Abstract
Background
Cryptococcal meningitis (CM) is the most common fungal infection of the central nervous system that can cause significant morbidity and mortality. Although several prognostic factors have been identified, their clinical efficacy and use in combination to predict outcomes in immunocompetent patients with CM are not clear. Therefore, we aimed to determine the utility of those prognostic factors alone or in combination in predicting outcomes of immunocompetent patients with CM.
Methods
The demographic and clinical data of patients with CM were collected and analyzed. The clinical outcome was graded by the Glasgow outcome scale (GOS) at discharge, and patients were divided into good (score of 5) and unfavorable (score of 1–4) outcome groups. Prognostic model was created and receiver-operating characteristic curve analyses were conducted.
Results
A total of 156 patients were included in our study. Patients with higher age at onset (p = 0.021), ventriculoperitoneal shunt placement (p = 0.010), Glasgow Coma Scale (GCS) score of less than 15(p< 0.001), lower CSF glucose concentration (p = 0.037) and immunocompromised condition (p = 0.002) tended to have worse outcomes. Logistic regression analysis was used to create a combined score which had a higher AUC (0.815) than those factors used alone for predicting outcome.
Conclusions
Our study shows that a prediction model based on clinical characteristics had satisfactory accuracy in prognostic prediction. Early recognition of CM patients at risk of poor prognosis using this model would be helpful in providing timely management and therapy to improve outcomes and to identify individuals who warrant early follow-up and intervention.
Similar content being viewed by others
Background
Cryptococcal meningitis (CM) is the most common fungal infection of the central nervous system (CNS) [1]. Despite advances in new anti-fungal agents, CM remains associated with a high morbidity and mortality among immunocompromised patients, such as HIV-infected patients, patients with hematological malignancies, solid-organ transplant recipients and so on [2,3,4]. Studies have shown no differences in hospital mortality or satisfactory outcomes between immunocompromised and immunocompetent patients [5]. However, the vast majority of research on identifying prognostic has factors of CM focused on immunocompromised patients, such as HIV-positive population, rather than immunocompetent patients. In our previous retrospective study, we found impaired consciousness and decreased glucose concentration in cerebrospinal fluid (CSF) were independent prognostic factors that predict the unsatisfactory outcome in immunocompetent patients with CM [6]. Although the clinical efficacy of combining of multiple prognostic factors in CM is not well investigated, our findings highlight the need to develop practical tools for early recognition of CM patients at risk of poor prognosis. This can facilitate timely management and therapy to improve outcome and identify individuals who warrant early follow-up and intervention.
In this study, we aimed to explore the clinical significance of those prognostic clinical signatures used alone or in combination in the prognostic prediction of patients with CM.
Methods
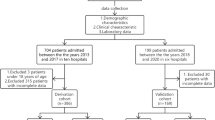
We conducted a review of hospitalized patients with CM from January 2003 to August 2022.The diagnosis of CM was based on clinical features and positive laboratory findings. The patients with CM were included if they had to meet one or more of the following criteria: (1) positive culture of cryptococcus from CSF, (2) positive CSF india ink testing, (3) positive CSF cryptococcal antigen testing, or (4) positive cerebral biopsy. Patients who had one or more identifiable underlying diseases were categorized as immunocompromised hosts, this included individuals with a history of autoimmune disorders, long-term glucocorticoids or other immunosuppressive therapies, idiopathic CD4 T-cell lymphopenia, HIV infection, malignant tumor, hepatic cirrhosis, end-stage renal failure or diabetes [6, 7]. Meanwhile, T-SPOT, tuberculosis ELISA, AFB stain, culture in CSF were performed in all cases to exclude the possibility of tuberculosis.
In this retrospective study, we collected demographic data, major symptoms and signs, neuroimaging features, laboratory findings and clinical outcome at discharge. The clinical outcome was evaluated by a neurological physician using Glasgow Outcome Scale (GOS) within 24 h prior to discharge. Score of 1–4, indicating death, vegetative status, severe disability and moderate disability, was considered “unfavorable” clinical outcomes. Score of 5, indicating no or mild disability, was considered “good” outcome [6]. The degree of impaired consciousness at admission was graded by the Glasgow Coma Scale (GCS), which evaluates motor responsiveness, verbal performance, and eye opening, and GCS score can range from 3 (completely unresponsive) to 15 (responsive) and provide a practical method for reflecting the level of consciousness [8].
Mean and standard deviations were presented for parametric variables, while medians and quartiles were used for non-parametric variables. Chi squared and fisher’s exact tests, Two-sample t-test (parametric) or Mann Whitney U test(non-parametric) were used to assess differences in demographic and clinical features between the good and unfavorable outcome groups. Variable selection was performed using the least absolute shrinkage and selection operator (LASSO) regression model, followed by multivariable logistic regression analysis to create a combined score for predicting the outcome utilizing the independent variable statistically significant at the univariate analysis, in which P value levels for inclusion and exclusion criteria were set as 0.05 and 0.10, respectively. Odds ratio (OR) and its 95% Confidence interval (CI) were estimated for each factor. To determine clinical efficacy of individual variables and the combined predictive score. The pairwise comparison of ROC curves was conducted with Delong’s test. The area under the curve (AUC) with 95% CI that evaluates the sensitivity and specificity, and cut-off values were calculated. P values < 0.05 were considered statistically significant. All statistical analyses in this study were conducted using SPSS (version 23.0, Chicago, IL, USA) and MedCalc Statistical Software (Ostend, Belgium).
Results
A total of 156 confirmed patients with CM (95 males; range: 16–87 years) with complete data were included, and 49 of them were considered immunocompromised.
In our study, the most frequent symptoms included headache (89.7%), fever (55.8%), vomiting (41.0%). Demographics and clinical manifestations of all cases in the training set are listed in Table 1.
87.8% of patients (n = 137) had the highest GCS score of 15. The median white blood cell (WBC) count in the blood was 8.2 (interquartile range, IQR 5.6, 12.0) × 109/L. The median WBC count in the CSF was 54.0(IQR 19.3, 149.0) 106/L, the median CSF glucose concentration was 2.18 (IQR 1.16, 2.94) mmol/L. The median CSF chloride concentration was 117.6 (IQR 114.6, 121.4) mmol/L. The median CSF protein concentration was 0.87 (IQR 0.52, 1.54) g/L. The sensitivity of the CSF India ink test/antigen test and culture in our study were 82.1% and 48.1%, respectively. One patient was diagnosed with CM by positive cerebral biopsy. Laboratory data and features of neuroimaging were presented in Table 2. At discharge, we assessed the outcome for all patients by using GOS, 60 patients (38.5%) obtained a good outcome.
In a univariate analysis comparing the good outcome group with the unfavorable outcome group, patients with higher age at onset (p = 0.021), ventriculoperitoneal shunt placement (p = 0.010), GCS score of less than 15(p< 0.001), lower CSF glucose concentration (p = 0.037) and immunocompromised condition (p = 0.002) tended to have worse outcomes (Table 3).
For variable selection, all variables(n = 8) with p-value of less than 0.1 in univariate analysis were included in LASSO, lambda.min and lambda.1se (standard error, SE) were 0.013 and 0.074. Optimal lambda.min resulted in 8 nonzero coefficients (Fig. 1). To identify the combined score, a multivariable logistic regression analysis was conducted and 8 variables with nonzero coefficients in LASSO were included. The result was the following: combined score = 1.587*(immunocompromised condition:0 for without, 1 for with) + 1.563*( ventriculoperitoneal shunt : 0 for without, 1 for with) + 0.025*(age at onset)-0.128*(CSF glucose concentration) + 3.129*(GCS score: 0 for = 15,1 for<15) + 0.602*(meningeal enhancement:0 for without,1 for with) + 0.851*(positive meningeal irritation: 0 for without, 1 for with) + 0.008*(Interval from onset to antifungal treatment). The results of a multivariable logistic regression were presented in Table 4. Mann Whitney U test confirmed that patients with higher combined score tended to have worse outcomes(p<0.001).
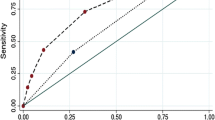
Receiver operating characteristic (ROC) curve was performed to investigate the predictive value of those markers used alone and combined score. We found that CSF glucose concentration(sensitivity of 50.0%, Specificity of 76.7%, AUC of 0.599), GCS score of <15(sensitivity of 26.0%, Specificity of 98.3%, AUC of 0.622), age at onset (sensitivity of 70.8%, Specificity of 48.3%, AUC of 0.610), ventriculoperitoneal shunt placement (sensitivity of 19.8%, Specificity of 95.0%, AUC of 0.574), immunocompromised condition (sensitivity of 40.6%, Specificity of 83.3%, AUC of 0.620) and combined score(sensitivity of 69.8%, Specificity of 78.3%, AUC of 0.815) had significant accuracy for predicting the unfavorable outcomes in patients with CM (p<0.05 for all, Table 5). According to multiple comparisons, the combined score provided higher AUC compared with the CSF glucose concentration, GCS score of <15, age at onset, immunocompromised condition and ventriculoperitoneal shunt placement (p<0.001 for all, Fig. 2), respectively. The cut‑off values and AUC values are displayed in Table 5.
Discussion
Previous studies have consistently confirmed that high CSF glucose concentration and age at onset are independent prognostic factors associated with favorable prognosis, mortality, survival time, regardless of presence of predisposing diseases [9,10,11,12,13,14]. Our study supports these findings.
Our study also confirms that ventriculoperitoneal shunt placement is an independent prognostic factor associated with unfavorable prognosis. This surgical procedure is commonly used in the management of CNS infection and is typically performed in patients with severe hydrocephalus or increased intracranial pressure, which lead to extensive brain injury and neurodisability[15].
Our present study identified a GCS score below 15 and immunocompromised conditions as independent prognostic factor. While previous studies have demonstrated that higher GCS score are associated with favorable outcomes [16] and lower mortality [17]. Li et al. found that no association between immunocompromised condition and worse prognosis or mortality in non-HIV population with CM [5]. One possible explanation for this difference is that our study included 5 AIDS patients (10% of the immunocompromised population) and more patients with long-term use of corticosteroid (46.9% vs. 23.2%, n = 23), which indicated that a greater degree of immunosuppression in our study.
In the present study, the AUC and cut-off values of individual and combined score have been analyzed. Compared with factors used alone, the combined score turned out to be a satisfactory predictor with an AUC of 0.815, which indicated that the patients with combined score of >2.67 had a significantly higher probability of unfavorable outcome. Besides that, the combined score was easy to be applied in practice, because these factors could be obtained from the routine examinations and scales without any additional costs in the diagnosis of CNS infections.
The prognostic model developed in the present study may draw the attention of clinicians to provide early specific measures, such as the admission of patients with a higher risk of poor outcome to intensive care units (ICU). Additionally, it could provide a helpful tool for risk assessment and decision-making in treatment strategy. Identifying patients with a higher risk of poor outcome could facilitate earlier, aggressive treatment (e.g., maximum dose and duration of Liposomal AmB in the induction phase) and potentially improve outcomes. Conversely, identifying patients with a lower risk of poor outcome could enable the use of moderate treatment to relieve the side effects and the financial burden caused by long-term antifungal therapy.
There were some limitations on the strength of this study. A larger sample size may allow findings to be more accurate. Moreover, no data of long-term clinical outcome after discharge were recruited, the utility of the clinical factors here analyzed on predicting long term prognosis was unattainable in this study. Because the vast majority of patients have not undergone CrAg test, the finding of the CrAg test was not included in our study. Therefore, a multicenter study with long term follow-up data and more variables should be performed for a more detailed study.
Conclusions
The present study established a prediction model based on clinical characteristics, which had statistically significant accuracy in prognostic prediction. Our findings may help clinicians early identify patients with poor outcomes and optimize treatment strategy.
Data availability
The data that support the findings of this study are available from the corresponding author via E-mail upon reasonable request.
References
Fang W, Fa Z, Liao W. Epidemiology of Cryptococcus and cryptococcosis in China. Fungal Genet Biol. 2015;78:7–15.
Guo L-y, Liu L-l, Liu Y, Chen T-m, Li S-y, Yang Y-h, Liu G. Characteristics and outcomes of cryptococcal meningitis in HIV seronegative children in Beijing, China, 2002–2013. BMC Infect Dis 2016, 16.
Chen C-H, Sy H-N, Lin L-J, Yen H-C, Wang S-H, Chen W-L, Chen Y-M, Chang Y-J. Epidemiological characterization and prognostic factors in patients with confirmed cerebral cryptococcosis in central Taiwan. J Venom Anim Toxins Incl Trop Dis 2015, 21.
Bandalizadeh Z, Shokohi T, Badali H, Abastabar M, Babamahmoudi F, Davoodi L, Mardani M, Javanian M, Cheraghmakani H, Sepidgar AA, et al. Molecular epidemiology and antifungal susceptibility profiles of clinical Cryptococcus neoformans/Cryptococcus gattii species complex. J Med Microbiol. 2020;69(1):72–81.
Li M, Chen Z, Xu L, Gan Z, Peng F, Liu J. A comparison of the clinical characteristics and outcomes of cryptococcal meningitis in HIV-negative individuals with and without immunosuppression. Neurologist. 2019;24(1):1–5.
Zhang C, Tan Z, Tian F. Impaired consciousness and decreased glucose concentration of CSF as prognostic factors in immunocompetent patients with cryptococcal meningitis. BMC Infect Dis 2020, 20(1).
Tan Z-R, Long X-Y, Li G-L, Zhou J-X, Long L. Spectrum of neuroimaging findings in cryptococcal meningitis in immunocompetent patients in China - A series of 18 cases. J Neurol Sci. 2016;368:132–7.
Barlow P. A practical review of the Glasgow coma scale and score. SURGEON-JOURNAL OF THE ROYAL COLLEGES OF SURGEONS OF EDINBURGH AND IRELAND 2012, 10(2):114–9.
Yao ZR, Liao WQ, Chen RG. Management of cryptococcosis in non-HIV-related patients. Med Mycol. 2005;43(3):245–51.
Cao W, Jian C, Zhang H, Xu S. Comparison of clinical features and prognostic factors of cryptococcal meningitis caused by Cryptococcus neoformans in patients with and without pulmonary nodules. Mycopathologia. 2019;184(1):73–80.
Zheng H, Chen Q, Xie Z, Wang D, Li M, Zhang X, Man Y, Lao J, Chen N, Zhou L. A retrospective research of HIV-negative cryptococcal meningoencephalitis patients with acute/subacute onset. Eur J Clin Microbiol Infect Dis. 2016;35(2):299–303.
Hakyemez IN, Erdem H, Beraud G, Lurdes M, Silva-Pinto A, Alexandru C, Bishop B, Mangani F, Argemi X, Poinot M et al. Prediction of unfavorable outcomes in cryptococcal meningitis: results of the multicenter infectious Diseases International Research Initiative (ID-IRI) cryptococcal meningitis study (vol 37, pg 1231, 2018). Eur J Clin Microbiol Infect Dis 2018, 37(7):1241–1242.
Xu L, Zhang X, Guo Y, Tao R, Dai X, Yang Z, Huang Y, Zhu B, Xu Y. Unique clinical features of cryptococcal meningitis among chinese patients without predisposing diseases against patients with predisposing diseases. Med Mycol. 2019;57(8):944–53.
Wu L, Xiao J, Song Y, Gao G, Zhao H. The clinical characteristics and outcome of cryptococcal meningitis with AIDS in a tertiary hospital in China: an observational cohort study. BMC Infect Dis 2020, 20(1).
Raut T, Garg RK, Jain A, Verma R, Singh MK, Malhotra HS, Kohli N, Parihar A. Hydrocephalus in tuberculous meningitis: incidence, its predictive factors and impact on the prognosis. J Infect. 2013;66(4):330–7.
Hakyemez IN, Erdem H, Beraud G, Lurdes M, Silva-Pinto A, Alexandru C, Bishop B, Mangani F, Argemi X, Poinot M, et al. Prediction of unfavorable outcomes in cryptococcal meningitis: results of the multicenter infectious Diseases International Research Initiative (ID-IRI) cryptococcal meningitis study. Eur J Clin Microbiol Infect Dis. 2018;37(7):1231–40.
Kitonsa J, Nsubuga R, Mayanja Y, Kiwanuka J, Nikweri Y, Onyango M, Anywaine Z, Ggayi AB, Kibengo FM, Kaleebu P, et al. Determinants of two-year mortality among HIV positive patients with cryptococcal meningitis initiating standard antifungal treatment with or without adjunctive dexamethasone in Uganda. PLoS Negl Trop Dis. 2020;14(11):e0008823.
Acknowledgements
We thank all our colleagues at the Department of Neurology.
Funding
This study was supported by the National key R&D program of China (2017YFC1310003) and Natural Science Foundation of China (81571276). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
CZ, ZT, and FT: conceptualization. ZT , ZH and CZ: methodology. CZ, ZT: investigation. CZ: formal analysis, resources, writing, and original draft. FT: writing-review & editing and Supervision. All authors have read and approved the manuscript. All authors had full access to all the data in the study, took responsibility for the integrity of the data, and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All subjects gave their verbal informed consent prior to their inclusion in the study. Written consent to participate was waived by the Research Ethics Committee of the Xiangya Hospital, as the present study was a retrospective study using de-identified data, and patients will spend additional time and transportation costs for obtaining written consent to participate after discharge, which will increase the burden on patients. This study was approved by the Research Ethics Committee of the Xiangya Hospital, and was conducted according to the principles expressed in the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, C., He, Z., Tan, Z. et al. The clinic-based predictive modeling for prognosis of patients with cryptococcal meningitis. BMC Infect Dis 23, 352 (2023). https://doi.org/10.1186/s12879-023-08337-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08337-2