Abstract
Background
Currently, some meta-analyses on COVID-19 have suggested that glucocorticoids use can reduce the mortality rate of COVID-19 patients, utilization rate of invasive ventilation, and improve the prognosis of patients. However, optimal regimen and dosages of glucocorticoid remain unclear. Therefore, the purpose of this network meta-analysis is to analyze the efficacy and safety of glucocorticoids in treating COVID-19 at regimens.
Methods
This meta-analysis retrieved randomized controlled trials from the earliest records to December 30, 2022, published in PubMed, Embase, Cochrane Library, CNKI Database and Wanfang Database, which compared glucocorticoids with placebos for their efficacy and safety in the treatment of COVID-19, Effects of different treatment regimens, types and dosages (high-dose methylprednisolone, very high-dose methylprednisolone, Pulse therapy methylprednisolone, medium-dose hydrocortisone, high-dose hydrocortisone, high-dose dexamethasone, very high-dose dexamethasone and placebo) on 28-day all-caused hospitalization mortality, hospitalization duration, mechanical ventilation requirement, ICU admission and safety outcome were compared.
Results
In this network meta-analysis, a total of 10,544 patients from 19 randomized controlled trials were finally included, involving a total of 9 glucocorticoid treatment regimens of different types and dosages. According to the analysis results, the 28-day all-cause mortality rate was the lowest in the treatment with pulse therapy methylprednisolone (OR 0.08, 95% CI 0.02, 0.42), but the use of high-dose methylprednisolone (OR 0.85, 95% CI 0.59, 1.22), very high-dose dexamethasone (OR 0.95, 95% CI 0.67, 1.35), high-dose hydrocortisone (OR 0.64, 95% CI 0.34, 1.22), medium-dose hydrocortisone (OR 0.80, 95% CI 0.49, 1.31) showed no benefit in prolonging the 28-day survival of patient. Compared with placebo, the treatment with very high-dose methylprednisolone (MD = -3.09;95%CI: -4.10, -2.08) had the shortest length of hospital stay, while high-dose dexamethasone (MD = -1.55;95%CI: -3.13,0.03) and very high-dose dexamethasone (MD = -1.06;95%CI: -2.78,0.67) did not benefit patients in terms of length of stay.
Conclusions
Considering the available evidence, this network meta‑analysis suggests that the prognostic impact of glucocorticoids in patients with COVID-19 may depend on the regimens of glucocorticoids. It is suggested that pulse therapy methylprednisolone is associated with lower 28-day all-cause mortality, very high-dose methylprednisolone had the shortest length of hospital stay in patients with COVID-19.
Trial registration
PROSPERO CRD42022350407 (22/08/2022).
Similar content being viewed by others
Introduction
SARS-Cov-2 was first discovered in Wuhan, China in 2019 [1]. COVID-19, caused by SARS-Cov-2 [2], has been declared as a global pandemic by world health organization (WHO) in March 2020. The main clinical manifestations of COVID-19 are fever, dry cough and fatigue, with a small number of patients accompanied by nasal congestion, runny nose, sore throat and diarrhea [3], patients with severe novel coronavirus pneumonia are characterized by a severe cytokine storm, in which the overproduction of pro-inflammatory cytokines leads to increased vascular permeability and multiple organ failure [4], poses severe challenges to not only to human health, but also global health care system [5, 6].
As effective anti-inflammatory drugs, glucocorticoids are often used as adjuvant treatment of viral pneumonias and ARDS treatments [7], such as severe acute respiratory syndrome (SARS) [8], middle east respiratory syndrome (MERS) [9], etc. National Institutes of Health in the United States have included glucocorticoids as a treatment for COVID-19 patient [10]. Glucocorticoid bind to the glucocorticoid receptors, thus affects many physiological pathways, including metabolism, cell apoptosis, and benefits COVID-19 patients through its immunosuppressive action [11]. Some recent studies suggest that the use of glucocorticoids can effectively reduce the mortality, increase ventilator-free days and improve the prognosis of COVID-19 patients [12, 13]. However, the glucocorticoid regimen and dosage used in those studies are different, so the optimal glucocorticoid regimen for COVID-19 patients remains unknown. Moreover, side effects of glucocorticoids, including hyperglycemia, electrolyte disorders, and water and sodium retention, and so on, make the safety and efficacy of their treatment of COVID-19 still controversial.
This network meta-analysis focuses on whether glucocorticoid therapy can improve the prognosis of COVID-19 patients, to find the optimal glucocorticoid regimen, so as to provide evidence for the clinical use of glucocorticoids in COVID-19 patients.
Methods
Protocol and search strategy
The study protocol of this network meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022350407) with basic principles of data extraction and the analysis method, the literature search results are reported according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) for NMA [14] (PRISMA checklist were provided in Additional file 2).
The retrieval languages of this network meta-analysis were Chinese and English, databases including PubMed, Web of Science, Cochrane Library, China National Knowledge Infrastructure Database (CNKI database), Wanfang Database, China Biology Medicine disc(CBMdisc) were searched for published randomized controlled trials. The retrieval period was from the establishment of the database to November 1, 2022. Medical Subject heading (MeSH) terms were used, including COVID-19, glucocorticoid, steroids, etc., while other keywords were limited to title and abstract (details of search strategies were provided in Additional file 1).
Study selection and data extraction
Only published randomized controlled trials of glucocorticoids for the treatment of COVID-19 were included, excluding studies including case-control studies, cohort studies, etc. Inclusion criteria included: adults(age ≥ 18 years old), confirmed COVID-19 and willingness to provide informed consent. Exclusion criteria included foreseeable and inevitable death, pregnancy, breast-feeding, and use of glucocorticoids for other needs. Full inclusion and exclusion criteria in the appendix (Additional file 1).
Articles included in this network meta-analysis was retrieved and identified by two authors (QH and CW). After full-text review, for articles that met inclusion criteria, patient characteristics, interventions, controls, and outcomes were extracted using Excel, opinions of a third author (MZ) were solicited if necessary.
Based on the literature retrieved, this network meta-analysis has divided glucocorticoid regimens into nine groups [15, 16]: pulse therapy methylprednisolone(> 200 mg/day), very high-dose methylprednisolone(> 80 mg /day, but ≤ 200 mg/day), high-dose methylprednisolone(> 24 mg /day, but ≤ 80 mg/day), very high-dose dexamethasone(> 12 mg /day, but ≤ 37.5 mg/day), high-dose dexamethasone(> 6 mg /day, but ≤ 12 mg/day), medium-dose dexamethasone(> 1.125 mg /day, but ≤ 6 mg/day), high-dose hydrocortisone(> 120 mg /day, but ≤ 400 mg/day), medium-dose hydrocortisone(> 30 mg /day, but ≤ 120 mg/day) and no glucocorticoid use.
Quality assessment
The risk of bias was assessed by the Cochrane Handbook for Systematic Reviews of Interventions [17, 18], and was assessed independently by two investigators. The evaluation contents including randomization bias, implementation of distribution concealment scheme, blind implementation; integrity of the result data, selective reporting bias and other sources of bias.
Outcome measures and definitions
The primary outcome of this network meta-analysis is all-cause mortality at 28 days, the secondary outcome is hospitalization duration, the utilization and duration of invasive mechanical ventilation, intensive care unit admission and duration and safety outcome.
Data analysis
All statistical analyses of this review were performed in STATA, version 17.0 (Stata Corporation, College Station, TX, USA), using frequentist framework. Relative odds ratio (OR) and 95% credible intervals were used as the effect indicators of binary outcome. For continuous variables, mean difference (MD) and 95% credible intervals were used. The level of significance for all analyses was p < 0.05, the heterogeneity of the included studies was evaluated by heterogeneity parameter tau-square (τ2). When P > 0.05 and τ2 ≤ 50%, the heterogeneity of the study was small, and the fixed effect model was used. On the contrary, if P < 0.05 and τ2 > 50%, the random effects model was used. The surface under the cumulative ranking curve (SUCRA) of each intervention was used to reflect the efficacy of different glucocorticoid treatment regimens. The closer it was to 100%, the more likely it was that the treatment regimen had the optimal efficacy. The funnel plot was drawn to determine whether there were publication bias or small sample effect. For studies that only reported the interquartile range and median, we used the methods that were introduced by literature to estimate the mean and standard deviation [19, 20].
Results
Study selection
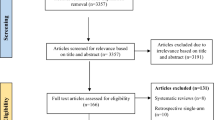
The selection process of included studies selection is shown in Fig. 1. A total of 3877 records were retrieved from PubMed, Embase, Cochrane Library, CNKI full-text database, Wanfang Database, CBMdisc and other sources. After removing duplicate literatures and further screening by reading their titles and abstracts, 1643 articles were excluded. After screening of the titles and abstracts, 1714 articles were excluded. A total of 271 articles were retrieved and under full-text reading and 74 of them were assessed for eligibility. Finally, 19 articles were included for this network meta-analysis.
Quality assessment
The quality of included 19 randomized controlled trials were assessed by the Cochrane risk of bias tool and showed by RevMan 5.4 software in Fig. 2. Five studies were considered to have a low risk of bias [21,22,23,24,25], while another 6 studies were assessed as having unclear risk of bias [13, 26,27,28,29,30]. In addition, 8 RCTs [12, 31,32,33,34,35,36,37] were considered to have a high risk of bias because of their performance bias, detection bias and attrition bias.
Study characteristics
A total of 19 randomized controlled trials were included in this meta-analysis. Eighteen of them were two-arm trials [12, 13, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] and one was a three-arm trial [21]. 10,544 patients with COVID-19 were included, with an average age of 61.47 years old, including 35.80% females and 64.20% males. The 28-day all-cause mortality was reported in 16 articles [12, 13, 22,23,24,25,26, 28,29,30,31,32,33,34,35, 37], 9 regimens and dosages of glucocorticoid were involved in the included study; 10 of them reported the length of stay [21, 22, 25, 26, 28, 29, 31,32,33, 36], while 6 each reported mechanical ventilation requirement [12, 21, 22, 24, 29, 32] and ICU admission [21, 27, 28, 31, 32, 35], the basic characteristics of the included study were shown in Table 1.
Hospital mortality
Sixteen articles have reported 28-day all-cause mortality [12, 13, 22,23,24,25,26, 28,29,30,31,32,33,34,35, 37] (n = 9536), and their network plots have shown in Fig. 3a. Each node indicates a treatment strategy. The edge represented the number of direct comparisons between two different dosage and regimen of glucocorticoid.
Network meta-analysis showed that, compared with other treatment regimens, pulse therapy methylprednisolone (PT-mp) significantly reduced patient 28-day all-cause mortality, except for very high-dose of methylprednisolone (VHD-mp) (OR 0.24, 95% CI 0.04, 1.62); compared with placebo, half of the treatment regimens can reduce 28-day all-cause mortality in patients with COVID-19, including PT-mp (OR 0.08, 95% CI 0.02, 0.42), VHD-mp (OR 0.34, 95% CI 0.13, 0.93), high-dose dexamethasone (HD-dm) (OR 0.70, 95% CI 0.53, 0.94) and medium-dose dexamethasone (MD-dm) (OR 0.86, 95% CI 0.76, 0.97). There was no significant difference in 28-day all-cause mortality among patients treated with other glucocorticoids regimens and dosages (Fig. 4a).
By analyzing the data of the included articles, the effectiveness of different doses and types of glucocorticoids in reducing 28-day all-cause mortality in patients with COVID-19 is ranked as follows: PT-mp (SUCRA = 98.8%) > VHD-mp (SUCRA = 82.9%) > high-dose hydrocortisone (HD-hc) (SUCRA = 60.3%) > HD-dm (SUCRA = 60%) > medium-dose hydrocortisone (MD-hc) (SUCRA = 42.2%) > high-dose methylprednisolone (HD-mp) (SUCRA = 37%) > MD-dm (SUCRA = 36%) > very high-dose dexamethasone (VHD-dm) (SUCRA = 21.6%) > placebo(SUCRA = 11.2%) (Fig. 5a). There are no comparisons with statistically significant inconsistencies were observed in the node-splitting model.
To assess publication bias, we performed funnel plot analyses of OR and SE (log [OR]) for 28-day all-cause mortality of 9 glucocorticoid regimens. The distribution on both sides of the funnel plot is basically symmetrical, and most of them are concentrated in the middle and upper part of the funnel plot, indicating that there is less possibility of small sample effect or publication bias (Fig. S2a).
Secondary outcome
Ten articles [21, 22, 25, 26, 28, 29, 31,32,33, 36] (n = 1696) that have been included reported the hospitalization duration of COVID-19 patients, 6 reported mechanical ventilation requirement [12, 21, 22, 24, 29, 32] (n = 6926), ICU admission [21, 27, 28, 31, 32, 35](n = 921), and their network plots have shown in Fig. 3b, c and d. Mechanical ventilation duration was reported in 3 studies [13, 21, 32] (n = 632) and ICU duration was reported in 5 studies [21, 29, 32, 33, 36] (n = 951).
Hospitalization duration
Compare with other treatments, very high-dose methylprednisolone significantly reduced the length of hospital duration of COVID-19 patients. Hospitalization duration in patient using MD-dm treatment regimen was shorter than other treatment regimen, except for VHD-mp (MD = 3.09;95%CI: 2.08, 4.10); compared with placebo, only VHD-mp (MD = -5.36;95%CI: -7.35, -3.37) and MD-dm (MD = -2.27;95%CI: -3.98, -0.56) could reduce hospitalization duration, and all treatment regimens were better than high-dose methylprednisolone. There was no significant difference among patients receiving other glucocorticoid regimens (Fig. 4b).
SUCRA graph indicated the ranking of 6 glucocorticoid regimens in shortening the length of hospital stay: VHD-mp (SUCRA = 100%) > MD-dm (SUCRA = 79.5%) > HD-dm (SUCRA = 58.0%) > VHD-dm (SUCRA = 39.2%) > placebo(SUCRA = 22.8%) > HD-mp (SUCRA = 0.50%) (Fig. 5b). There are no comparisons with statistically significant inconsistencies were observed in the node-splitting model.
Mechanical ventilation requirement
In terms of the need for mechanical ventilation, VHD-mp (OR 0.26, 95% CI 0.10, 0.72) and MD-dm (OR 0.73, 95% CI 0.58, 0.91) reduce mechanical ventilation requirement compared to placebo, VHD-mp reduced the probability of intubation better than MD-dm (OR 0.36, 95% CI 0.13, 0.97). In addition, it is also superior to VHD-dm (OR 0.23, 95% CI 0.07, 0.75). The remaining glucocorticoids showed no significant difference in reducing the need for intubation (Fig. 4c). The SUCRA graph is sorted as follows: VHD-mp (SUCRA = 90.8%) > HD-hc (SUCRA = 79.8%) > MD-dm (SUCRA = 57.5%) > placebo (SUCRA = 26.8%) > HD-dm (SUCRA = 23.6%) > VHD-dm (SUCRA = 21.5%) (Fig. 5c). There are no comparisons with statistically significant inconsistencies were observed in the node-splitting model.
Other outcomes
We also conducted network meta-analysis of mechanical ventilation duration, ICU admission and ICU duration. Their network plots have shown in Fig. S1. For mechanical ventilation duration, we found that, neither HD-dm (MD = 0.40;95%CI: -0.15, 0.95), nor VHD-dm (MD = 0.40;95%CI: -0.96, -0.16) can shorten the duration of mechanical ventilation in patients with COVID-19. The MD-dm significantly increased the length of mechanical ventilation (MD = 4.63;95%CI: 3.02, 6.23) (Fig. S3a). For ICU admission and length of stay in the ICU, glucocorticoid regimens did not reduce the rate of admission or length of stay in the ICU compared with placebo (Figs. 4d and 5d, S3b). SUCRA graph were shown in Fig. S4.
Safety outcomes
A total of 9 articles [13, 21, 23, 24, 27, 30, 34, 36, 37] (n = 2881) reported serious adverse effects caused by different treatment regimens, including 8 glucocorticoid regimens as follows: VHD-MP, HD-dm, VHD-dm, MD-dm, PT-mp, placebo, MD-hc, HD-hc, however, our analysis showed no significant difference in serious adverse reactions in patients with severe COVID-19 compared to SOC or placebo among the eight treatment regimens (Fig. S5). Hyperglycemia is one of the common side effects of glucocorticoid, and was reported in 6 RCTs [13, 21, 28, 31, 32, 35] (n = 919), there regimens include HD-dm, MD-dm, VHD-dm, placebo, HD-mp. Similarly, we did not find that glucocorticoid use increased the incidence of hyperglycemia (Fig. S5).
Discussion
Although glucocorticoids are commonly prescribed for SARS [8] and MERS [9], the efficacy of using glucocorticoids to treat COVID-19 patients remains controversial. The largest clinical trial evidence to date has shown that dexamethasone at a medium-dose (6 mg/day) reduces 28-day mortality in patients with COVID-19. However, the merits and disadvantages of other doses and types of glucocorticoids for COVID-19 treatment have not been fully explored.
This network meta-analysis was based on 19 randomized controlled trials, involving 10,544 COVID-19 patients randomly assigned to nine glucocorticoids or to placebo groups. Similar to the previous meta-analysis [38, 39], a medium-dose of dexamethasone (6 mg/day) did reduce 28-day all-cause mortality, length of hospitalization, and the need for mechanical ventilation in patients with COVID-19. We further found that very high-dose methylprednisolone (80-200 mg/day) not only reduces the above outcomes, but also has better efficacy than dexamethasone (6 mg/day).
The use of pulse therapy methylprednisolone was only reported in one RCT [34]. The analysis showed that pulse therapy methylprednisolone was better than any other dose and type of glucocorticoid, including very high-dose methylprednisolone methylprednisolone, in reducing patient’s death within 28 days. However, the duration of mechanical ventilation use and duration of ICU admission were not reported in Edalatifard et al.’s study, therefore it could not be compared with other glucocorticoid protocols.
Due to the following limitations, this network meta-analysis should be interpreted with caution. First, SARS-Cov-2 is a highly variable virus, the time span of RCTs included in our study was 2 years, during which different RCTs may enroll patients with different virus subspecies. Different virus subspecies may have different virulence and different clinical symptoms. However, the RCTs included in this network meta-analysis did not report the subspecies of virus patients were infected with, which may be a potential source of bias [40, 41]. Second, our study was conducted at the study level and may not reflect variables at the patient level, limited by the quantity and quality of the included article, further studies are needed to determine the optimal type and dosage of glucocorticoids, and to take these results into account with long-term clinical efficacy and safety to provide a basis for clinical use. Third, not all glucocorticoid treatment regimens reported the outcomes we wanted to explore. For example, pulse therapy methylprednisolone did not report the length of hospital stay, invasive ventilation utilization, and ICU admission that we were interested in.
Despite these limitations, our study has two key advantages. We divided glucocorticoid treatment regimens into 9 groups, further revealing the role of glucocorticoid type and dose in the prognosis of COVID-19 patients. Secondly, we only included randomized controlled trials on glucocorticoid therapy for COVID-19, the number of included articles was larger than the previous meta-analysis, therefore, the results were more credible.
In conclusion, all included glucocorticoid regimens were superior to placebo in reducing 28-day mortality, and methylprednisolone and medium or high-dose dexamethasone were significantly superior to other treatments, among which pulse therapy methylprednisolone was the best. In terms of length of hospital stay, glucocorticoids were superior to placebo except for unreported glucocorticoid regimens and high-dose methylprednisolone, and methylprednisolone was the best. In terms of mechanical ventilation utilization, methylprednisolone (80-200 mg/day), hydrocortisone (120-400 mg/day), dexamethasone (1.125-6 mg/day) can reduce the probability of mechanical ventilation. The sequence from high to low that glucocorticoids reduced ICU admission was: high-dose dexamethasone; medium-dose dexamethasone; very high-dose methylprednisolone; very high-dose dexamethasone; high-dose methylprednisolone, but there was no statistical significance. In terms of adverse effects, glucocorticoid use did not increase the occurrence of adverse reactions.
Different regimens of glucocorticoids have variable pleiotropic effects in the treatment of COVID-19. In order to better interpret our conclusions, we had discussed commonly used clinical dose of the above glucocorticoids in the treatment of COVID-19, the most common dosage of dexamethasone was medium dose, and the common dosage of methylprednisolone and hydrocortisone were both high dose. Their primary and secondary outcomes in the treatment of COVID-19 were: only medium dose dexamethasone can both reduce the 28-day all-cause mortality, hospitalization duration and mechanical ventilation requirement of patients, but could not improve ICU admission rate; high dose methylprednisolone was not reported in terms of mechanical ventilation requirement, there was no significant improvement in the other three outcomes. No RCTs had been reported on hospitalization duration and ICU admission in high dose hydrocortisone, and it didn’t improve 28-day all-cause mortality and mechanical ventilation requirement in COVID-19 patients.
To compare the effects of different types of glucocorticoids on the primary and secondary outcomes of the treatment of COVID-19 at the equivalent dose, we took the most commonly used glucocorticoid regimen as an example: medium dose dexamethasone, and other equivalent doses of glucocorticoids were: medium dose methylprednisolone and hydrocortisone, the type of glucocorticoids with the best performance was medium dose dexamethasone, which can significantly reduce the 28-day all-cause mortality and other secondary outcomes, including hospitalization duration and mechanical ventilation requirement of patients. While no RCTs have been conducted on methylprednisolone at this dosage till the literature retrieval was completed in this meta-analysis. As for medium dose hydrocortisone, it was only reported in the 28-day all-cause mortality and had no improvement on it.
From the above point of view, we can conclude that medium dose dexamethasone was the most commonly used glucocorticoid regimen for the treatment of COVID-19, and it has the best effect among the commonly used and equivalent doses of other glucocorticoids. The reason for the different results of the same equivalent dose of glucocorticoids used in the treatment of COVID-19 is still unclear, and we speculate that it may be due to the different types of glucocorticoids have different metabolism and half-life: dexamethasone is a long-acting glucocorticoids, methylprednisolone is a medium-acting glucocorticoids, and hydrocortisone is a short-acting glucocorticoids.
Availability of data and materials
The datasets used during the current study are available from the corresponding author upon reasonable request.
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–44.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–74.
Bueno-Notivol J, Gracia-García P, Olaya B, Lasheras I, López-Antón R, Santabárbara J. Prevalence of depression during the COVID-19 outbreak: a meta-analysis of community-based studies. Int J Clin Health Psychol. 2021;21(1):100196.
Mertens G, Gerritsen L, Duijndam S, Salemink E, Engelhard IM. Fear of the coronavirus (COVID-19): predictors in an online study conducted in March 2020. J Anxiety Disord. 2020;74:102258.
Chang X, Li S, Fu Y, Dang H, Liu C. Safety and efficacy of corticosteroids in ARDS patients: a systematic review and meta-analysis of RCT data. Respir Res. 2022;23(1):301.
Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343.
Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–67.
COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids. Accessed 20 Dec 2022.
Ahmed MH, Hassan A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Compr Clin Med. 2020;2(12):2637–46.
Recovery Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva M, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–16.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Buttgereit F, da Silva JA, Boers M, Burmester GR, Cutolo M, Jacobs J, Kirwan J, Köhler L, Van Riel P, Vischer T, et al. Standardised nomenclature for glucocorticoid dosages and glucocorticoid treatment regimens: current questions and tentative answers in rheumatology. Ann Rheum Dis. 2002;61(8):718–22.
Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43(11):1216–27.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–805.
Toroghi N, Abbasian L, Nourian A, Davoudi-Monfared E, Khalili H, Hasannezhad M, Ghiasvand F, Jafari S, Emadi-Kouchak H, Yekaninejad MS. Comparing efficacy and safety of different doses of dexamethasone in the treatment of COVID-19: a three-arm randomized clinical trial. Pharmacol Rep. 2022;74(1):229–40.
Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, Erfani A, Khodamoradi Z, Gholampoor Saadi MH. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21(1):337.
Munch MW, Meyhoff TS, Helleberg M, Kjaer MN, Granholm A, Hjortso CJS, Jensen TS, Moller MH, Hjortrup PB, Wetterslev M, et al. Low-dose hydrocortisone in patients with COVID-19 and severe hypoxia: the COVID STEROID randomised, placebo-controlled trial. Acta Anaesthesiol Scand. 2021;65(10):1421–30.
Dequin PF, Heming N, Meziani F, Plantefeve G, Voiriot G, Badie J, Francois B, Aubron C, Ricard JD, Ehrmann S, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298–306.
Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Safe IP, Borba MGS, Netto RLA, Maciel ABS, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2021;72(9):e373–81.
Wu H, Daouk S, Kebbe J, Chaudry F, Harper J, Brown B. Low-dose versus high-dose dexamethasone for hospitalized patients with COVID-19 pneumonia: a randomized clinical trial. PLoS One. 2022;17(10):e0275217.
Salvarani C, Massari M, Costantini M, Merlo DF, Mariani GL, Viale P, Nava S, Guaraldi G, Dolci G, Boni L, et al. Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia: a double-blind, randomised, placebo-controlled trial. Eur Respir J. 2022;60(4):2200025.
Dastenae ZH, Bahadori A, Dehghani M, Asadi-Samani M, Izadi I, Shahraki HR. Comparison of the effect of intravenous dexamethasone and methylprednisolone on the treatment of hospitalized patients with COVID-19: a randomized clinical trial. Int J Infect Dis. 2022;122:659–64.
Jamaati H, Hashemian SM, Farzanegan B, Malekmohammad M, Tabarsi P, Marjani M, Moniri A, Abtahian Z, Haseli S, Mortaz E, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947.
Covid Steroid Trial Group, Munch MW, Myatra SN, Vijayaraghavan BKT, Saseedharan S, Benfield T, Wahlin RR, Rasmussen BS, Andreasen AS, Poulsen LM, et al. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326(18):1807–17.
Tang X, Feng YM, Ni JX, Zhang JY, Liu LM, Hu K, Wu XZ, Zhang JX, Chen JW, Zhang JC, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration. 2021;100(2):116–26.
Taboada M, Rodriguez N, Varela PM, Rodriguez MT, Abelleira R, Gonzalez A, Casal A, Diaz Peromingo JA, Lama A, Dominguez MJ, et al. Effect of high versus low dose of dexamethasone on clinical worsening in patients hospitalised with moderate or severe COVID-19 pneumonia: an open-label, randomised clinical trial. Eur Respir J. 2022;60(2):2102518.
Maskin LP, Bonelli I, Olarte GL, Palizas F Jr, Velo AE, Lurbet MF, Lovazzano P, Kotsias S, Attie S, Lopez Saubidet I, et al. High- versus low-dose dexamethasone for the treatment of COVID-19-related acute respiratory distress syndrome: a multicenter, randomized open-label clinical trial. J Intensive Care Med. 2022;37(4):491–9.
Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, Najafizadeh SR, Farhadi E, Jalili N, Esfahani M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808.
Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gomez-Barquero J, Abadia-Otero J, Garcia-Ibarbia C, Mora V, Cerezo-Hernandez A, Hernandez JL, Lopez-Muniz G, et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: an open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr. 2021;133(7–8):303–11.
Bouadma L, Mekontso-Dessap A, Burdet C, Merdji H, Poissy J, Dupuis C, Guitton C, Schwebel C, Cohen Y, Bruel C, et al. High-dose dexamethasone and oxygen support strategies in intensive care unit patients with severe COVID-19 acute hypoxemic respiratory failure: the COVIDICUS randomized clinical trial. JAMA Intern Med. 2022;182(9):906–16.
Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, van Bentum-Puijk W, Berry L, Bhimani Z, Bonten M, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–29.
Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–41.
Beran A, Ayesh H, Mhanna M, Srour O, Musallam R, Sayeh W, Khokher W, Altorok N, Noori Z, Assaly R, et al. Methylprednisolone versus dexamethasone in COVID-19: a meta-analysis of nonrandomized studies. Am J Ther. 2022;29(3):e354–7.
Halfmann PJ, Iida S, Iwatsuki-Horimoto K, Maemura T, Kiso M, Scheaffer SM, Darling TL, Joshi A, Loeber S, Singh G, et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature. 2022;603(7902):687–92.
Shuai H, Chan JF, Hu B, Chai Y, Yuen TT, Yin F, Huang X, Yoon C, Hu JC, Liu H, et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature. 2022;603(7902):693–9.
Acknowledgements
We are grateful to Wensen Chen (Office of Infection Management, the First Affiliated Hospital of Nanjing Medical University) for revising the manuscript. We would like to thank the authors of the studies that were included in this analysis. Their work is very much appreciated.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81971807), the Science and Technology Commission of Shanghai Municipality (grant numbers 20XD1420800), and Science and Technology Commission of Shanghai Municipality (20DZ2261200).
Author information
Authors and Affiliations
Contributions
QH and MZ had full access to all the data in the study and accept responsibility for the integrity of the work as a whole, including the data and the analysis, and the entire submission process from inception to publication. QH and MZ were also responsible for the study design, study selection, data analysis, interpretation of the data, and drafting and revision of the manuscript. YW was responsible for the conception and design of the study. CW and GC assisted with the electronic search and data acquisition. YZ and YW contributed to interpretation of the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Network plot of different glucocorticoid regimens. Figure S2. Comparison-correction funnel plot. Figure S3. The results of the network meta-analysis. Figure S4. SUCRA ranking charts of different regimen of glucocorticoid. Figure S5. Forest plot of different glucocorticoid regimens.
Additional file 2.
PRISMA 2020 Checklist.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, Q., Wang, C., Wang, Y. et al. Efficacy and safety of glucocorticoids use in patients with COVID-19: a systematic review and network meta‑analysis. BMC Infect Dis 23, 896 (2023). https://doi.org/10.1186/s12879-023-08874-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08874-w