Abstract
Background
Neutralizing monoclonal antibodies (mAbs) are highly effective in reducing hospitalization and mortality among early symptomatic COVID-19 patients in clinical trials and real-world data. While resistance to some mAbs has since emerged among new variants, characteristics associated with treatment failure of mAbs remain unknown.
Methods
This multicenter, observational cohort study included patients with COVID-19 who received mAb treatment between November 20, 2020, and December 9, 2021. We utilized electronic health records from a statewide health system plus state-level vaccine and mortality data. The primary outcome was mAb treatment failure, defined as hospitalization or death within 28 days of a positive SARS-CoV-2 test.
Results
COVID-19 mAb was administered to 7406 patients. Hospitalization within 28 days of positive SARS-CoV-2 test occurred in 258 (3.5%) of all patients who received mAb treatment. Ten patients (0.1%) died within 28 days, and all but one were hospitalized prior to death. Characteristics associated with treatment failure included having two or more comorbidities excluding obesity and immunocompromised status (adjusted odds ratio [OR] 3.71, 95% confidence interval [CI] 2.52–5.56), lack of SARS-CoV-2 vaccination (OR 2.73, 95% CI 2.01–3.77), non-Hispanic black race/ethnicity (OR 2.21, 95% CI 1.20–3.82), obesity (OR 1.79, 95% CI 1.36–2.34), one comorbidity (OR 1.68, 95% CI 1.11–2.57), age ≥ 65 years (OR 1.62, 95% CI 1.13–2.35), and male sex (OR 1.56, 95% CI 1.21–2.02). Immunocompromised status (none, mild, or moderate/severe), pandemic phase, and type of mAb received were not associated with treatment failure (all p > 0.05).
Conclusions
Comorbidities, lack of prior SARS-CoV-2 vaccination, non-Hispanic black race/ethnicity, obesity, age ≥ 65 years, and male sex are associated with treatment failure of mAbs.
Similar content being viewed by others
Introduction
Persistent surges of coronavirus disease 2019 (COVID-19) necessitate novel therapeutics, especially for unvaccinated persons, those with waning vaccine immunity, or older adults with chronic medical conditions [1]. Neutralizing monoclonal antibodies (mAbs) are widely seen as an important tool for managing surge caseloads. They provide immediate, passive immunity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the virus that causes COVID-19. Phase III clinical trials [2,3,4,5] and real-world data [6,7,8] demonstrated the effectiveness of neutralizing mAbs in reducing hospitalization and mortality among early symptomatic COVID-19 patients. Based on the strength of these trials, the US Food and Drug Administration (FDA) currently recommends mAb therapy for non-hospitalized patients when both ritonavir-boosted nirmatrelvir (Paxlovid) and remdesivir are not available, feasible to use, or clinically appropriate [9].
While some mAbs have lost effectiveness due to resistance of newer variants [10, 11], other mAb agents and antivirals have maintained effectiveness. When these agents are effective, hospitalization or death (e.g., treatment failure) occurs infrequently but are important outcome measures to understand which patients might be less likely to benefit from mAb therapies. These data could inform alternate treatment, combination therapy, or intensified follow-up. Prior studies did not observe enough adverse events to analyze the factors associated with treatment failure [4, 5, 12]. Our group established a real-world evidence platform in 2021 to assess the ongoing clinical impact of mAb therapies in high-risk outpatients with early symptomatic COVID-19. We recently reported the effectiveness of mAbs in significantly reducing hospitalization and 28-day mortality among such outpatients [6, 7]. Patient characteristics associated with treatment failure of mAbs, however, remain unknown.
Accordingly, our objective was to evaluate the characteristics associated with treatment failure among high-risk outpatients treated with mAbs during different pandemic phases, such as Alpha and Delta, and broad SARS-CoV-2 vaccination. We included patients prior to the emergence of resistance to many mAbs by newer variants such as Omicron.
Methods
Study oversight and data sources
We completed a secondary analysis of a multicenter observational cohort study collaborating with leaders from the University of Colorado Hospital, University of Colorado Health (UCHealth), and the Colorado Department of Public Health and Environment (CDPHE). The study was approved by the Colorado Multiple Institutional Review Board (COMIRB) with a waiver of informed consent. We previously reported methods for data collection [7]. In brief, we accessed patient data from the electronic health record (EHR; Epic, Verona, WI) of UCHealth, the largest health system in Colorado. UCHealth consists of 13 hospitals across the state and accounts for approximately 141,000 annual hospital admissions. Data from the EHR were merged with statewide data on vaccination status from the Colorado Comprehensive Immunization Information System and mortality from Colorado Vital Records.
Patient population studied
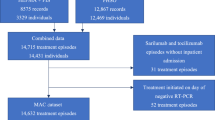
We included adult patients (≥ 18 years of age) diagnosed with SARS-CoV-2 between November 20, 2020, and December 9, 2021, who received mAb treatment within 10 days of a positive SARS-CoV-2 test (n = 7974). We identified patients using EHR-based date of SARS-CoV-2 positive testing (by polymerase chain reaction or antigen tests) or date of administration of mAb treatment (if no SARS-CoV-2 test result date was available). The decision to utilize mAb treatment was made by patients and clinicians. The CDPHE established a statewide referral system to facilitate patient referrals to facilities for mAb infusion [13]. We excluded patients who died before their SARS-CoV-2 test results, patients missing both a mAb administration date and a SARS-CoV-2 positive date, and patients who tested positive either while in the hospital or on the same day as admission as these patients were not eligible to receive mAbs and not at risk for the primary study outcome. We also excluded patients with a positive SARS-CoV-2 test after December 9, 2021, to avoid comparing patients infected with the Delta variant with those infected with the Omicron variant, which had a much lower rate of hospitalization or death [14]. We also excluded patients who had more than 10 days between their SARS-CoV-2 positive test and the date of mAb administration, in accordance with the FDA’s Emergency Use Authorization (EUA) criteria (Fig. 1). For patients who were missing a documented SARS-CoV-2 test date, a test date was imputed based on the distribution of observed times between SARS-CoV-2 positive test to mAb administration.
Outcomes
The primary outcome was mAb treatment failure, defined as hospitalization or death within 28 days of a positive SARS-CoV-2 test obtained from EHR data. Hospitalization was defined as an inpatient or observation encounter documented in the EHR. Death was defined as all-cause mortality whether the patient was hospitalized or not.
Variable definitions
The presence and status of comorbid conditions (cardiovascular disease, hypertension, pulmonary disease, renal disease, diabetes) were determined using the Charlson and Elixhauser comorbidity indices [15] for EHR data and a 90-day lookback period. Immunocompromised status was further validated by manual chart reviews and was categorized as “Not Immunocompromised,” “Mild,” or “Moderate/Severe,” based on chronic medications or specific conditions. Mild criteria included the administration of tumor necrosis factor-alpha inhibitors, or azathioprine, as well as human immunodeficiency virus (HIV) without acquired immunodeficiency syndrome (AIDS). Moderate/Severe patients were NIH tier 1 immunocompromised individuals, including those receiving chemotherapeutic agents or antirejection medications, and HIV with AIDS (Additional file 5: Table S1). If a patient met at least one defining element for “Moderate/Severe,” they were categorized as “Moderate/Severe” immunocompromised. Systemic corticosteroids, excluding dexamethasone, were classified as “Mild” immunocompromised. The number of comorbid conditions was created by summing all conditions excluding immunocompromised status or obesity because these two were the key pre-specified conditions we chose to evaluate. Pandemic phase was divided based on SARS-CoV-2 positive date and in accordance with the prevalent variant in Colorado. Phases included Pre-Alpha (November 2020–February 2021), Alpha (March 2021–June 2021), and Delta (June 2021–December 2021). Virus sequencing results were not available at an individual patient level. Vaccination status at the time of SARS-CoV-2 positive date was categorized by the number of vaccine doses received (zero, one, two, or more) at least 2 weeks before infection. mAb treatments included bamlanivimab (Eli Lilly), casirivimab + imdevimab (Regeneron), bamlanivimab + etesevimab (Eli Lilly), and sotrovimab (GlaxoSmithKline).
Statistical analysis
Patient characteristics by treatment failure status were compared using t-tests and Chi-squared test statistics, as appropriate. Due to the infrequent nature of our primary outcome, Firth’s bias-reduced multiple logistic regression model was used to investigate the association between patient risk factors and treatment failure [16]. For each of the risk factors in the model, we computed the adjusted odds ratio (OR) and 95% confidence intervals (95% CI) via penalized profile likelihood. A separate model was fitted for each risk factor along with a final multivariable model including age, sex, race/ethnicity, insurance status, presence of obesity, insurance status, immunocompromised status, number of comorbidities, pandemic phase, and vaccination status to adjust association estimates for potential confounding effects between risk factors. In addition, we computed the absolute risk difference along with 95% CI. Kaplan–Meier curves were estimated to visually assess temporal trends in cumulative incidence by treatment status for secondary outcomes.
We performed two sensitivity analyses. For the first, we employed a more conservative imputation method for missing SARS-CoV-2 positive test dates by assuming all missing positive test dates were 10 days prior to the mAb administration date (the maximum time difference allowed by the EUA). Second, we included only patients with complete dates for both SARS-CoV-2 positive test and mAb administration dates verified by EHR data. All statistical analyses were performed using R Statistical Software (version 3.6.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Treatment failure of mAbs
A total of 7406 patients with confirmed SARS-CoV-2 infection received mAbs between November 20, 2020, and December 9, 2021. A CONSORT flow diagram of applied exclusion criteria is presented in Fig. 1. We excluded 47 patients who died before their SARS-CoV-2 test results, 377 patients missing both a mAb administration date and a SARS-CoV-2 positive date, and 109 patients who tested positive for SARS-CoV-2 either while in the hospital or on the same day as admission. We also excluded 35 patients who had more than 10 days between their SARS-CoV-2 positive test and the date of mAb administration, in accordance with EUA criteria. Treatment failure within 28 days of a positive SARS-CoV-2 test occurred in 258 (3.5%) patients who received mAbs. Ten patients (0.1%) died within 28 days of their positive test, and all but one of these patients were hospitalized prior to death. The maximum level of oxygenation support required during the index hospitalization for the 258 patients who experienced treatment failure is displayed in Additional file 1: Figure S1.
Characteristics of hospitalized and not-hospitalized cohorts
Patients who experienced treatment failure were older (47% vs. 33% were age ≥ 65 years), more likely to be male (55% vs. 44%), more likely to be obese (46% vs. 27% with body mass index [BMI] ≥ 30 kg/m2), more likely to be at least mildly immunocompromised (37% vs. 23%) and more likely to have two or more additional comorbidities (63% vs. 30%) (Table 1).
Primary analysis
All patient risk factors were significantly associated with treatment failure during initial (unadjusted) analysis (Table 1). After accounting for potential confounding of insurance status and different comorbid conditions, the following risk factors remained as significant independent predictors of treatment failure: age ≥ 65 years old compared to those younger than 45 years old (adjusted OR 1.62, 95% CI 1.13–2.35, p = 0.008), male versus female sex (OR 1.56, 95% CI 1.21–2.02, p = 0.001), Non-Hispanic black race versus non-Hispanic white race (OR 2.21, 95% CI 1.20–3.82, p = 0.013), and obesity (OR 1.79, 95% CI 1.36–2.34, p < 0.001) (Fig. 2). The cumulative number of additional comorbid conditions was also significantly associated with hospitalization: patients with one additional comorbidity had OR 1.68 (95% CI 1.11–2.57, p = 0.014), and those with two or more additional comorbidities had OR 3.71 (95% CI 2.52–5.56, p < 0.001) compared to those with no additional comorbidities. Compared to patients who received two or more doses of vaccines, those who received no SARS-CoV-2 vaccine had a nearly three times higher likelihood of experiencing treatment failure (adjusted OR 2.73, 95% CI 2.01–3.77, p < 0.001).
Adjusted risk difference and adjusted odds ratio (OR) for treatment failure for each risk factor from the full model. Risk differences were calculated via Firth’s bias-reduced multiple regression logistic regression. Adjusted ORs and 95% confidence intervals (95% CI) were computed by penalized profile likelihood
Presence of at least mild immunocompromised status was significantly more likely in hospitalized patients compared to non-hospitalized patients in univariate analysis (37% vs. 23% in Table 1; unadjusted OR 2.02, 95% CI 1.55–2.61, p < 0.001). However, after adjusting for other risk factors, neither mild immunocompromised status (OR 1.39, 95% CI 0.94–1.99) nor moderate/severe immunocompromised status (OR 1.36, 95% CI 0.97–1.91) was significantly associated with treatment failure, compared to non-immunocompromised patients (Fig. 2). Pandemic phase and type of mAb received were also not associated with treatment failure. The cumulative incidence of hospitalization stratified by immunocompromised status is displayed in Additional file 2: Figure S2, by sex in Fig. 3, and by patient age in Fig. 4. After adjusting for relevant characteristics, increasing age was significantly associated with treatment failure; however, age 45–64 years was not (Fig. 2).
Sensitivity analyses
We first employed a more conservative imputation method for missing SARS-CoV-2 positive test dates by assuming all missing positive test dates were 10 days prior to the mAb administration date (the maximum time difference allowed by the EUA). In that sensitivity analysis, we found that Hispanic ethnicity (adjusted OR 1.63, 95% CI 1.13–2.31, p = 0.01) and moderate/severe immunocompromised status (OR 1.42, 95% CI 1.02–1.97; p = 0.04) were significantly associated with hospitalization (Additional file 3: Figure S3). We did not observe any other differences between our primary analysis and this analysis for other risk factors. In this analysis, the overall sample size changed slightly because SARS-CoV-2 positive test date was used in defining our initial eligibility criteria. For the second sensitivity analysis, we included only patients with confirmed dates for both SARS-CoV-2 positive test and mAb administration. In this cohort, we found that age ≥ 65 years was not associated with treatment failure (OR 1.65, 95% CI 0.98–2.82), and patients who received only one dose of SARS-CoV-2 vaccine had a higher risk of experiencing treatment failure compared to those who had two or more doses of vaccine (OR 2.55, 95% CI 1.19–5.16, p = 0.02) (Additional file 4: Figure S4). No other differences were observed between this analysis and our primary analysis.
Discussion
Our study, using real-world data in the pre-Omicron variant period, demonstrates novel results that age ≥ 65 years, male sex, non-Hispanic black race/ethnicity, obesity, increasing number of comorbidities, and lack of prior SARS-CoV-2 vaccination are significantly associated with treatment failure (hospitalization or death) following treatment of COVID-19 with mAbs. While some neutralizing mAbs have lost effectiveness to Omicron and its sub-variants, mAbs that have maintained effectiveness are widely regarded as critical tools in combating surges in COVID-19 cases. However, previous studies have not evaluated the patient characteristics associated with treatment failure of mAbs. Our present study fills this key knowledge gap.
Hospitalization among all patients who received mAbs was uncommon (3.5%), as was 28-day mortality (0.1%). However, these patients represent an important subgroup. Identifying patients more likely to experience mAb treatment failure helps to identify those who may benefit from alternative treatment or additional follow-up. For instance, some patients may require other antiviral treatments—either as an alternative or in combination with mAb treatment. These alternative strategies could be particularly relevant during periods of hospital and ICU strain during the Covid-19 pandemic [17]. To our knowledge, our study is the first to address this critical gap in the understanding of treatment failure of mAbs. Our large sample size affords a high degree of precision in our estimates and may assist clinicians in making treatment decisions based on patient characteristics [18].
Treatment failure was progressively less likely among patients who had received more SARS-CoV-2 vaccine doses. Notably, the odds of 28-day hospitalization among mAb-treated patients who received two (or more) vaccine doses was approximately one-third that of patients who received zero doses (i.e., unvaccinated). These data support the policy that SARS-CoV-2 vaccination should remain the first-line intervention to prevent Covid-19 hospitalization. Treatment with mAbs should therefore be reserved as supplemental therapy for breakthrough COVID-19 in the high-risk patients highlighted in our study.
Interestingly, immunocompromised status was not significantly associated with treatment failure. The presence of immunocompromising conditions has previously been associated with worsened disease severity among Covid-19 patients [19,20,21]. Univariate analysis did reveal a strong association between at least mild immunocompromised status and treatment failure (OR 2.02, 95% CI 1.55–2.61). One of two sensitivity analyses demonstrated an association between moderate/severe immunocompromised status and treatment failure (OR 1.42, 95% CI 1.02–1.97) (Additional file 3: Figure S3). However, there appears to be collinearity between an immunocompromised status and other comorbidities, most notably cardiovascular disease (OR 12.98, 95% CI 11.31–14.91). This relationship could make sense clinically as patients with a compromised immune system may be more likely to have co-existing disease processes. However, understanding the etiology of this relationship is beyond the scope of our study. Notably, our pre-specified definition of comorbidities did not include immunocompromised status or obesity because these were key pre-specified subgroups to evaluate separately.
Limitations
Our study has several potential limitations. We analyzed patients from a single health care system. While this large system encompasses both urban and rural settings, and community and academic hospitals, it is located entirely within one US state. Ethnic and racial minority representation was lower than national averages, which potentially limited our ability to detect differences across these subgroups. Duration of immunocompromised status was not captured; therefore, contrasting timing or duration of immunocompromising conditions with regard to treatment failure was not possible. Since our study was observational, associations may have been influenced by unmeasured confounding. Data for mortality and vaccination status were collected using a statewide database, but hospitalizations were only collected within the single health care system. Therefore, discrepancies between mAb-treated patients and hospitalization status (i.e., patients who were hospitalized outside of the health care system) may exist. In addition, death was rare, and only one additional patient died without evidence of hospital admission. However, the composite approach to treatment failure—including both hospitalization and death—is most clinically relevant. Clinical indication for hospital admission was not captured. Therefore, it is possible some of the hospitalized patients were admitted for non-COVID-19 indications. Finally, we included patients prior to the emergence of the Omicron variant. This variant has proven resistant to treatment with many mAbs [22, 23]. Patients infected with the Omicron variant may have a greater likelihood of experiencing treatment failure for some mAb and antiviral agents.
Conclusion
Using real-world data, we found that age ≥ 65 years, male sex, non-Hispanic black race/ethnicity, increasing number of comorbidities, and lack of prior SARS-CoV-2 vaccination are significantly associated with treatment failure and hospitalization following treatment with mAbs. Understanding factors associated with treatment failure of mAbs allows for identification of patients who may require additional treatments and/or close follow-up.
Availability of data and materials
We accessed patient data from the electronic health record (EHR; Epic, Verona, WI) of the University of Colorado Health (UCHealth), the largest health system in Colorado. UCHealth consists of 13 hospitals across the state and accounts for approximately 141,000 annual hospital admissions. Data from the EHR were merged with statewide data on vaccination status from the Colorado Comprehensive Immunization Information System and mortality from Colorado Vital Records.
The data that support the findings of this study are available from Health Data Compass Warehouse Project (healthdatacompass.org), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the Health Data Compass Warehouse Project (healthdatacompass.org).
References
Centers for Disease Control and Prevention. Science brief: background rationale and data for public health recommendations for fully vaccinated people. 2021. https://www.cdc.gov/coronavirus/2019-ncov/more/fully-vaccinated-people.html. Published Online 8 March 2021.
Lundgren JD, Grund B, Barkauskas CE, et al. A neutralizing monoclonal antibody for hospitalized patients with covid-19. N Engl J Med. 2020;384:905–14.
Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–37.
Weinreich DM, Sivapalasingam S, Norton T, et al. REGEN-COV antibody combination and outcomes in outpatients with covid-19. N Engl J Med. 2021;385(23):e81.
Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–50.
Aggarwal NR, Beaty LE, Bennett TD, et al. Real world evidence of the neutralizing monoclonal antibody sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients. J Infect Dis. 2022. https://doi.org/10.1093/infdis/jiac206.
Wynia MK, Beaty LE, Bennett TD, et al. Real world evidence of neutralizing monoclonal antibodies for preventing hospitalization and mortality in COVID-19 outpatients. medRxiv. 2022:2022.01.09.22268963.
Huang DT, McCreary EK, Bariola JR, et al. Effectiveness of casirivimab-imdevimab and sotrovimab during a SARS-CoV-2 delta variant surge: a cohort study and randomized comparative effectiveness trial. JAMA Netw Open. 2022;5(7):e2220957.
National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. Bethesda, MD. 2022. https://www.covid19treatmentguidelines.nih.gov. Accessed 26 Sept 2022.
Aggarwal NR, Beaty LE, Bennett TD, Carlson NE, Ginde AA. Change in effectiveness of sotrovimab for preventing hospitalization and mortality in COVID-19 outpatients during the omicron phase. medRxiv. 2022:2022.06.17.22276575.
United States Food and Drug Administration (FDA). COVID-19 update: FDA limits use of certain monoclonal antibodies to treat COVID-19 due to the omicron variant. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-covid-19-due-omicron. Accessed 1 Oct 2022.
Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med. 2021;385(15):1382–92.
Colorado Department of Public Health and Environment. Treatments for COVID-19. https://covid19.colorado.gov/for-coloradans/covid-19-treatments#collapse-accordion-40911-4. Accessed 31 Jan 2022.
Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. The Lancet. 2022;399(10332):1303–12.
van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–33.
Puhr R, Heinze G, Nold M, Lusa L, Geroldinger A. Firth’s logistic regression with rare events: accurate effect estimates and predictions? Stat Med. 2017;36(14):2302–17.
Douin DJ, Ward MJ, Lindsell CJ, et al. ICU bed utilization during the coronavirus disease 2019 pandemic in a multistate analysis—March to June 2020. Crit Care Explor. 2021;3(3):e0361.
National Academies of Sciences E, and Medicine News Release. Strategies to allocate scarce COVID-19 monoclonal antibody treatments to eligible patients examined in new rapid response to government. Accessed 1 Feb 2022. https://www.nationalacademies.org/news/2021/01/strategies-to-allocate-scarce-covid-19-monoclonal-antibody-treatments-to-eligible-patients-examined-in-new-rapid-response-to-government.
Krause M, Douin DJ, Kim KK, Fernandez-Bustamante A, Bartels K. Characteristics and outcomes of mechanically ventilated COVID-19 patients—an observational cohort study. J Intensive Care. 2020;36(3):271–6.
Roth GA, Emmons-Bell S, Alger HM, et al. Trends in patient characteristics and COVID-19 in-hospital mortality in the United States during the COVID-19 pandemic. JAMA Netw Open. 2021;4(5):e218828.
Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of severe acute respiratory syndrome Coronavirus 2 messenger RNA vaccines for preventing Coronavirus Disease 2019 hospitalizations in the United States. Clin Infect Dis. 2022;74(9):1515–24.
Gandhi RT, Malani PN, del Rio C. COVID-19 therapeutics for nonhospitalized patients. JAMA. 2022;327(7):617–8.
VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;28(3):490–5.
Acknowledgements
Not applicable.
Funding
This study was funded by National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) Grants UL1TR002535, UL1TR002535-03S3 and UL1TR002535-04S2. Our study was also supported by the Health Data Compass Data Warehouse project (healthdatacompass.org).
Author information
Authors and Affiliations
Contributions
Study concept and design: DJD, AFW, LEB, AAG. Acquisition of data: TDB, DAM, TCO, SR, JS, AAG. Statistical analysis: AFW, LEB, NEC. Interpretation of data: DJD, AFW, LEB, NEC, TDB, NA, DAM, TCO, SR, JS, JLP, KCM, MKW, AAG. Drafting of the manuscript: DJD. Critical revision of the manuscript for important intellectual content: DJD, AFW, LEB, NEC, TDB, NA, DAM, TCO, SR, JS, JLP, KCM, MKW, AAG. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the Declaration of Helsinki and were approved by the Colorado Multiple Institutional Review Board (COMIRB) with a waiver of informed consent.
Consent for publication
Not applicable.
Competing interests
Dr. Douin received research grant funding from the NIH / National Institute of General Medical Sciences (NIGMS) T32GM135169. Dr. Ginde received other Covid-19 research grants from NIH, DoD, CDC, AbbVie, and Faron Pharmaceuticals, outside the current work. Dr. Wynia received research funding from PCORI and ASPR and is an unpaid advisor to NASEM, including on crisis standards of care during the Covid-19 pandemic, and to DARPA, the Hastings Center, and the Lancet on projects unrelated to mAbs. Dr. Bennett, Dr. Aggarwal, and Dr. Carlson received research grants from the NIH outside of the current work. Other authors have no disclosures to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Maximum level of oxygenation support during the index hospitalization for patients who experienced treatment failure. IV: invasive mechanical ventilation; HFC: high flow nasal cannula; NIV: non-invasive ventilation.
Additional file 2: Figure S2.
Cumulative incidence of hazard for hospitalization by immunocompromised status.
Additional file 3: Figure S3.
Adjusted risk difference and adjusted odds ratio (OR) for treatment failure for each risk factor from a conservative imputation model. In this model, we assumed all missing SARS-CoV-2 positive test dates were ten days prior to the mAb administration date. Risk differences were calculated via Firth's bias-reduced multiple regression logistic regression. Adjusted ORs and 95% confidence intervals (95% CI) were computed by penalized profile likelihood.
Additional file 4: Figure S4.
Adjusted risk difference and adjusted odds ratio (OR) for treatment failure for each risk factor from a conservative imputation model. In this model, we included only patients with confirmed dates for both SARS-CoV-2 positive test and mAb administration. Risk differences were calculated via Firth's bias-reduced multiple regression logistic regression. Adjusted ORs and 95% confidence intervals (95% CI) were computed by penalized profile likelihood.
Additional file 5: Table S1.
Medications and conditions used to stratify Mild versus Moderate/Severe immunocompromised status.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Douin, D.J., Wogu, A.F., Beaty, L.E. et al. Association between treatment failure and hospitalization after receipt of neutralizing monoclonal antibody treatment for COVID-19 outpatients. BMC Infect Dis 22, 818 (2022). https://doi.org/10.1186/s12879-022-07819-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07819-z