA:
-
Eradication
- ESBL:
-
Extended Spectrum-Β-Lactamase
- FAO:
-
Food Agriculture Organization
- Fig:
-
Figure
- HAART:
-
Highly Active Antiretroviral Therapy
- HIV:
-
Human Immunodeficiency Virus
- H. pylori :
-
Helicobacter pylori
- HSV:
-
Herpes Simpex Virus
- ID:
-
Infectious Diarrhea
- IgA:
-
Immunoglobulin A
- I:
-
Intervention
- LAB:
-
Lactic Acid Bacillus
- LMIC:
-
Low and Middle-Income Countries
- MDs:
-
Median Differences
- MDR:
-
Multidrug-Resistant
- MSP2:
-
Merozoite Surface Protein-2
- NR:
-
Not Reported
- NK:
-
Natural Killer
- NS:
-
Not significant (p value > 0.050)
- P :
-
p Value
- PPI:
-
Proton Pump Inhibitor
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- PROSPERO:
-
Prospective Register of Systematic Reviews
- RCT:
-
Randomized Controlled Trials
- RKL:
-
Richella Khansa Lauditta
- RR:
-
Risk Ratio
- S:
-
Significant (p value ≤ 0.050)
- SMY:
-
Syarif Maulana Yusuf
- SQT:
-
Standard Quadruple Therapy
- STT:
-
Standard Triple Therapy
- TLR:
-
Toll-Like Receptors
- TV:
-
Trichomonas vaginalis
- UBT:
-
Urea Breath Test
- USA:
-
United State of America
- URTI:
-
Upper Respiratory Tract Infection
- UTI:
-
Urinary Tract Infections
- VRE:
-
Vancomycin-Resistant Enterococcus spp.
- WHO:
-
World Health Organization
References
Ahn J, Boettiger D, Law M, Kumarasamy N, Yunihastuti E, Caiwarith R, Lee M, Sim B, Oka S, Wong W, Kamarulzaman A, Kantipong P, Phanuphak P, Tek O, Kiertiburanakul S, Zhang F, Pujari S, Ditangco R, Ratanasuwan W, Choi J. Effects of CD4 monitoring frequency on clinical endpoints in clinically stable HIV infected patients with viral suppression. Physiology & Behavior. 2016;176(1):139–48. https://doi.org/10.1097/QAI.0000000000000634.Effects.
Akgül T, Karakan T. The role of probiotics in women with recurrent urinary tract infections. Turkish J Urolog. 2018;44(5):377–83. https://doi.org/10.5152/tud.2018.48742.
Amara AA, Shibl A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharmaceutical Journal. 2015;23(2):107–14. https://doi.org/10.1016/j.jsps.2013.07.001.
Blake M, Raker J, Whelan K. Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea‐predominant irritable bowel syndrome. 2016. (pp. 693–703). https://doi.org/10.1111/apt.13746
Carter GM, Esmaeili A, Shah H, Indyk D, Johnson M, Andreae M, Sacks HS. Probiotics in human immunodeficiency virus infection: a systematic review and evidence synthesis of benefits and risks. Open Forum Infect Dis. 2016;3(4):1–13. https://doi.org/10.1093/ofid/ofw164.
Çekin AH, Şahintürk Y, Harmandar FA, Uyar S, Yolcular BO, Çekin Y. Use of probiotics as an adjuvant To sequential H. pylori eradication therapy: impact on eradication rates, treatment resistance, treatment-related side effects, and patient compliance. Turkish J Gastroenterol. 2017;28(1):3–11.
Chapman CMC, Gibson GR, Todd S, Rowland I. Comparative in vitro inhibition of urinary tract pathogens by single- and multi-strain probiotics. Eur J Nutr. 2013;52(6):1669–77. https://doi.org/10.1007/s00394-013-0501-2.
Chen L, Xu W, Lee A, He J, Huang B, Zheng W, Su T, Lai S, Long Y, Chu H, Chen Y, Wang L, Wang K, Si J, Chen S. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: an open-label, randomized clinical trial. EBioMedicine. 2018;35(2018):87–96. https://doi.org/10.1016/j.ebiom.2018.08.028.
Chitapanarux T, Thongsawat S, Pisespongsa P, Leerapun A, Kijdamrongthum P. Effect of Bifidobacterium longum on PPI-based triple therapy for eradication of Helicobacter pylori: a randomized, double-blind placebo-controlled study. J Functional Foods. 2015;13(2015):289–94. https://doi.org/10.1016/j.jff.2015.01.003.
Cohen C, Wierzbicki M, French A, Morris S, Newmann S, Reno H, Green L, Miller S, Powell J, Parks T, Hemmerling A. Randomized trial of Lactin V to prevent recurrence of bacterial vaginosis. 2020. (pp. 1–10). https://doi.org/10.1056/NEJMoa1915254.
Dajani AI, Abu Hammour AM, Yang DH, Chung PC, Nounou MA, Yuan KY, Zakaria MA, Schi HS. Do probiotics improve eradication response to Helicobacter pylori on standard triple or sequential therapy? Saudi J Gastroenterol. 2013;19(3):113–20. https://doi.org/10.4103/1319-3767.111953.
Deguchi R, Nakaminami H, Rimbara E, Noguchi N, Sasatsu M, Suzuki T, Matsushima M, Koike J, Igarashi M, Ozawa H, Fukuda R, Takagi A. Effect of pretreatment with Lactobacillus gasseri OLL2716 on first-line Helicobacter pylori eradication therapy. J Gastroenterol Hepatol (Australia). 2012;27(5):888–92. https://doi.org/10.1111/j.1440-1746.2011.06985.x.
Devi TB, Devadas K, George M, Gandhimathi A, Chouhan D, Retnakumar RJ, Alexander SM, Varghese J, Dharmaseelan S, Chandrika SK, Jissa VT, Das B, Nair GB, Chattopadhyay S. Low Bifidobacterium abundance in the lower gut microbiota Is associated with Helicobacter pylori-related gastric ulcer and gastric cancer. Front Microbiol. 2021;12(February):1–14. https://doi.org/10.3389/fmicb.2021.631140.
Dore MP, Bibbò S, Loria M, Salis R, Manca A, Pes GM, Graham DY. Twice-a-day PPI, tetracycline, metronidazole quadruple therapy with Pylera® or Lactobacillus reuteri for treatment naïve or for retreatment of Helicobacter pylori. Two Randomized Pilot Stud Helicobacter. 2019;24(e12659):1–6. https://doi.org/10.1111/hel.12659.
Emara MH, Mohamed SY, Abdel Aziz HR. Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: a double-blind placebo-controlled randomized clinical trial. Ther Adv Gastroenterol. 2014;7(1):4–13. https://doi.org/10.1177/1756283X13503514.
FAO/WHO. (2002). FAO WHO 2002. In WHO working group report on drafting guidelines for the evaluation of probiotics in food. https://www.fao.org/documents/card/en?details=Y6000EN.
Francavilla R, Polimeno L, Demichina A, Maurogiovanni G, Principi B, Scaccianoce G, Ierardi E, Russo F, Riezzo G, Di Leo A, Cavallo L, Francavilla A, Versalovic J. Lactobacillus reuteri strain combination in helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. J Clin Gastroenterol. 2014;48(5):407–13. https://doi.org/10.1097/MCG.0000000000000007.
Goodman C, Keating G, Georgousopoulou E, Hespe C, Levett K. Probiotics for the prevention of antibiotic-associated diarrhoea: a systematic review and meta-analysis. BMJ Open. 2021;11(8):e043054. https://doi.org/10.1136/bmjopen-2020-043054.
Gori A, Tincati C, Rizzardini G, Torti C, Quirino T, Haarman M, Amor KB, Van Schaik J, Vriesema A, Knol J, Marchetti G, Welling G, Clerici M. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol. 2008;46(2):757–8. https://doi.org/10.1128/JCM.01729-07.
Greuter T, Michel MC, Thomann D, Weigmann H, Vavricka SR. Randomized, placebo-controlled, double-blind and open-label studies in the treatment and prevention of acute diarrhea with enterococcus faecium SF68. Front Med. 2020;7(June):1–9. https://doi.org/10.3389/fmed.2020.00276.
Grgov S, Tasić T, Radovanović-Dinić B, Benedeto-Stojanov D. Can probiotics improve efficiency and safety profile of triple Helicobacter pylori eradication therapy? A prospective randomized study. Vojnosanitetski Pregled. 2016;73(11):1044–9. https://doi.org/10.2298/VSP150415127G.
Haghdoost M, Taghizadeh S, Montazer M, Poorshahverdi P, Ramouz A, Fakour S. Double strain probiotic effect on Helicobacter pylori infection treatment: a double-blinded randomized controlled trial. Caspian J Int Med. 2017;8(3):165–71. https://doi.org/10.22088/cjim.8.3.165.
Happel AU, Singh R, Mitchev N, Mlisana K, Jaspan HB, Barnabas SL, Passmore JAS. Testing the regulatory framework in South Africa - a single-blind randomized pilot trial of commercial probiotic supplementation to standard therapy in women with bacterial vaginosis. BMC Infect Dis. 2020;20(1):1–13. https://doi.org/10.1186/s12879-020-05210-4.
Hauser G, Salkic N, Vukelic K, JajacKnez A, Stimac D. Probiotics for standard triple helicobacter pylori eradication: a randomized, double-blind, placebo-controlled trial. Medicine (United States). 2015;94(17):1–6. https://doi.org/10.1097/MD.0000000000000685.
Hemsworth JC, Hekmat S, Reid G. Micronutrient supplemented probiotic yogurt for HIV-infected adults taking HAART in London. Canada Gut Microbes. 2012;3(5):414–9. https://doi.org/10.4161/gmic.21248.
Homan M, Orel R. Are probiotics useful in Helicobacter pylori eradication? World J Gastroenterol. 2015;21(37):10644–53. https://doi.org/10.3748/wjg.v21.i37.10644.
Imrey PB. Limitations of meta-analyses of studies with high heterogeneity. JAMA Netw Open. 2020;3(1):2019–21. https://doi.org/10.1001/jamanetworkopen.2019.19325.
Ismail NI, Nawawi KNM, Hsin DCC, Hao KW, Mahmood NRKN, Chearn GLC, Wong Z, Tamil AM, Joseph H, Raja Ali RA. Probiotic containing Lactobacillus reuteri DSM 17648 as an adjunct treatment for Helicobacter pylori infection: a randomized, double-blind, placebo-controlled trial. Helicobacter. 2023;28(6):1–11. https://doi.org/10.1111/hel.13017.
Isolauri E. Probiotics for infectious diarrhoea. Gut. 2003;52(3):436–7. https://doi.org/10.1136/gut.52.3.436.
Ji J, Yang H. Using probiotics as supplementation for Helicobacter pylori antibiotic therapy. Int J Mol Sci. 2020;21(3):1136. https://doi.org/10.3390/ijms21031136.
Ji W, Chen W-Q, Tian X. Efficacy of compound Lactobacillus acidophilus tablets combined with quadruple therapy for Helicobacter pylori eradication and its correlation with pH value in the stomach: a study protocol of a randomised, assessor-blinded, single-centre study. BMJ Open. 2018;8(10):e023131. https://doi.org/10.1136/bmjopen-2018-023131.
Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM. Health benefits of probiotics: a review. ISRN Nutrition. 2013;2013:1–7. https://doi.org/10.5402/2013/481651.
Laue C, Papazova E, Liesegang A, Pannenbeckers A, Arendarski P, Linnerth B, Domig KJ, Kneifel W, Petricevic L, Schrezenmeir J. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women - a double-blind, randomised, controlled clinical pilot trial. Beneficial Microbes. 2018;9(1):35–50. https://doi.org/10.3920/BM2017.0018.
Li X, Wang Q, Hu X, Liu W. Current status of probiotics as supplements in the prevention and treatment of infectious diseases. Front Cell Infect Microbiol. 2022;12:789063. https://doi.org/10.3389/fcimb.2022.789063.
Liang B, Yuan Y, Peng XJ, Liu XL, Hu XK, Xing DM. Current and future perspectives for Helicobacter pylori treatment and management: from antibiotics to probiotics. Front Cell Infect Microbiol. 2022;12(November):1–13. https://doi.org/10.3389/fcimb.2022.1042070.
Liou JM, Fang YJ, Chen CC, Bair MJ, Chang CY, Lee YC, Chen MJ, Chen CC, Tseng CH, Hsu YC, Lee JY, Yang TH, Luo JC, Chang CC, Chen CY, Chen PY, Shun CT, Hsu WF, Hu WH, Wu MS. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. The Lancet. 2016;388(10058):2355–65. https://doi.org/10.1016/S0140-6736(16)31409-X.
Maity C, Gupta AK. A prospective, interventional, randomized, double-blind, placebo-controlled clinical study to evaluate the efficacy and safety of Bacillus coagulans LBSC in the treatment of acute diarrhea with abdominal discomfort. Eur J Clin Pharmacol. 2019;75(1):21–31. https://doi.org/10.1007/s00228-018-2562-x.
Markowiak P, Ślizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9(1021):1–30. https://doi.org/10.3390/nu9091021.
Mazziotta C, Tognon M, Martini F, Torreggiani E, Rotondo JC. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. In Cells. 2023. (Vol. 12, Issue 1). https://doi.org/10.3390/cells12010184.
McFarland LV. Efficacy of single-strain probiotics versus multi-strain mixtures: systematic review of strain and disease specificity. Dig Dis Sci. 2021;66(3):694–704. https://doi.org/10.1007/s10620-020-06244-z.
McNicholl AG, Molina-Infante J, Lucendo AJ, Calleja JL, Pérez-Aisa Á, Modolell I, Aldeguer X, Calafat M, Comino L, Ramas M, Callejo Á, Badiola C, Serra J, Gisbert JP. Probiotic supplementation with Lactobacillus plantarum and Pediococcus acidilactici for Helicobacter pylori therapy: a randomized, double-blind, placebo-controlled trial. Helicobacter. 2018;23(5):1–9. https://doi.org/10.1111/hel.12529.
Mehling H, Busjahn A. Non-viable lactobacillus reuteri DSMZ 17648 (Pylopass™) as a new approach to Helicobacter pylori control in humans. Nutrients. 2013;5(8):3062–73. https://doi.org/10.3390/nu5083062.
Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: Prediction for in vivo efficacy. PLoS ONE. 2009;4(9):1–16. https://doi.org/10.1371/journal.pone.0007056.
Navarro-Rodriguez T, Silva FM, Barbuti RC, Mattar R, Moraes-Filho JP, de Oliveira MN, Bogsan CS, Chinzon D, Eisig JN. Association of a probiotic to a Helicobacter pylori eradication regimen does not increase efficacy or decreases the adverse effects of the treatment: a prospective, randomized, double-blind, placebo-controlled study. BMC Gastroenterol. 2013;13(56):1–8. https://doi.org/10.1186/1471-230X-13-56.
Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6(4):1–20. https://doi.org/10.1371/journal.ppat.1000852.
Qureshi N, Li P, Gu Q. Probiotic therapy in Helicobacter pylori infection: a potential strategy against a serious pathogen? Appl Microbiol Biotechnol. 2019;103(4):1573–88. https://doi.org/10.1007/s00253-018-09580-3.
Russo R, Karadja E, De Seta F. Evidence-based mixture containing Lactobacillus strains and lactoferrin to prevent recurrent bacterial vaginosis: a double blind, placebo controlled, randomised clinical trial. Beneficial Microbes. 2019;10(1):19–26. https://doi.org/10.3920/BM2018.0075.
Seddik H, Boutallaka H, Elkoti I, Nejjari F, Berraida R, Berrag S, Loubaris K, Sentissi S, Benkirane A. Saccharomyces boulardii CNCM I-745 plus sequential therapy for Helicobacter pylori infections: a randomized, open-label trial. Eur J Clin Pharmacol. 2019;75(5):639–45. https://doi.org/10.1007/s00228-019-02625-0.
Senok AC, Ismaeel AY, Botta GA. Probiotics: facts and myths. Clin Microbiol Infect. 2005;11(12):958–66. https://doi.org/10.1111/j.1469-0691.2005.01228.x.
Sgibnev A, Kremleva E. Probiotics in addition to metronidazole for treatment Trichomonas vaginalis in the presence of BV: a randomized, placebo-controlled, double-blind study. Eur J Clin Microbiol Infect Dis. 2020;39(2):345–51. https://doi.org/10.1007/s10096-019-03731-8.
Shavakhi A, Tabesh E, Yaghoutkar A, Hashemi H, Tabesh F, Khodadoostan M, Minakari M, Shavakhi S, Gholamrezaei A. The effects of multistrain probiotic compound on bismuth-containing quadruple therapy for Helicobacter pylori infection: a randomized placebo-controlled triple-blind study. Helicobacter. 2013;18(4):280–4. https://doi.org/10.1111/hel.12047.
Shim YH, Lee SJ, Lee JW. Antimicrobial activity of lactobacillus strains against uropathogens. Pediatr Int. 2016;58(10):1009–13. https://doi.org/10.1111/ped.12949.
Shirasawa Y, Shibahara-Sone H, Iino T, Ishikawa F. Bifidobacterium bifidum BF-1 suppresses Helicobacter pylori-induced genes in human epithelial cells. J Dairy Sci. 2010;93(10):4526–34. https://doi.org/10.3168/jds.2010-3274.
Srinarong C, Siramolpiwat S, Wongcha-um A, Mahachai V, Vilaichone R. Improved eradication rate of standard triple therapy by adding bismuth and probiotic supplement for Helicobacter pylori treatment in Thailand. Asian Pacific J Cancer Prevention. 2014;15(22):9909–13. https://doi.org/10.7314/apjcp.2014.15.22.9909.
Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–29. https://doi.org/10.1038/s41591-019-0439-x.
Tang B, Tang L, Huang C, Tian C, Chen L, He Z, Yang G, Zuo L, Zhao G, Liu E, Wang S, Lin H, He J, Yang S. The effect of probiotics supplementation on gut microbiota after helicobacter pylori eradication: a multicenter randomized controlled trial. Infect Dis Ther. 2021;10(1):317–33. https://doi.org/10.1007/s40121-020-00372-9.
Tongtawee T, Dechsukhum C, Leeanansaksiri W, Kaewpitoon S, Kaewpitoon N, Loyd RA, Matrakool L, Panpimanmas S. Effect of pretreatment with lactobacillus delbrueckii and streptococcus thermophillus on tailored triple therapy for helicobacter pylori eradication: a prospective randomized controlled clinical trial. Asian Pacific J Cancer Prevent. 2015;16(12):4885–90. https://doi.org/10.7314/apjcp.2015.16.12.4885.
Tongtawee T, Dechsukhum C, Leeanansaksiri W, Kaewpitoon S, Kaewpitoon N, Loyd RA, Matrakool L, Panpimanmas S. Improved Helicobacter pylori eradication rate of tailored triple therapy by adding Lactobacillus delbrueckii and Streptococcus thermophilus in Northeast Region of Thailand: a prospective randomized controlled clinical trial. Gastroenterol Res Pract. 2015;2015(518018):1–7. https://doi.org/10.1155/2015/518018.
Wang X, Zhang P, Zhang X. Probiotics regulate gut microbiota: an effective method to improve immunity. Molecules. 2021;26(19):1–15. https://doi.org/10.3390/molecules26196076.
West NP, Horn PL, Barrett S, Warren HS, Lehtinen MJ, Koerbin G, Brun M, Pyne DB, Lahtinen SJ, Fricker PA, Cripps AW. Supplementation with a single and double strain probiotic on the innate immune system for respiratory illness. E-SPEN Journal. 2014;9(5):178–84. https://doi.org/10.1016/j.clnme.2014.06.003.
Yadav M, Chauhan NS. Microbiome therapeutics: Exploring the present scenario and challenges. Gastroenterol Rep. 2022;10(July):1–19. https://doi.org/10.1093/gastro/goab046.
Yang OO, Kelesidis T, Cordova R, Khanlou H. Immunomodulation of antiretroviral drug-suppressed chronic HIV-1 infection in an oral probiotic double-blind placebo-controlled trial. AIDS Res Hum Retroviruses. 2014;30(10):988–95. https://doi.org/10.1089/aid.2014.0181.
Zhang M, Zhang C, Zhao J, Zhang H, Zhai Q, Chen W. Meta-analysis of the efficacy of probiotic-supplemented therapy on the eradication of H. pylori and incidence of therapy-associated side effects. Microbial Pathogenesis. 2020;147(104403):1–10. https://doi.org/10.1016/j.micpath.2020.104403.
Zhang Y, Lyu J, Ge L, Huang L, Peng Z, Liang Y, Zhang X, Fan S. Probiotic lacticaseibacillus rhamnosus GR-1 and limosilactobacillus reuteri RC-14 as an adjunctive treatment for bacterial vaginosis do not increase the cure rate in a chinese cohort: a prospective, parallel-group, randomized, controlled study. Front Cell Infect Microbiol. 2021;11(669901):1–13. https://doi.org/10.3389/fcimb.2021.669901.
Zhao Y, Dong BR, Hao Q. Probiotics for preventing acute upper respiratory tract infections (Review). Cochrane Database Syst Rev. 2022;2022(8):1–119. https://doi.org/10.1002/14651858.CD006895.pub4.
Zhao Y, Yang Y, Aruna Xiao J, Song J, Huang T, Li S, Kou J, Huang L, Ji D, Xiong S, Peng W, Xu S, Cheng B. Saccharomyces boulardii combined with quadruple therapy for helicobacter pylori eradication decreased the duration and severity of diarrhea: a multi-center prospective randomized controlled trial. Front Med. 2021;8(776955):1–8. https://doi.org/10.3389/fmed.2021.776955.
Zojaji H, Ghobakhlou M, Rajabalinia H, Ataei E, Sherafat S, Dekhordi B, Bahreiny R. The efficacy and safety of adding the probiotic Saccharomyces. 2013. (pp. 1–6). https://pubmed.ncbi.nlm.nih.gov/24834296/.
Zuccotti GV, Meneghin F, Raimondi C, Dilillo D, Agostoni C, Riva E, Giovannini M. Probiotics in clinical practice: an overview. J Int Med Res. 2008;36(SUPPL. 1):1–53. https://doi.org/10.1177/14732300080360s101.
Acknowledgements
Not applicable.
Funding
This study was funded by a University Indonesia Grant (PUTI 2022).
Author information
Authors and Affiliations
Contributions
EJN contributed to the research idea, design of the study, editing of the first draft, writing and editing of the final manuscript; AH, AGIK, RKL, and SMY contributed to data extraction, writing of the first draft, and statistical analysis interpretation; ES contributed to the design of the study, statistical analysis and interpretation, risk of bias assessment, editing of the first draft, and writing and editing of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Since the article is a systematic review, the PROSPERO PDF was provided in Supplementary Data 7.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nelwan, E.J., Herdiman, A., Kalaij, A.G.I. et al. Role of probiotic as adjuvant in treating various infections: a systematic review and meta-analysis. BMC Infect Dis 24, 505 (2024). https://doi.org/10.1186/s12879-024-09259-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09259-3