Abstract
Background
While others have reported severe acute respiratory syndrome-related coronavirus 2(SARS-CoV-2) seroprevalence studies in health care workers (HCWs), we leverage the use of a highly sensitive coronavirus antigen microarray to identify a group of seropositive health care workers who were missed by daily symptom screening that was instituted prior to any epidemiologically significant local outbreak. Given that most health care facilities rely on daily symptom screening as the primary method to identify SARS-CoV-2 among health care workers, here, we aim to determine how demographic, occupational, and clinical variables influence SARS-CoV-2 seropositivity among health care workers.
Methods
We designed a cross-sectional survey of HCWs for SARS-CoV-2 seropositivity conducted from May 15th to June 30th 2020 at a 418-bed academic hospital in Orange County, California. From an eligible population of 5,349 HCWs, study participants were recruited in two ways: an open cohort, and a targeted cohort. The open cohort was open to anyone, whereas the targeted cohort that recruited HCWs previously screened for COVID-19 or work in high-risk units. A total of 1,557 HCWs completed the survey and provided specimens, including 1,044 in the open cohort and 513 in the targeted cohort. Demographic, occupational, and clinical variables were surveyed electronically. SARS-CoV-2 seropositivity was assessed using a coronavirus antigen microarray (CoVAM), which measures antibodies against eleven viral antigens to identify prior infection with 98% specificity and 93% sensitivity.
Results
Among tested HCWs (n = 1,557), SARS-CoV-2 seropositivity was 10.8%, and risk factors included male gender (OR 1.48, 95% CI 1.05–2.06), exposure to COVID-19 outside of work (2.29, 1.14–4.29), working in food or environmental services (4.85, 1.51–14.85), and working in COVID-19 units (ICU: 2.28, 1.29–3.96; ward: 1.59, 1.01–2.48). Amongst 1,103 HCWs not previously screened, seropositivity was 8.0%, and additional risk factors included younger age (1.57, 1.00-2.45) and working in administration (2.69, 1.10–7.10).
Conclusion
SARS-CoV-2 seropositivity is significantly higher than reported case counts even among HCWs who are meticulously screened. Seropositive HCWs missed by screening were more likely to be younger, work outside direct patient care, or have exposure outside of work.
Similar content being viewed by others
Background
Protecting health care workers (HCWs) during the coronavirus disease 2019 (COVID-19) pandemic is essential to mounting an effective response, as outbreaks among this population could potentially cripple health care delivery. Current case identification relies on symptom and temperature screening with follow-up testing by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR). This approach underestimates disease prevalence by missing cases of asymptomatic infection and false negatives due to suboptimal timing, flawed specimen collection, or low viral load [1, 2]. Given the importance of asymptomatic persons in the transmission of SARS-CoV-2, estimated to account for 59% of overall transmission, including 24% from asymptomatic persons and 35% from pre-symptomatic persons [3], identification of risk factors that may augment identification of asymptomatic infection in HCWs is crucial to protecting patients and the health care system [1].
Serologic testing can help to determine the true prevalence of COVID-19 by identifying previously infected persons who had minimal symptoms so were missed by the current testing paradigm [4, 5]. Multiple COVID-19 seroprevalence studies have been performed in different populations but are limited by low specificity in low-prevalence populations or potential selection bias from the use of convenience sampling, with estimated seroprevalence of comparable populations during the study period ranging from 1.0 to 11.2% [6,7,8,9,10,11]. Estimated seroprevalence among HCW varies widely, with some studies finding similar or even lower prevalence compared to the surrounding community, but most studies noting a significant proportion of asymptomatic infections [8, 12,13,14,15,16,17,18].
Risk factors for SARS-CoV-2 infection amongst HCW were initially extrapolated from studies of hospitalized patients with severe disease that may not be generalizable to the population at large [19]. Previous studies to identify risk factors amongst HCWs were performed in early outbreak setting prior to current infection control practices so may not be currently applicable [20, 21]. More recent studies have conflicting results as to whether occupational exposures confer an increased risk of SARS-CoV-2 seropositivity and may be limited by suboptimal performance of the assays upon external validation, heterogeneity in the study populations, and lack of control for confounding due to the use of univariate analyses [22,23,24,25].
This study measured SARS-CoV-2 seropositivity amongst 1,557 HCWs at the University of California-Irvine Health, a 418-bed academic medical center in Orange County, California, from May 15th to June 30th 2020, using a novel coronavirus antigen microarray (CoVAM). This CoVAM utilizes 11 SARS-CoV-2 IgG and IgM antigens to determine prior infection with 98% specificity and 93% sensitivity based on validation in 91 rt-PCR-positive cases and 88 pre-pandemic negative controls. The CoVAM also is used to distinguish SARS_CoV-2 infection from prior infection with other human coronaviruses [26]. This performance and level of validation compares favorably to other serologic assays based on a single antigen [27, 28], and the predictive model based on multiple antigens outperformed models based on any single antigen during validation of the COVAM [26]. A multivariable analysis was used to probe associations among demographic, clinical, and occupational risk factors and SARS-CoV-2 seropositivity among HCWs.
Methods
Study design, setting, and population
The study, a prospective cohort study, with the first time point presented here as a cross-sectional analysis, was approved by the Institutional Review Board of the University of California-Irvine under Protocol HS 2020–5818. All 5,349 employees who worked in the hospital were eligible. Universal daily symptom and temperature screening was initiated on April 14, 2020, with subsequent immediate rt-PCR testing for any HCW with symptoms or fever or disclosure of a confirmed or suspected COVID-19 contact. A primary study site in the main hospital building of University of California Irvine Health (Orange, California, USA) was open from May 15 to May 29, 2020 to all employees who provided electronic consent (open enrollment cohort). In addition, all employees who had been tested for SARS-CoV-2 by rt-PCR at the primary study site due to symptoms or possible exposure, or who provided direct patient care in COVID-19 clinical units or similar control units, were invited via email and provided electronic consent to participate at a secondary study open from May 15 to June 30, 2020 (targeted enrollment cohort). This second cohort was included to enrich the study for HCW with COVID-19 infection, symptoms, or exposure.
Study procedures
Participants were given a unique study identifier and a mobile phone link to a Research Electronic Data Capture (REDCap, Vanderbilt University, Nashville, TN) survey to collect data on demographic, clinical, and occupational risk factors (Supplementary Fig. 2 ). At the primary study site, participants then underwent capillary blood collection via fingerstick using a disposable lancet into microfuge capillary tubes (BD Microtainer). After centrifugation at 1500 x g for 10 min, supernatant plasma was collected, frozen, and transported for laboratory analysis (for testing for SARS-CoV-2 antibodies on the CoVAM). At the secondary study site, participants underwent phlebotomy into gold-top tubes (BD Biosciences, San Jose, CA) for centrifugation and collection of serum, from which an aliquot was frozen at 0 C° and transported for laboratory analysis (for testing for SARS-CoV-2 antibodies on the CoVAM). All specimens were labeled with unique identifiers accessible only via a secure key.
Laboratory assay
The CoVAM includes 67 antigens from respiratory viruses, including 11 antigens from SARS-CoV-2 (Sino Biological U.S. Inc., Wayne, PA). Antigens were printed onto microarrays in quadruplicate, probed with serum specimens and secondary antibodies for IgM and IgG, and imaged to determine background-subtracted median fluorescence intensity [29,30,31]. Briefly, CoVAM data for each specimen were compared with 91 rt-PCR-positive cases with blood collected ≥ 7 days (range 7–50, median 11) post-symptom onset and 88 pre-pandemic controls with blood collected prior to November 1, 2019, which were split randomly into 70% training set and 30% testing set for model development. Based on IgM and IgG antibodies against the 11 SARS-CoV-2 antigens on the array, a logistic regression model was trained on positive and negative controls in the training set to determine optimal weighted combinations of reactive antigens to calculate composite SARS-CoV-2 antibody titers that discriminate the two groups, with reactivity thresholds selected to achieve maximum sensitivity while maintaining ≥ 98% specificity. The model was tested on the testing set and achieved 92.7% sensitivity and 97.7% specificity for detecting prior SARS-CoV-2 infection based on composite IgM or IgG positivity [26]. A detailed description of the development and validation of the CoVAM has been previously published [26].
Statistical analysis
The prevalence of SARS-CoV-2 seropositivity was calculated in the study population as the proportion of HCWs who were classified as seropositive, and the prevalence of SARS-CoV-2 seropositivity was calculated within categories of each demographic, clinical and occupational risk factor and compared to the study population by expressing as odds ratio (OR) with 95% confidence interval. In order to assess the associations between clinical and occupational risk factors and seropositivity, multivariable logistic regression models were constructed to control for potential confounding due to demographic and health-related factors associated with both occupational exposure and underlying risk for seropositivity; potential covariates were chosen by the authors based on known association with COVID-19 epidemiology and relevance to occupational health. We included in the model demographic variables (age, gender, and race/ethnicity) and health-related covariates (asthma or chronic obstructive pulmonary disease, diabetes mellitus, hypertension, self-reported smoking or vaping) which have known associations with COVID-19 epidemiology [32], in addition to known COVID-19 exposure outside of work and occupation-related variables of interest (self-reported role, location, and contact with COVID-19 patients). A targeted cohort was included in addition to the open cohort in order to gain data on healthcare workers who may be more prone to exposure- those in high-risk areas (e.g. ICU healthcare workers), a particular interest to this study. Health- and occupation-related exposures were selected based on bivariate associations with seropositivity using a p < 0.1 criterion for inclusion and added to the model in a forward stepwise regression. The final model for clinical and occupational risk factors was adjusted for age (quartiles), gender, race/ethnicity (Asian, White, Latino, Black, and Mixed/other/not reported), known COVID-19 exposure outside of work, and workplace role, location, and COVID-19 patient contact. Adjusted analyses were conducted among the entire sample and the same model was applied to the subgroup of HCWs not tested previously via rt-PCR. Model fit was evaluated using the Hosmer-Lemeshow goodness-of-fit test and C-statistic. All analyses were conducted using R software v4.0.3 (R Consortium for Statistical Computing, Vienna, Austria).
Results
SARS-CoV-2 seropositivity in the study population
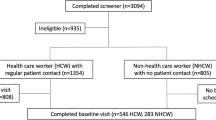
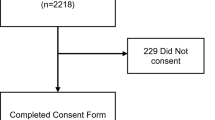
From an eligible population of 5,349 HCWs, 1,841 (34.4%) consented to participate, including 1,108 in the open enrollment cohort and 733 in the targeted enrollment cohort. Of the targeted cohort, 343 had been tested by rt-PCR for COVID-19, 237 worked in a COVID-19 unit and 153 worked in a matched control unit of similar acuity. A total of 1,557 HCWs completed the survey and provided blood specimens to be analyzed by CoVAM, including 1,044 in the open enrollment cohort and 513 in the targeted cohort.
SARS-CoV-2 seropositivity was 10.8% in the overall HCW cohort (Table 1). Seropositivity was 17.7% amongst the 419 HCWs who had been tested by rt-PCR previously, and 8.0% among the 1,138 HCW who had not. Seropositivity in the targeted versus open enrollment cohorts matched closely the seropositivity among HCWs tested previously versus not tested by rt-PCR respectively. Of the 413 HCW tested by rt-PCR, results were available for 360 HCWs, among these 38 were PCR + and 322 PCR-, with 36 (94.7%) of the PCR + testing seropositive whereas 30 (9.3%) of the PCR- were seropositive, higher than the 8.0% seropositivity rate of those not tested by rt-PCR (Supplementary Table 4 ).
Potential demographic risk factors identified by bivariate analysis included age, gender, race/ethnicity, and co-morbid conditions, as well as confirmed SARS-CoV-2 exposure outside the hospital (Table 1). No significant effect of age was noted among HCWs overall; however, a non-significant increase in seropositivity was observed for younger HCWs who were not previously tested by rt-PCR. Male gender was associated with increased seropositivity, whereas race/ethnicity and co-morbidities were not. Confirmed COVID-19 exposure outside the hospital was the most significant demographic risk factor for seropositivity.
Impact of occupational risk factors on SARS-CoV-2 seropositivity
The multivariate model included the non-occupational covariates discussed above, in addition to role and location within the hospital (Table 2). The Hosmer-Lemeshow goodness-of-fit test was non-significant (p = 0.55) and area under the receiver-operating characteristic curve showed moderate discriminant ability (C-statistic = 0.62).
Among roles within the hospital, only HCWs working in food services or environmental services showed significantly increased seropositivity (OR 4.85) as compared to the overall HCW population, and the effect was restricted to those not tested by rt-PCR. Similarly, working in administration was associated with increased seropositivity (OR 2.69) only amongst HCWs not tested by rt-PCR. Among locations in the hospital, working in COVID-19 units was associated with increased seropositivity (OR 2.28 for ICU, 1.59 for ward), whereas working in labor and delivery units was associated with decreased seropositivity (OR 0.24). COVID-19 patient contact and participation in aerosol-generating procedures on these patients were not associated with seropositivity (OR 0.70).
Correlation of COVID-19 symptoms with seropositivity
A separate multivariable model was constructed that included non-occupational covariates discussed above, in addition to symptoms of COVID-19, but not including occupational covariates (Table 3). Overall, multiple symptoms, specifically fatigue (OR 1.77), myalgias (OR 1.76), fever (OR 1.67), chills (OR 1.79), and anosmia were associated with increased seropositivity, with the strongest association observed for anosmia (OR 5.34). No association between symptoms and seropositivity was observed for HCW who were not previously tested by rt-PCR (i.e. not previously identified by occupational screening).
Discussion
This study provides several insights into the relationships between non-occupational and occupational risk factors and COVID-19 seropositivity among HCWs (Fig. 1). The study hospital was able to maintain infection prevention best practices consistent with guidance from the U.S. Centers for Disease Control and Prevention (CDC), including continuous, ample availability of PPE throughout the pandemic, which is relevant to the question of whether these measures fully prevent in-hospital transmission of SARS-CoV-2. Exposure to COVID-19 outside of work was a greater risk factor for seropositivity than any occupational exposure other than working in food or environmental services. The HCW roles associated with the greatest odds of seropositivity did not involve direct patient care. Nurses, who have the most direct and sustained patient contact, were not at significantly increased odds. The only locations associated with increased seropositivity were the dedicated COVID-19 ICU and floor units. The operating room, an area of great concern due to intubation of multiple patients, was not associated with increased risk. Performing aerosol-generating procedures on known COVID-19 patients was also not significantly associated with seropositivity, which is reassuring given that perceived risk of transmission during these procedures can delay patient care.
Forest plot of adjusted odds ratios (OR) of hypothesized predictors of COVID-19 seropositivity (AB+) among HCW study population and subgroups segregated by prior rt-PCR testing. ORs are adjusted for sex, age, race/ethnicity, known COVID-19 exposure outside of work, role, location, and COVID-19 patient contact. (EVS, environmental services)
Stratification of HCWs based on whether or not they were tested previously by rt-PCR yielded several additional insights into the strengths and weaknesses of universal symptom screening. The study hospital was conducting universal symptom screening of all HCWs during the study period, and HCW who screened positive were captured in the subgroup tested by rt-PCR; those HCW who did not screen positive for symptoms and were not tested by rt-PCR but were found to be seropositive likely reflect asymptomatic infections or lack of reporting of symptoms. Although the hospital’s mandatory occupational health screening was only implemented one month prior to this study, relatively few COVID-19 infections would have occurred prior to this screening based on the local prevalence of COVID-19 (Fig. 2). The association between COVID-19 symptoms and seropositivity was restricted to HCW tested previously by rt-PCR, indicating that universal screening was effective in identifying symptomatic infections among the HCW in the study population. Younger HCWs who were COVID-19-seropositive were more likely to be missed by occupational screening, which is consistent with the increased prevalence of minimally symptomatic infection among younger individuals [33]. Decreased seropositivity among HCW in labor and delivery units may be due to increased vigilance amongst HCW who care for pregnant patients or low disease prevalence among these patients. Exposure to COVID-19 outside of work was associated with increased odds of seropositivity among HCWs not previously tested by rt-PCR, indicating that these exposures were not being universally reported during screening as they should have prompted rt-PCR testing.
Epidemiologic context of HCW study with respect to community prevalence and hospital burden of COVID-19. This study was performed between May 15th to June 30th, 2020 (top graph), a period in which total cases in Orange County were on a rise (bottom graph). Key events for the University Hospital system are outlined in the bottom
While no data were available for SARS-CoV-2 seropositivity in the surrounding community at the time that the study was performed, the prevalence of COVID-19 in Orange County was 0.2% at that time (based on case reporting to the Orange County Health Department) and increased subsequently (Fig. 2). The overall seropositivity rate was 10.8% in this study, but the 8.0% seropositivity amongst HCW not previously identified by screening, which matches the seropositivity in the open enrollment cohort, is most appropriate for comparison to community prevalence to avoid the enrichment effect of the targeted enrollment cohort. This estimated seropositivity is 40-fold higher than community prevalence based on public health case reporting confirmed by rt-PCR testing. This large discrepancy is likely explained in part by the waning of PCR positivity over time, as viral shedding is short-term whereas seropositivity is relatively sustained. Whereas more recent seroprevalence studies show a lower increase compared to case counts, our result is most comparable to early seroprevalence studies prior to significant local outbreaks of COVID-19 that have found larger disparities between seropositivity rates and case counts [6,7,8,9,10, 16]. Subsequently, a community study sampled 2,979 random participants in Orange County from July 10 through August 16, 2020 and found a seropositivity rate of 11.5% (95% CI: 10.5–12.4%) using an updated version of the CoVAM with a more stringent threshold for seropositivity [34]. The seroprevalence estimates imply that HCWs have SARS-CoV-2 seropositivity similar to the surrounding community (although seropositivity in both studies is much higher than prevalence based on public health case reporting confirmed by rt-PCR testing), with the caveat that the two studies were performed during different time periods.
The findings of this study are largely consistent with recently published studies of SARS-CoV-2 seropositivity among HCWs [22,23,24,25]. In particular, the lack of association of either race/ethnicity or co-morbidities with seropositivity in our study is consistent with these prior HCW studies and differs from a prior community study that did observe such associations [35]. This study provides additional insight compared to prior studies by examining specific roles in the hospital and controlling for multiple likely sources of confounding. For example, nurses had significantly elevated seropositivity in bivariate analyses (unadjusted OR [CI] = 1.52 [1.10–2.10]) but this finding did not persist after adjusting for work location; in contrast, null associations between seropositivity and roles without direct patient care became significant and positive after adjusting for location (unadjusted OR [CI] for administrative = 1.08 [0.66, 1.69]; food/environmental = 1.54 [0.62–3.29]).
Strengths of this study include the validated test performance of the CoVAM, which compares favorably to currently available single-antigen assays; the large sample size with inclusion of 34.4% of HCW at the hospital; and the use of multivariable analysis to control for confounding. The weaknesses of this study include the non-random enrollment methodology as the targeted enrollment cohort was invited from groups expected to have higher seroprevalence and the open enrollment cohort was subject to self-selection, which both could lead to sampling bias. Also, different blood sampling methodology was used in the open and targeted enrollment cohorts due to institutional interest in banking specimens from the latter group. The subgroup analysis based on prior rt-PCR testing was used to control for the heterogeneous sampling, as prior testing was the primary driver of increased prevalence in the targeted enrollment cohort. When the study population is stratified based on method of recruitment (Supplementary Tables 1–3 and Supplementary Fig. 1 ), the results are largely similar to stratification based on rt-PCR testing (Tables 1, 2 and 3; Fig. 1).
Conclusion
The results of this study have several implications for the local and global responses to the COVID-19 pandemic. The finding of a significantly increased SARS-CoV-2 prevalence by serology as compared with rt-PCR provides evidence that the reported counts of confirmed COVID-19 cases are significant underestimates. The observations that HCWs who are younger, work in non-patient care roles, or have COVID-19 exposure outside of work are more likely to have COVID-19 seropositivity without prior testing indicates that screening and vaccination efforts targeting these groups can be particularly effective. While we do not observe an association of aerosol-generating procedures with SARS-CoV-2 seropositivity in the context of adequate availability and presumably appropriate use of PPE, we do observe increased seropositivity in COVID-19 units, but this may potentially be related to geographical factors other than patient care given that caring for COVID-19 patients was not a significant risk factor. Further studies are needed to confirm these observations.
Of note, this study was performed prior to the availability of vaccines against SARS-CoV-2, which is now required for workers in most healthcare facilities. While these early pandemic observations are therefore less relevant to the current epidemiology of the COVID-19 pandemic in healthcare facilities, they can be used to inform the hospital epidemiology response to future epidemics of viral respiratory infections.
Data Availability
The dataset generated by testing specimens on the coronavirus antigen microarray and the analysis code applied to this dataset is available upon request. The associated clinical data with removal of all identifying information is also available upon request, please contact Dr. Saahir Khan at Saahir.khan@med.usc.edu for data requests.
References
Lavezzo E, Franchin E, Ciavarella C, Cuomo-Dannenburg G, Barzon L, Del Vecchio C, et al. Suppression of a SARS-CoV-2 outbreak in the italian municipality of Vo’. Nature. 2020;584(7821):425–9.
Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J Clin Microbiol. 2020;58(6).
Johansson MA, Quandelacy TM, Kada S, Prasad PV, Steele M, Brooks JT, et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open. 2021;4(1):e2035057.
Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients with Novel Coronavirus Disease 2019. Clin Infect Dis. 2020;71(16):2027–34.
To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–74.
Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, Baysson H, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–9.
Sood N, Simon P, Ebner P, Eichner D, Reynolds J, Bendavid E, et al. Seroprevalence of SARS-CoV-2-Specific antibodies among adults in Los Angeles County, California, on April 10–11, 2020. JAMA. 2020;323(23):2425–7.
Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jimenez A, et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large spanish reference hospital. Nat Commun. 2020;11(1):3500.
Bendavid E, Mulaney B, Sood N, Shah S, Bromley-Dulfano R, Lai C, et al. COVID-19 antibody seroprevalence in Santa Clara County, California. Int J Epidemiol. 2021;50(2):410–9.
Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, Hall AJ et al. Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020.
Vogel G. First antibody surveys draw fire for quality, bias. Science. 2020;368(6489):350–1.
Rivett L, Sridhar S, Sparkes D, Routledge M, Jones NK, Forrest S et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9.
Schmidt SB, Gruter L, Boltzmann M, Rollnik JD. Prevalence of serum IgG antibodies against SARS-CoV-2 among clinic staff. PLoS ONE. 2020;15(6):e0235417.
Stubblefield WB, Talbot HK, Feldstein LR, Tenforde MW, Ur Rasheed MA, Mills L, et al. Seroprevalence of SARS-CoV-2 among Frontline Healthcare Personnel during the First Month of caring for patients with COVID-19-Nashville, Tennessee. Clin Infect Dis. 2021;72(9):1645–8.
Self WH, Tenforde MW, Stubblefield WB, Feldstein LR, Steingrub JS, Shapiro NI, et al. Seroprevalence of SARS-CoV-2 among Frontline Health Care Personnel in a Multistate Hospital Network – 13 Academic Medical Centers, April-June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(35):1221–6.
Korth J, Wilde B, Dolff S, Anastasiou OE, Krawczyk A, Jahn M, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437.
Schwartz J, King CC, Yen MY. Protecting Healthcare Workers during the Coronavirus Disease 2019 (COVID-19) outbreak: Lessons from Taiwan’s severe Acute Respiratory Syndrome Response. Clin Infect Dis. 2020;71(15):858–60.
Chu J, Yang N, Wei Y, Yue H, Zhang F, Zhao J, et al. Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. J Med Virol. 2020;92(7):807–13.
Liang W, Liang H, Ou L, Chen B, Chen A, Li C, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–9.
Boccia S, Ricciardi W, Ioannidis JPA. What other countries can learn from Italy during the COVID-19 pandemic. JAMA Intern Med. 2020;180(7):927–8.
Lai X, Wang M, Qin C, Tan L, Ran L, Chen D, et al. Coronavirus Disease 2019 (COVID-2019) infection among Health Care Workers and Implications for Prevention Measures in a Tertiary Hospital in Wuhan, China. JAMA Netw Open. 2020;3(5):e209666.
Iversen K, Bundgaard H, Hasselbalch RB, Kristensen JH, Nielsen PB, Pries-Heje M, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20(12):1401–8.
Rudberg AS, Havervall S, Manberg A, Jernbom Falk A, Aguilera K, Ng H, et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11(1):5064.
Moscola J, Sembajwe G, Jarrett M, Farber B, Chang T, McGinn T, et al. Prevalence of SARS-CoV-2 antibodies in Health Care Personnel in the New York City Area. JAMA. 2020;324(9):893–5.
Steensels D, Oris E, Coninx L, Nuyens D, Delforge ML, Vermeersch P, et al. Hospital-wide SARS-CoV-2 antibody screening in 3056 staff in a Tertiary Center in Belgium. JAMA. 2020;324(2):195–7.
de Assis RR, Jain A, Nakajima R, Jasinskas A, Felgner J, Obiero JM, et al. Analysis of SARS-CoV-2 antibodies in COVID-19 convalescent blood using a coronavirus antigen microarray. Nat Commun. 2021;12(1):6.
Zhou W, Wang W, Wang H, Lu R, Tan W. First infection by all four non-severe acute respiratory syndrome human coronaviruses takes place during childhood. BMC Infect Dis. 2013;13:433.
Liu W, Liu L, Kou G, Zheng Y, Ding Y, Ni W et al. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58(6).
Khan S, Jain A, Taghavian O, Nakajima R, Jasinskas A, Supnet M et al. Use of an Influenza Antigen Microarray to Measure the Breadth of Serum Antibodies Across Virus Subtypes. J Vis Exp. 2019(149).
Nakajima R, Supnet M, Jasinskas A, Jain A, Taghavian O, Obiero J et al. Protein Microarray Analysis of the Specificity and Cross-Reactivity of Influenza Virus Hemagglutinin-Specific Antibodies. mSphere. 2018;3(6).
Jain A, Taghavian O, Vallejo D, Dotsey E, Schwartz D, Bell FG, et al. Evaluation of quantum dot immunofluorescence and a digital CMOS imaging system as an alternative to conventional organic fluorescence dyes and laser scanning for quantifying protein microarrays. Proteomics. 2016;16(8):1271–9.
Mehta HB, Li S, Goodwin JS. Risk factors Associated with SARS-CoV-2 infections, hospitalization, and Mortality among US nursing home residents. JAMA Netw Open. 2021;4(3):e216315.
Davies NG, Klepac P, Liu Y, Prem K, Jit M, group, CC-w et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-11.
Bruckner TA, Parker DM, Bartell SM, Vieira VM, Khan S, Noymer A, et al. Estimated seroprevalence of SARS-CoV-2 antibodies among adults in Orange County, California. Sci Rep. 2021;11(1):3081.
Rosenberg ES, Tesoriero JM, Rosenthal EM, Chung R, Barranco MA, Styer LM, et al. Cumulative incidence and diagnosis of SARS-CoV-2 infection in New York. Ann Epidemiol. 2020;48:23–9. e4.
Acknowledgements
None.
Funding
This work was supported by two intramural research grants from the COVID-19 Basic, Translational, and Clinical Research Fund of the University of California Irvine and by the Emergency COVID-19 Research Seed Funding Opportunity from the University of California Office of the President [research grants R00RG2646, R01RG3745]. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or policy of the University of California. This work utilized the Chao Family Comprehensive Cancer Center Experimental Tissue Shared Resource, supported by the National Cancer Institute of the National Institutes of Health [award number P30CA062203]. S. Khan was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health [grant KL2 TR001416]. The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health [grant UL1 TR001414]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The initial design and construction of the CoVAM was supported by the Prometheus-UMD contract sponsored by the Defense Advanced Research Projects Agency (DARPA) BTO under the auspices of Col. Matthew Hepburn [agreements N66001-17-2-4023, N66001-18-2-4015]. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position or policy of the funding agencies and no official endorsements should be inferred.
Author information
Authors and Affiliations
Contributions
SS, CF, AP, RA, AJ, SA, FZ, RE, AA, MJ, PB, PB, PF, and SK concieved and designed research. RN, AJ, DB, SH, AN, OD, AR, SS, JC, AK, KL, WZ, and AG collected samples. PH, DT, and SA prepared and stored samples. PF and SK designed the microarray. RA, AaJ, RN, and AaJ constructed the microarray and probed samples. AP, RA, AJ, RN, and AJ analyzed and interpreted data. SS, CF, RA, AJ, PF, and SK drafted the manuscript. SH and SK edited and revised the manuscript. SS, PF, and SK obtained funding for the project. All authors approved final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The coronavirus antigen microarray is intellectual property of the Regents of the University of California that is licensed for commercialization to Nanommune Inc. (Irvine, CA), a private company for which P. Felgner is the largest shareholder and several co-authors (R. de Assis, A. Jain, R. Nakajima, and S. Khan) also own shares. Nanommune Inc. has a business partnership with Sino Biological Inc. (Beijing, China) which expressed and purified the antigens used in this study. A. Amin reports serving as a clinical trials investigator for NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli-Lilly, PTC Therpeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, and has served as a consultant and/or speaker for Bristol-Meyers-Squibb, Pfizer, Boehringer Ingelheim, Portola, Sunovion, Mylan, Salix, Alexion, Astra Zeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achogen, LaJolla, Millenium, Aseptiscope, HeartRite, and Sprightly Health. S. Schubl, C. Figueroa, A. Palma, A. Jasinkas, D. Brabender, S. Hosseinian, A. Naaseh, O. Dominguez, A. Runge, S. Skochko, J. Chinn, A. Kelsey, K. Lai, W. Zhao, P. Horvath, D. Tifrea, A. Grigorian, A. Gonzales, S. Adelsohn, F. Zaldivar, R. Edwards, M. Stamos, and P. Barie have no competing interests. AA has been a principal investigator or co-investigator of clinical trials sponsored by NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, Alexion, Gilead and a speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achogen LaJolla, Ferring, Seres, Spero, Eli Lilly, Gilead, Millenium, HeartRite, Aseptiscope, and Sprightly; these relationships were unrelated to the current work.
Ethics Approval and consent to participate
The study was approved by the Institutional Review Board of the University of California-Irvine under Protocol HS 2020–5818. All subjects were underwent electronic or written informed consent as outlined under IRB protocol HS 2020–5818. All methods and procedures were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Schubl, S.D., Figueroa, C., Palma, A.M. et al. Risk factors for SARS-CoV-2 seropositivity in a health care worker population during the early pandemic. BMC Infect Dis 23, 330 (2023). https://doi.org/10.1186/s12879-023-08284-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08284-y