Abstract
Objective
The preferred agent of glucocorticoids in the treatment of patients with severe COVID-19 is still controversial. This study aimed to compare the efficacy and safety of methylprednisolone and dexamethasone in the treatment of patients with severe COVID-19.
Methods
By searching the electronic literature database including PubMed, Cochrane Central Register of Controlled Trials, and Web of Science, the clinical studies comparing methylprednisolone and dexamethasone in the treatment of severe COVID-19 were selected according to the inclusion criteria and exclusion criteria. Relevant data were extracted and literature quality was assessed. The primary outcome was short-term mortality. The secondary outcomes were the rates of ICU admission and mechanical ventilation, PaO2/FiO2 ratio, plasma levels of C-reactive protein (CRP), ferritin, and neutrophil/lymphocyte ratio, hospital stay, and the incidence of severe adverse events. Statistical pooling applied the fixed or random effects model and reported as risk ratio (RR) or mean difference (MD) with the corresponding 95% confidence interval (CI). Meta-analysis was performed using Review Manager 5.1.0.
Results
Twelve clinical studies were eligible, including three randomized controlled trials (RCTs) and nine non-RCTs. A total of 2506 patients with COVID-19 were analyzed, of which 1242 (49.6%) received methylprednisolone and 1264 (50.4%) received dexamethasone treatment. In general, the heterogeneity across studies was significant, and the equivalent doses of methylprednisolone were higher than that of dexamethasone. Our meta-analysis showed that methylprednisolone treatment in severe COVID-19 patients was related to significantly reduced plasma ferritin and neutrophil/lymphocyte ratio compared with dexamethasone, and that no significant difference in other clinical outcomes between the two groups was found. However, subgroup analyses of RCTs demonstrated that methylprednisolone treatment was associated with reduced short-term mortality, and decreased CRP level compared with dexamethasone. Moreover, subgroup analyses observed that severe COVID-19 patients treated with a moderate dose (2 mg/kg/day) of methylprednisolone were related to a better prognosis than those treated with dexamethasone.
Conclusions
This study showed that compared with dexamethasone, methylprednisolone could reduce the systemic inflammatory response in severe COVID-19, and its effect was equivalent to that of dexamethasone on other clinical outcomes. It should be noted that the equivalent dose of methylprednisolone used was higher. Based on the evidence of subgroup analyses of RCTs, methylprednisolone, preferably at a moderate dose, has an advantage over dexamethasone in the treatment of patients with severe COVID-19.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), is considered to be the third outbreak of β coronaviruses in the twentieth and twenty-first centuries, after SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) [1]. As of 13 November 2022, 632 million confirmed COVID-19 cases and 6.5 million deaths have been reported globally [2]. Approximately 5% of patients with COVID-19 need transferring to the intensive care unit (ICU) for respiratory support and are associated with high mortality [3, 4]. The therapeutic drugs investigated for COVID -19, such as hydroxychloroquine [5], lopinavir/ritonavir [6], azithromycin [7], and ivermectin [8], could not bring definitely favorable clinical effects.
The pathophysiology of severe COVID-19 involves a host-mediated inflammatory response that leads to serious endothelial injury and alveolar damage [4, 9]. Many studies showed that patients with severe COVID-19 tended to have a higher level of inflammatory cytokines, including white blood cells, neutrophils, procalcitonin, C-reactive protein (CRP), interleukin, ferritin, and so on [3, 10, 11]. To prevent tissue damage caused by an excessive inflammatory response, glucocorticoid, as the classic anti-inflammatory drug, has been rapidly applied. The RECOVERY trial conducted in the UK has demonstrated that dexamethasone can significantly decrease mortality in cases with severe COVID-19, especially in patients receiving mechanical ventilation support, as compared to standard care without corticosteroids [12]. A multicenter randomized controlled trial (RCT) conducted by Villar et al. found that dexamethasone could reduce overall mortality and duration of mechanical ventilation in COVID-19 patients with moderate-to-severe acute respiratory distress syndrome (ARDS) [13]. Based on these studies, World Health Organization (WHO) recommends the use of systemic corticosteroids in patients with severe COVID-19 [14].
However, it is well known that the preferred glucocorticoid for the treatment of ARDS in ICU is methylprednisolone rather than dexamethasone. Several studies have revealed that compared with no glucocorticoid treatment, methylprednisolone also showed a mortality advantage in the treatment of severe COVID-19 [15,16,17]. Some researchers have compared the clinical effects of the two glucocorticoids during the current epidemic [18,19,20,21,22,23,24,25,26,27,28,29]. Nevertheless, the results are inconsistent.
The purpose of this meta-analysis was to compare the efficacy and safety of methylprednisolone and dexamethasone in the treatment of patients with severe COVID-19, and to provide evidence-based proof for the optimal management of COVID-19 pneumonia.
Materials and methods
This study was carried out in line with the Statement of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [30]. All stages of literature search, study selection, data extraction, and quality assessment were performed independently by two authors. Any disagreements between the two authors were resolved by discussion or arbitration by a third author.
Search strategy
The literature search was performed as we described previously [17]. The following electronic databases were searched: PubMed, Cochrane Central Register of Controlled Trials, and Web of Science. The following search strategy was used in PubMed and changes depending on the rules of each database: (COVID-19) AND (((corticosteroids) OR (methylprednisolone)) OR (dexamethasone)). The latest search was conducted on 10 August 2022. No language or geographical restrictions were applied during literature searches. All references cited in the relevant articles were screened to identify eligible studies.
Study selection
Studies comparing methylprednisolone with dexamethasone in the treatment of severe COVID-19 met the inclusion criteria of our meta-analysis. According to the guideline specified by the Chinese National Health Commission [23], severe COVID-19 is defined as meeting any of the following items: ①Respiratory rate ≥ 30 breaths per minute; ②Oxygen saturation ≤ 93% on room air at rest; ③Arterial oxygen pressure / inspired oxygen fraction ≤ 300 mmHg; ④Clinical symptoms are gradually aggravated, and the pulmonary imaging shows that the lesions progress more than 50% within 24–48 h. In cases of duplicates, the most recent or the most complete publication was used. Studies comparing methylprednisolone or dexamethasone with no corticosteroid therapy for patients with COVID-19 were excluded. Reviews, case reports, letters, editorials, and comparative studies which presented insufficient data were excluded.
Data extraction and quality assessment
The following information was extracted using standardized data extraction forms for each study: the first author’s last name; year of publication; study design; country; study interval; sample size, gender composition, mean age, intervention method in each group; inclusion criteria, primary outcome and other study features and data needed for quality assessment. Mean and standard deviation were used for extracting continuous variables. If studies reported continuous data as median and/or range values, the standard deviation was calculated using statistical algorithms by Hozo et al. [31]. The number of events and the total number of participants in each group were used for extracting binary variables. The primary outcome of our meta-analysis was short-term mortality, which involves in-hospital, 28-day, 30-day and 50-day mortality corresponding to the definition used in each study. The secondary outcomes included the rates of ICU admission and mechanical ventilation, PaO2/FiO2 ratio, systemic inflammatory markers (CRP, ferritin, and neutrophil/lymphocyte ratio), hospital stay, and the incidence of severe adverse events. The methodological quality of the RCTs was assessed according to the criteria specified by the Cochrane Collaboration [32]. For non-RCTs, the methodological index for non-randomized studies (MINORS) was used for quality assessment [33].
Statistical analysis
The outcomes were pooled as an estimate of the overall effect for the meta-analysis conducted using Review Manage, version 5.1.0 (The Cochrane Collaboration, 2011). As we previously reported [17, 34], for dichotomous variables, the pooled risk ratio (RR) with corresponding 95% confidence interval (CI) was aggregated in Mantel–Haenszel method, and the mean difference (MD) with corresponding 95% CI was calculated in inverse variance method for continuous variables. Clinical heterogeneity was discussed when appropriate. Subgroup analysis was performed on the basis of different types of study design, the daily dosage of methylprednisolone, and the treatment course of corticosteroids. Statistical heterogeneity was assessed by Cochran’s Q test and I2 statistic with p < 0.1 or I2 > 50% considered as significant. An I2 value of 0% indicates no observed statistical heterogeneity. If the statistical heterogeneity was not significant, then the fixed-effect model was used; otherwise, the random effects model would be applied. Sensitivity analysis was carried out, if applicable, by excluding studies to remove heterogeneity when it was statistically significant. A forest plot was constructed to graphically assess the statistical heterogeneity by displaying effect estimates and 95% CI for both individual studies and meta-analyses. The p value threshold for statistical significance was set at 0.05 for effect sizes. Publication bias was explored by Begg’s funnel plot and Egger’s regression test with p < 0.05 considered as significant (STATA 12.0).
Results
Study selection
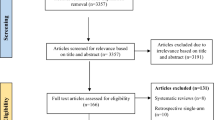
The flowchart of study selection is presented in Fig. 1. Overall, we identified 5281 citations by the initial literature searches. Among them, 710 were removed as duplicates, and 4513 were excluded after titles and abstracts screening. We retrieved 58 full-text articles for detailed evaluation. After reviewing, 43 references were excluded for the following reasons: reviews (n = 24), case reports (n = 10), letters (n = 8), and published erratum (n = 1). Then, 15 studies were included for qualitative synthesis. Of them, two studies [35, 36] in which the methylprednisolone group involved dexamethasone treatment were further excluded, and one study [37] providing insufficient data was also excluded. Finally, 9 non-RCTs [18,19,20,21,22,23,24,25,26] and 3 RCTs [27,28,29] matched the inclusion criteria and were suitable for our meta-analysis. A total of 2506 patients with severe COVID-19 were analyzed, of which 1242 (49.6%) received methylprednisolone and 1264 (50.4%) received dexamethasone treatment.
Study characteristics
The characteristics of the twelve included studies are summarized in Table 1. Three studies were conducted in Pakistan [18,19,20], two in Egypt [28, 29], and the remaining were respectively carried out from Italy [21], South Africa [22], Morocco [23], USA [24], Spain [25], Colombia [26] and Iran [27]. The study interval in each study ranged from March 2020 to October 2021. The percentage of females in each arm ranged from 24.0% to 53.3%, without significant difference between the groups (RR 0.89; 95% CI 0.75–1.04; p = 0.14). The mean age of the patients varied between 52 years and 74.4 years across the studies and was not significantly different between the two groups (MD -0.16 years; 95% CI − 1.47 to 1.15; p = 0.81). As shown in Table 1, the severity of COVID-19 patients included in all studies was at least severe. The doses and treatment courses of corticosteroids were not consistent across the studies, and the equivalent doses of methylprednisolone were higher than that of dexamethasone.
Quality assessment and publication bias
The methodological quality assessments of the included literature are briefly described in Table 1, and separately summarized in Supplementary Table 1 and Supplementary Fig. 1. Based on the MINORS scoring system, the scores of 9 non-RCTs ranged from 13 to 18 points. According to the criteria of risk of bias, the 3 RCTs were classified as low risk. Overall, the included studies were of moderate to high quality. Begg’s funnel plot constructed for the visual evaluation of publication bias revealed a slight asymmetry (Fig. 2). However, Egger’s regression test demonstrated that the visual asymmetry was not statistically significant (95% CI of intercept -6.32 to 5.31; p = 0.855) (Fig. 3).
Primary outcome
Ten included studies [18, 21,22,23,24,25,26,27,28,29] provided the data of short-term mortality after corticosteroids therapy. When these studies were pooled, the overall mortality was 30.2%. There were 293 deaths (25.0%) among 1170 patients receiving methylprednisolone and 421 deaths (35.3%) among 1191 patients receiving dexamethasone. Due to the significant heterogeneity across studies (p < 0.1, I2 = 91%), random effects model was applied. Our meta-analysis showed that the mortality was not significantly decreased in the methylprednisolone group compared with the dexamethasone group (RR 0.75; 95% CI 0.48–1.18; p = 0.21) (Fig. 4). This result was consistent with the subgroup analysis of non-RCTs, while in the subgroup analysis of RCTs, the mortality of the methylprednisolone group was significantly lower than that of the dexamethasone group (Fig. 4). In the subgroup analysis of shorter or longer courses of glucocorticoid treatment, the difference in mortality between methylprednisolone and dexamethasone was not significant (Supplementary Fig. 2). Besides, methylprednisolone at a dose of 1 mg/kg/day or > 2 mg/kg/day had the same effect on mortality as dexamethasone, while its dose of 2 mg/kg/day could significantly reduce the mortality of patients with severe COVID-19 (Supplementary Fig. 3).
Secondary outcomes
ICU admission
There were five non-RCTs [18, 20, 21, 25, 26] reporting the information about ICU admission. Overall, the rate of ICU admission in this analysis was 19.9%, with 110 cases (20.5%) in the methylprednisolone group and 95 cases (19.2%) in the dexamethasone group. Random effects model was used owing to a significant heterogeneity across studies (p < 0.1, I2 = 77%). Our pooling results revealed that the difference in ICU admission rate between the two groups was not statistically significant (RR 0.87; 95% CI 0.50–1.54; p = 0.64) (Fig. 5). This result was in accordance with the outcomes of subgroup analyses on different courses of glucocorticoids treatment (Supplementary Fig. 4).
Mechanical ventilation
Information regarding the need for mechanical ventilation after corticosteroids administration was described in seven studies [18, 22, 23, 25,26,27, 29]. In this analysis, the general rate of mechanical ventilation in patients with severe COVID-19 was 22.7%. The number of patients receiving mechanical ventilation was 156 (22.0%) in the methylprednisolone group and 188 (23.3%) in the dexamethasone group, respectively. Random effects model was used to synthesize the data because of a significant heterogeneity among studies (p < 0.1, I2 = 83%). No significant difference in mechanical ventilation rate between the groups was detected in our meta-analysis (RR 0.90; 95% CI 0.54–1.51; p = 0.69) (Fig. 6) and subgroup analyses of different treatment courses (Supplementary Fig. 5).
PaO2/FiO2 ratio
There were three studies [19, 26, 29] recording the data of the PaO2/FiO2 ratio after corticosteroids treatment. Random effects model was employed since the heterogeneity among studies was significant (p < 0.1, I2 = 79%). The difference between the treatments in PaO2/FiO2 ratio was not statistically significant in our meta-analysis (MD 3.08; 95% CI -7.23 to 13.40; p = 0.56) (Fig. 7) and subgroup analysis of non-RCTs. Since only one RCT provided data, subgroup analysis for RCTs cannot be performed. However, this RCT [29] showed the benefit of methylprednisolone in increasing the PaO2/FiO2 ratio.
Systemic inflammatory markers
Data about plasma CRP levels following corticosteroids application was collected from five studies [18, 23, 26, 28, 29]. As heterogeneity across studies was significant (p < 0.1, I2 = 99%), random effects model was adopted. Our meta-analysis found no significant difference in plasma CRP level between two groups (MD -20.02; 95% CI -43.36 to -3.32; p = 0.09) (Fig. 8). Nevertheless, subgroup analysis of RCTs showed that the plasma CRP level was significantly reduced in patients treated with methylprednisolone (Fig. 8).
Four studies [19, 26, 28, 29] measured the plasma ferritin value after the intervention. Random effects model was chosen due to a significant heterogeneity among studies (p < 0.1, I2 = 98%). Meta-analysis indicated that the plasma ferritin value was significantly lower in the methylprednisolone group than in the dexamethasone group (MD -124.43; 95% CI -218.44 to -30.41; p = 0.009), although subgroup analyses of RCTs and non-RCTs both implied no significant difference between two groups in this outcome (Fig. 9).
Two included RCTs [28, 29] calculated the neutrophil/lymphocyte ratio after steroids treatment. Meta-analysis on random effects model demonstrated that this ratio was significantly reduced in patients receiving methylprednisolone than in those receiving dexamethasone (MD -6.97; 95% CI -12.09 to -1.84; p = 0.008) (Fig. 10).
Hospital stay
There were four studies [21, 22, 25, 27] reporting the length of hospital stay. Owing to a significant heterogeneity among studies (p < 0.1, I2 = 97%), random effects model was used. Our meta-analysis observed that the length of hospital stay was not significantly different between the two groups (MD 0.13; 95% CI -1.38 to 1.64; p = 0.87) (Fig. 11). This finding was consistent with the result of subgroup analysis of non-RCT. Interestingly, an included RCT [27] supported the effect of methylprednisolone on shortening the length of hospitalization. However, due to the limited number of studies, subgroup analysis of RCTs cannot be conducted.
Severe adverse events
Data about hyperglycemia after corticosteroids treatment was recorded in four non-RCTs [18, 21, 23, 26]. Meta-analysis on random effects model demonstrated that the incidences of hyperglycemia in the methylprednisolone group (19.2%) and the dexamethasone group (21.9%) were comparable (RR 0.72; 95% CI 0.39–1.31; p = 0.28) (Fig. 12).
In our analysis, there were six studies [18, 21,22,23, 26, 29] providing the data of nosocomial infections. Overall, 89 (14.5%) cases treated with methylprednisolone and 117 (17.3%) cases treated with dexamethasone suffered from nosocomial infections. Random effects model was utilized due to a significant heterogeneity across studies (p < 0.1, I2 = 71%). This meta-analysis failed to show the significant difference in the incidence of nosocomial infections between the two groups (RR 0.85; 95% CI 0.43–1.66; p = 0.62) (Fig. 13).
Sensitivity analysis
We performed sensitivity analyses on all interested outcomes due to the significant heterogeneity among studies. However, sensitivity analyses did not change the significance of statistical heterogeneity and had no effect on the results of the meta-analysis, except for the incidence of hyperglycemia. After excluding the study by Fatima et al. [18], the statistical heterogeneity between studies was eliminated (p = 0.73, I2 = 0%). Sensitivity analysis indicated that the risk of hyperglycemia in the methylprednisolone group was significantly lower than that in the dexamethasone group (RR 0.61; 95% CI 0.44–0.85; p = 0.004) (Supplementary Fig. 6).
Discussion
This meta-analysis including 12 clinical studies found that methylprednisolone could significantly reduce the systemic inflammatory response in severe COVID-19 patients, and its effect was equivalent to that of dexamethasone on other clinical outcomes. Nevertheless, subgroup analyses of RCTs demonstrated that methylprednisolone treatment in severe COVID-19 was associated with reduced short-term mortality compared with dexamethasone. Moreover, severe COVID-19 patients treated with a moderate dose (2 mg/kg/day) of methylprednisolone were related to a better prognosis than those treated with dexamethasone.
It is reported that excessive inflammatory reaction is closely related to the severity of patients with severe COVID-19. In brief, when the body suffers from hyper-inflammatory reaction, immune cells such as T cells, macrophages and natural killer cells are over activated and proliferated. These cells produce a large number of inflammatory mediators, which cause damage to lung epithelium and endothelial cells. The consequence of this pathological change is the occurrence of ARDS, which is the main cause of death of patients with COVID-19 [38,39,40]. Thereby, according to this theory, the comprehensive treatment strategy of COVID-19 should include measures to resist hyperinflammation.
Glucocorticoids can inhibit the transcription and secretion of various cytokines, reduce systemic inflammation and exudate in lung tissue, and minimize the risk of respiratory failure [41]. They have been used previously in respiratory diseases such as asthma, chronic obstructive pulmonary disease, severe bacterial pneumonia, and ARDS. During the SARS epidemic, Zhao et al. [42] conducted a comparative study and observed that early administration of high-dose methylprednisolone was associated with reduced mortality compared with other treatment regimens. However, this mortality benefit of glucocorticoids did not appear in the MERS epidemic. Arabi et al. [43] reported in their study that corticosteroid therapy in critically ill patients with MERS was not associated with lower 90-day mortality but was related to delayed MERS-CoV RNA clearance, as compared to no corticosteroid therapy. Nevertheless, the mortality advantage of glucocorticoids has been found again in the current epidemic of coronavirus pneumonia [12]. A meta-analysis collected data from 7 randomized clinical trials to evaluate the effectiveness of glucocorticoids in 1703 critically ill patients with COVID-19 [44]. The results showed that administration of systemic corticosteroids was associated with lower 28-day all-cause mortality compared with usual care or placebo. Another meta-analysis including 44 studies and 20,197 patients with COVID-19 also demonstrated a beneficial effect of corticosteroids on short-term mortality [45].
Although this beneficial effect of glucocorticoids in severe COVID-19 is confirmed by WHO [14], it is still unclear which glucocorticoid drug is preferred. Some scholars suggest that corticosteroids in general are not expected to help as a class of drugs, but rather each steroid should be assessed individually because different drugs can be associated with a different number of genes [46]. Many published meta-analyses have evaluated the role of glucocorticoids in the COVID-19 epidemic, but they have not evaluated the comparison between glucocorticoids [45, 47,48,49,50,51].
The glucocorticoids used in the reported studies are mainly methylprednisolone and dexamethasone. For the treatment of severe patients, the National Health Commission of the China suggests methylprednisolone 0.5–1 mg/kg/day for 3–5 days [52], while the WHO and the National Institutes of Health of the United States recommend the use of dexamethasone 6 mg/day for 7–10 days [14, 53]. Buso et al. retrospectively analyzed 246 COVID-19 patients who needed oxygen supplementation and had received glucocorticoids therapy. They found that the choice of different glucocorticoids did not affect the main clinical outcomes [21]. In contrast, Ko et al. compared the effectiveness of methylprednisolone to dexamethasone in patients requiring intensive care, and demonstrated a mortality benefit in the methylprednisolone group [24]. This finding is consistent with the results of our subgroup analysis of RCTs, although our meta-analysis failed to confirm such mortality benefit of methylprednisolone. However, our meta-analysis observed that methylprednisolone was more effective in reducing the systemic inflammatory response than dexamethasone.
The reasons for such different clinical outcomes may be related to the inflammatory pathways and characteristics of the different glucocorticoids. Generally, the therapeutic effects of glucocorticoids can be mediated through genomic and more rapid-onset non-genomic mechanisms [54]. A study by Draghici et al. [46] described an initial characterization of the main pro-inflammatory pathways induced by SARS-Cov-2 infection on human lung epithelial cells, and detected methylprednisolone as the most effective agent that targets critical components of the inflammatory pathway responsible for ARDS. The study also suggested that methylprednisolone would revert the largest number of the gene perturbed by COVID-19, followed by dexamethasone. The non-genomic mechanism is dose-dependent with glucocorticoids, and the response of methylprednisolone was higher than that of dexamethasone in vitro [55, 56]. In addition, methylprednisolone could obtain a higher lung tissue-to-plasma ratio than dexamethasone in the animal model [57, 58]. From the perspective of mechanism, the combination of the above factors may make methylprednisolone more effective in reducing hyper-inflammation of the lung. In that case, the reduction of inflammation may lead to the rapid improvement of lung injury and easier relief of symptoms. The decrease of inflammatory markers found in this meta-analysis and the improvement of PaO2/FiO2 ratio and hospital stay shown in subgroup analyses just prove the advantage of methylprednisolone. These may partly explain the potential mortality advantage of methylprednisolone in the treatment of severe patients with COVID-19 [17]. Interestingly, we noticed in the subgroup analysis that such an advantage of methylprednisolone was associated with its dose of 2 mg/kg/day. We speculate that this may be related to the aforementioned dose-dependent characteristics of the hormones. Perhaps this moderate dose is the optimal dose of methylprednisolone for severe COVID-19 treatment.
It should be noted that the doses of the two glucocorticoids used in the included studies are not equivalent. The daily dose of methylprednisolone was at least 60 mg, if calculated according to the adult weight of 60 kg. In contrast, the dose of dexamethasone was 6–8 mg, equivalent to 32–42 mg of methylprednisolone. One may point out that this advantage of methylprednisolone is due to its high equivalent dose. However, there is no direct data in our meta-analysis to prove that the difference of outcomes is completely caused by the difference between the two hormone doses. The dose–effect of a hormone may be easily assessed, while the dose–effect between different types of hormones cannot be evaluated, because the factor in the characteristics of hormones cannot be completely excluded. We suggest that this issue needs further research.
When therapeutic drugs achieve good efficacy, safety is also a significant consideration. One of the adverse events of glucocorticoids treatment is hyperglycemia. Glucocorticoids can promote lipolysis, increase liver glucose output and raise insulin resistance, leading to elevation of blood glucose. In addition, patients receiving glucocorticoids therapy may also suffer from fluid retention, adrenal suppression, and secondary bacterial and fungal infections [54, 59]. In our meta-analysis, no difference was detected in the incidences of hyperglycemia and nosocomial infections between the two glucocorticoid treatments. Nevertheless, the results from sensitivity analysis suggest that methylprednisolone is safer because the incidences of adverse events in this group were significantly lower than that in the dexamethasone group.
It has been reported that the use of glucocorticoids would delay viral clearance in patients with COVID-19 [60, 61]. However, evidence from a recently published meta-analysis showed that low-dose corticosteroids did not have a significant impact on the duration of SARS-CoV-2 viral shedding [62]. The retrospective study by Buso et al. discovered no significant difference in virus clearance between the two glucocorticoids, while the time of virus clearance in the methylprednisolone group tended to be relatively shorter [21]. Although our meta-analysis showed a benefit of methylprednisolone in reducing inflammatory markers, whether it has an impact on virus clearance at high equivalent doses remains unresolved. To say the least, even if glucocorticoids are not conducive to virus clearance, their potential survival benefit in severe COVID-19 will outweigh the risk of prolonged viral shedding.
It is worth noting that the safety of glucocorticoids discussed in this meta-analysis is only for ordinary adult patients with COVID-19, but not for some special populations, such as pregnant women, because there is a lack of research in such groups that requires more strict ethics and highly careful study design [63].
Coincidentally, some systematic reviews [64,65,66] on the same topic have been published recently. One review [65] published in the form of "Letters" also included the three RCTs analyzed in our study. The information provided in that article was relatively incomplete and only three outcomes were analyzed. The authors of that article carried out another review [66] on non-RCTs, however, the number of included studies was relatively small. The evidence in the present study is significantly different from that in these reviews. In contrast, the number of included studies and the total sample size in our meta-analysis are larger. We believe that the larger the sample size, the lower the probability of false-negative results, and the more reliable the conclusions. Moreover, our meta-analysis investigated more interested outcomes, which is more meaningful for clinical guidance.
There are limitations to the present meta-analysis which need to be mentioned. First, the number of RCTs included in the meta-analysis is relatively restricted. We are aware that the pooling of data from non-RCTs is a debated topic in the field of meta-analysis as it may exaggerate the effect magnitude of an intervention. However, during the current COVID-19 epidemic, RCTs comparing methylprednisolone and dexamethasone in the treatment of severe COVID-19 are scarce. Many people are still infected and die every day, which creates a great threat to human society. In order to find valuable evidence-based proof in the treatment of severe COVID-19 as soon as possible, we collected non-RCTs for meta-analysis to increase the sample size as much as possible. In this way, the possibility of false-negative results can be minimized. Second, we are aware that there is a significant clinical heterogeneity across the included studies. Clinical factors such as the study design, the inconsistent inclusion criteria, and the dosage and course of glucocorticoids treatment of each study may have inordinately influenced the results of this systematic analysis. Given this consideration, we chose the random effects model for data synthesis and performed subgroup analyses to minimize the interference of these factors. Third, the meta-analysis of partial outcomes is based on a limited number of studies, which may reduce the power of the results. However, this does not mean that these results have little clinical value. We hope that the findings of this study may attract the attention of clinicians who use glucocorticoids to treat severe patients, and provide reference for similar clinical studies in the future. Despite this, we emphasize that these results need to be interpreted with caution. Finally, we note that some RCTs [27, 29] have shown the advantages of methylprednisolone, such as increasing PaO2/FiO2 ratio, shortening hospital stay, and reducing nosocomial infections. Therefore, RCTs with a larger sample size are required to confirm the effectiveness and safety of methylprednisolone in the treatment of patients with severe COVID-19.
Conclusions
The present meta-analysis showed that compared with dexamethasone, methylprednisolone could significantly reduce the systemic inflammatory response in severe COVID-19 patients, and its effect was equivalent to that of dexamethasone on other clinical outcomes. It should be noted that the heterogeneity across studies was significant, and the equivalent dose of methylprednisolone used was relatively higher. Based on the evidence of subgroup analyses of RCTs, it is believed that methylprednisolone treatment has the potential to improve the prognosis of patients with severe COVID-19, which needs to be verified by high-quality RCTs with a larger sample size.
Availability of data and materials
The datasets analyzed during the present study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CI:
-
Confidence interval
- CRP:
-
C-reactive protein
- ICU:
-
Intensive care unit
- MD:
-
Mean difference
- MINORS:
-
Methodological index for non-randomized studies
- MERS-CoV:
-
Middle East respiratory syndrome coronavirus
- RCT:
-
Randomized controlled trial
- RR:
-
Risk ratio;
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- WHO:
-
World Health Organization
References
Izda V, Jeffries MA, Sawalha AH. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin Immunol. 2021;222:108634.
World Health Organization. Weekly epidemiological update on COVID-19 - 16 November 2022. World Health Organization, 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---16-november-2022. Accessed 20 Nov 2022.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506.
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A Review. JAMA. 2020;324(8):782–93.
Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, et al. Effect of Hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030–40.
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–99.
Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo L, Veiga VC, Avezum A, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383(21):2041–52.
Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. Bmc Infect Dis. 2021;21(1):635.
Kumar M, Al KS. Pathophysiology and treatment strategies for COVID-19. J Transl Med. 2020;18(1):353.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20.
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323(11):1061–9.
Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–76.
World Health Organization. Corticosteroids for COVID-19. World Health Organization, 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1. Accessed 9 Nov 2020.
Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808.
Hamed DM, Belhoul KM, Al MN, Ghayoor F, Moin M, Al SM, et al. Intravenous methylprednisolone with or without tocilizumab in patients with severe COVID-19 pneumonia requiring oxygen support: A prospective comparison. J Infect Public Health. 2021;14(8):985–9.
Hong S, Wang H, Zhang Z, Qiao L. The roles of methylprednisolone treatment in patients with COVID-19: A systematic review and meta-analysis. Steroids. 2022;183:109022.
Fatima SA, Asif M, Khan KA, Siddique N, Khan AZ. Comparison of efficacy of dexamethasone and methylprednisolone in moderate to severe covid 19 disease. Ann Med Surg (Lond). 2020;60:413–6.
Rana MA, Hashmi M, Qayyum A, Pervaiz R, Saleem M, Munir MF, et al. Comparison of efficacy of dexamethasone and methylprednisolone in improving PaO2/FiO2 ratio among COVID-19 patients. Cureus. 2020;12(10):e10918.
Aslam J, Masroor M, Ahmad S, Hussain W, Mansoor H, Khan L. Efficacy of methylprednisolone with dexamethasone in patients with severe Covid pneumonia in term of clinical and biochemical improvement. Pakistan J Med Health Sci. 2021;15(10):2635–6.
Buso R, Cinetto F, Dell’Edera A, Veneran N, Facchini C, Biscaro V, et al. Comparison between dexamethasone and methylprednisolone therapy in patients with COVID-19 pneumonia admitted to non-intensive medical units. J Clin Med. 2021;10(24):5812.
Du Plessis EM, Lalla U, Allwood BW, Louw EH, Nortje A, Parker A, et al. Corticosteroids in critical COVID-19: are all corticosteroids equal? S Afr Med J. 2021;111(6):550–3.
El MS, El AG, Merbouh M, El KA, Aftiss FZ, Berrichi S, et al. Dexamethasone or methylprednisolone therapy in covid-19 pneumonia: a retrospective and comparative study of 513 cases. Ann Med Surg (Lond). 2021;70:102858.
Ko JJ, Wu C, Mehta N, Wald-Dickler N, Yang W, Qiao R. A Comparison of methylprednisolone and dexamethasone in intensive care patients with COVID-19. J Intensive Care Med. 2021;36(6):673–80.
Mora-Lujan JM, Tuells M, Montero A, Formiga F, Homs NA, Alba-Albalate J, et al. High-Dose Methylprednisolone Pulses for 3 days vs. low-dose dexamethasone for 10 days in severe, non-critical COVID-19: a retrospective propensity score matched analysis. J Clin Med. 2021;10(19):4465.
Pinzon MA, Ortiz S, Holguin H, Betancur JF, Cardona AD, Laniado H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS ONE. 2021;16(5):e252057.
Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. Bmc Infect Dis. 2021;21(1):337.
Saeed M, Mohamed AH, Owaynat AH. Comparison between methylprednisolone infusion and dexamethasone in COVID-19 ARDS mechanically ventilated patients. Egypt J Intern Med. 2022;34(1):19.
Soliman OM, Moeen SM, Abbas YA, Kamel EZ. The impact of dexamethasone versus methylprednisolone upon neutrophil/lymphocyte ratio in COVID-19 patients admitted to ICU and its implication upon mortality. Egypt J Anaesthesia. 2022;38(1):78–84.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Chou R, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. Bmc Med Res Methodol. 2005;5:13.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://handbook.cochrane. org. Accessed 5 May 2013.
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. Anz J Surg. 2003;73(9):712–6.
Hong S, Wang H, Tian Y, Qiao L. The roles of noninvasive mechanical ventilation with helmet in patients with acute respiratory failure: a systematic review and meta-analysis. PLoS ONE. 2021;16(4):e250063.
Salvarani C, Massari M, Costantini M, Franco MD, Lucia MG, Viale P, et al. Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia, A double-blind, randomised, placebo-controlled trial. Eur Respir J. 2022;60(4):2200025.
Dafni M, Karampeli M, Michelakis I, Manta A, Spanoudaki A, Mantzos D, et al. Treatment with 3-day methylprednisolone pulses in severe cases of COVID-19 compared with the standard regimen protocol of dexamethasone. J Investig Med. 2022;70(6):1423–8.
Krishnaswamy P, Bs N, Hegde SV. A Comparison of the efficacy of adjuvant parenteral methylprednisolone and dexamethasone in reducing Covid-19 disease severity and mortality among the moderate to critical patients -a retrospective study. J Assoc Physicians India. 2022;70(4):11–2.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–2.
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–13.
Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–8.
Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–23.
Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou. PR China J Med Microbiol. 2003;52(Pt 8):715–20.
Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, et al. Corticosteroid therapy for critically ill patients with middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197(6):757–67.
Sterne J, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–41.
van Paassen J, Vos JS, Hoekstra EM, Neumann K, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24(1):696.
Draghici S, Nguyen TM, Sonna LA, Ziraldo C, Vanciu R, Fadel R, et al. COVID-19: disease pathways and gene expression changes predict methylprednisolone can improve outcome in severe cases. Bioinformatics. 2021;37(17):2691–8.
Langarizadeh MA, Ranjbar TM, Abiri A, Ghasempour A, Rezaei M, Ameri A. A review on function and side effects of systemic corticosteroids used in high-grade COVID-19 to prevent cytokine storms. Excli J. 2021;20:339–65.
Dolci G, Cassone G, Venturelli F, Besutti G, Revelli M, Corsini R, et al. High-dose glucocorticoids pulse-therapy for beta-coronaviridae pneumonia: a systematic literature review and case-series of Coronavirus disease-2019. Clin Exp Rheumatol. 2021;39(5):1119–25.
Raju R, V P, Biatris PS, J SJUC. Therapeutic role of corticosteroids in COVID-19: a systematic review of registered clinical trials. Futur J Pharm Sci. 2021;7(1):67.
Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8:D14963.
Yu GQ, Jiang ZH, Yang ZB, Jiang SQ, Quan XQ. The effect of glucocorticoids on mortality in severe COVID-19 patients: Evidence from 13 studies involving 6612 cases. Medicine (Baltimore). 2021;100(40):e27373.
National Health Commission of the People's Republic of China. Diagnosis and treatment plan for new coronavirus pneumonia (trial version 8). National Health Commission of the People's Republic of China, 2020. http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a.shtml. Accessed 25 Aug 2020.
National Institutes of Health. COVID-19 treatment guidelines, corticosteroids. National Institutes of Health, 2020. https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/. Accessed 25 Oct 2020.
Spies CM, Strehl C, van der Goes MC, Bijlsma JW, Buttgereit F. Glucocorticoids. Best Pract Res Clin Rheumatol. 2011;25(6):891–900.
Meduri GU, Siemieniuk R, Ness RA, Seyler SJ. Prolonged low-dose methylprednisolone treatment is highly effective in reducing duration of mechanical ventilation and mortality in patients with ARDS. J Intensive Care. 2018;6:53.
Ayyar VS, Jusko WJ. Transitioning from basic toward systems pharmacodynamic models: lessons from corticosteroids. Pharmacol Rev. 2020;72(2):414–38.
Annane D, Pastores SM, Arlt W, Balk RA, Beishuizen A, Briegel J, et al. Critical illness-related corticosteroid insufficiency (CIRCI): a narrative review from a Multispecialty Task Force of the Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM). Intensive Care Med. 2017;43(12):1781–92.
Ayyar VS, Song D, DuBois DC, Almon RR, Jusko WJ. Modeling corticosteroid pharmacokinetics and pharmacodynamics, Part I: determination and prediction of dexamethasone and methylprednisolone tissue binding in the rat. J Pharmacol Exp Ther. 2019;370(2):318–26.
Oray M, Abu SK, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016;15(4):457–65.
Huang R, Zhu C, Jian W, Xue L, Li C, Yan X, et al. Corticosteroid therapy is associated with the delay of SARS-CoV-2 clearance in COVID-19 patients. Eur J Pharmacol. 2020;889:173556.
Tang X, Feng YM, Ni JX, Zhang JY, Liu LM, Hu K, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind. Randomized Control Trial Resp. 2021;100(2):116–26.
Cano EJ, Fonseca FX, Corsini CC, O’Horo JC, Abu SO, Odeyemi Y, et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest. 2021;159(3):1019–40.
Fogacci S, Fogacci F, Favari E, Toth PP, Borghi C, Cicero A. Management of pregnancy-related hypertensive disorders in patients infected with SARS CoV-2: pharmacological and clinical issues. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):346–51.
Mohanty RR, Das S, Padhy BM, Meher BR. Comparison of clinical outcome between dexamethasone and methyl prednisolone in treatment of moderate to severe COVID-19: a systematic review and meta-analysis. J Assoc Physicians India. 2022;70(1):11–2.
Beran A, Ayesh H, Mhanna M, Srour O, Musallam R, Sayeh W, et al. Methylprednisolone may be superior to dexamethasone in COVID-19: a meta-analysis of randomized controlled trials. Am J Ther. 2022;29(3):e351–4.
Beran A, Ayesh H, Mhanna M, Srour O, Musallam R, Sayeh W, et al. Methylprednisolone versus dexamethasone in COVID-19: a meta-analysis of nonrandomized studies. Am J Ther. 2022;29(3):e354–7.
Acknowledgements
Not applicable.
Funding
This work was supported by Medicine and Health Science Technology Development Plan Project of Shandong Province. Grant number: 2019WS042. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
SH and HW contributed equally to this work. SH designed the study protocol. SH and HW performed the literature search and study selection. SL and JL performed data extraction and quality assessment. SH and LQ performed the statistical analyses. SH and HW drafted the manuscript. All authors carefully revised the manuscript and approved the final version for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA checklist.
Additional file 2: Supplementary Table 1.
Score of methodological items for non-randomized studies (MINORS). Supplementary Figure 1. Summary of risk of bias for randomized controlled trials. Supplementary Figure 2. Subgroup analysis of different courses of glucocorticoids treatment for the comparison of short-term mortality. Supplementary Figure 3. Subgroup analysis of different doses of methylprednisolone for the comparison of short-term mortality. Supplementary Figure 4. Subgroup analysis of different courses of glucocorticoids treatment for the comparison of ICU admission rate. Supplementary Figure 5. Subgroup analysis of different courses of glucocorticoids treatment for the comparison of mechanical ventilation rate. Supplementary Figure 6. Sensitivity analysis for the comparison of hyperglycemia rate.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hong, S., Wang, H., Li, S. et al. A systematic review and meta-analysis of glucocorticoids treatment in severe COVID-19: methylprednisolone versus dexamethasone. BMC Infect Dis 23, 290 (2023). https://doi.org/10.1186/s12879-023-08280-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08280-2