Abstract
Background
The SARS-CoV-2 variant B.1.1.529 (Omicron) was first described in November 2021 and became the dominant variant worldwide. Existing data suggests a reduced disease severity with Omicron infections in comparison to B.1.617.2 (Delta). Differences in characteristics and in-hospital outcomes of COVID-19 patients in Germany during the Omicron period compared to Delta are not thoroughly studied. ICD-10-code-based severe acute respiratory infections (SARI) surveillance represents an integral part of infectious disease control in Germany.
Methods
Administrative data from 89 German Helios hospitals was retrospectively analysed. Laboratory-confirmed SARS-CoV-2 infections were identified by ICD-10-code U07.1 and SARI cases by ICD-10-codes J09-J22. COVID-19 cases were stratified by concomitant SARI. A nine-week observational period between December 6, 2021 and February 6, 2022 was defined and divided into three phases with respect to the dominating virus variant (Delta, Delta to Omicron transition, Omicron). Regression analyses adjusted for age, gender and Elixhauser comorbidities were applied to assess in-hospital patient outcomes.
Results
A total cohort of 4,494 inpatients was analysed. Patients in the Omicron dominance period were younger (mean age 47.8 vs. 61.6; p < 0.01), more likely to be female (54.7% vs. 47.5%; p < 0.01) and characterized by a lower comorbidity burden (mean Elixhauser comorbidity index 5.4 vs. 8.2; p < 0.01). Comparing Delta and Omicron periods, patients were at significantly lower risk for intensive care treatment (adjusted odds ratio 0.72 [0.57–0.91]; p = 0.005), mechanical ventilation (adjusted odds ratio 0.42 [0.31–0.57]; p < 0.001), and in-hospital mortality (adjusted odds ratio 0.42 [0.32–0.56]; p < 0.001). This also applied mostly to the separate COVID-SARI group. During the Delta to Omicron transition, case numbers of COVID-19 without SARI exceeded COVID-SARI for the first time in the pandemic’s course.
Conclusion
Patient characteristics and outcomes differ during the Omicron dominance period as compared to Delta suggesting a reduced disease severity with Omicron infections. SARI surveillance might play a crucial role in assessing disease severity of future SARS-CoV-2 variants.
Similar content being viewed by others
Introduction
Various variants of SARS-CoV-2 continue to emerge during the COVID-19 pandemic. The European Centre for Disease Prevention and Control (ECDC) and the World Health Organization (WHO) currently list several virus lineages under close monitoring including the circulating variants of concern (VoC) B.1.617.2 (Delta) and B.1.1.529 (Omicron) [1, 2]. First described in South Africa and reported to the WHO on November 24, 2021 [3], Omicron has become the predominant SARS-CoV-2 variant worldwide throughout the first two months of 2022, with a share of sequences in European countries of almost 100% [4]. Several sub-lineages of Omicron exist with BA.1 and BA.2 being the most common ones [5]. Numbers of BA.2 increased rapidly worldwide as of January 2022 [6] with a proportion of up to 72% among Omicron sequences recently reported for Germany by the federal government agency Robert-Koch-Institute (RKI) [7].
Although Omicron carries several previously observed mutations, its genome markedly differs from those of other VoC which leads to significant differences in viral behaviour in comparison to Delta with respect to transmissibility and immune evasion [8,9,10]. However, early data from South Africa suggested a reduced disease severity in adults with Omicron infections [11]. In accordance therewith, reduced odds for hospitalisation or death were reported for patient cohorts in Norway [12] Canada [13] and the US [14]. Most recently, Nyberg et al. presented data of over 1.5 million laboratory-confirmed COVID-19 cases in the United Kingdom and found a substantially decreased risk for hard outcomes like hospital admission or death in patients infected with Omicron as compared to Delta [15]. The findings were consistent even when only unvaccinated individuals were analysed suggesting a “real” reduction in viral pathogenicity.
So far, outcome data of hospitalized patients in Germany infected during the Omicron dominance period is scarce. Weekly reports with descriptive data on COVID-19 incidence, hospitalizations and mortality are presented by the RKI [16]. Authors of one recently published article presenting German RKI surveillance data postulate reduced disease severity with BA.1 and BA.2 as compared to Delta [17]. However, to the best of our knowledge, structured analyses focusing on hospitalized patients in Germany during the fourth and fifth pandemic wave that also take specific patient characteristics into account are still lacking.
The aim of this study was to evaluate characteristics and outcomes of hospitalized patients with COVID-19 utilizing data derived from a nationwide German hospital network, estimate the disease severity of Omicron and investigate temporal trends during the Delta to Omicron transition phase. To serve this purpose, we assessed the frequency of severe acute respiratory infections (SARI) in COVID-19 patients.
Methods
We conducted a retrospective claims data-based analysis of inpatients treated in 89 German Helios hospitals with laboratory confirmed SARS-CoV-2 infection. ICD-10-GM-code U07.1 (International Statistical Classification of Diseases and Related Health Problems Version 10, German Modification) as main or secondary diagnosis was used to identify COVID-19. Present SARS-CoV-2 infection was linked to the occurrence of SARI, commonly defined by the WHO as an acute respiratory illness with a history of fever, cough, onset within the past 10 days and requiring hospitalization [18]. SARI cases were identified by ICD-10-codes J09-J22 (main or secondary diagnosis) in accordance with common methods of SARI surveillance [19]. ICD-10-codes J09-J22 comprise influenza and pneumonia (J09-J18), acute bronchitis (J20), acute bronchiolitis (J21) and unspecified acute lower respiratory tract infection (J22). To evaluate of temporal trends of SARI and COVID-19, we analysed the whole pandemic period from March 2020 to February 2022. Patients with full inpatient treatment during the observational period of nine weeks between December 6, 2021 and February 6, 2022 underwent a detailed analysis. Relevant treatments and patient characteristics were analysed according to the Operation and Procedure Classification System (OPS) and ICD-10-codes based on the Elixhauser comorbidity index [20] (Supplemental Table 1). The following outcomes and treatments were analysed: intensive care treatment (OPS-codes 8-980, 8-98f or duration of intensive-care stay > 0), mechanical ventilation (OPS-codes 8-70x, 8-71x or duration of ventilation > 0), in-hospital mortality, length of stay (in days), length of stay at intensive care unit (ICU, in days), duration of mechanical ventilation (hours). For the analysis of in-hospital mortality, we excluded cases with discharge due to hospital transfer or unspecified reasons.
Based on data made available by the RKI [21] but assuming a one week delay between infection and consecutive hospital admission, three cohorts and time periods were defined with respect to the dominating virus variant: Delta dominance (2021-12-06 to 2021-12-26), Delta to Omicron transition phase (2021-12-27 to 2022-01-16) and Omicron dominance (2022-01-17 to 2022-02-06).
For the description of the patient characteristics of the cohorts, we employed χ2-tests for binary variables and analysis of variance for numeric variables. We report proportions, means, standard deviations, and p-values.
Inferential statistics were based on generalized linear mixed models (GLMM) specifying hospitals as random factor in a random-intercept model [22]. We employed logistic GLMMs function for dichotomous data and linear mixed models (LMM) for continuous data. Effects were estimated with the lme4 package (version 1.1–26) [23] in the R environment for statistical computing (version 4.0.2, 64-bit build).
The analysis of the outcomes variables intensive care treatment, mechanical ventilation and in-hospital mortality was performed via logistic GLMMs with a logit link function. We report proportions, odds ratios (95% CI) and associated p-values. The analysis of the outcome variables length of stay, length of ICU stay, and duration of mechanical ventilation was performed via LMMs. For these analyses, we log-transformed the dependent variables. We report means (standard deviations), medians (interquartile ranges), models estimates (95%CI) and associated p-values. For the computation of p-values, we used the Satterthwaite approximation to estimate degrees of freedom for the t statistics. For all tests we apply a two-tailed 5% error criterion for significance. GLMMs and LMMs were fitted with the outcome as an explained variable and the cohort (Delta, Delta to Omicron and Omicron) as an explanatory variable. Models were also adjusted by adding age, gender and Elixhauser comorbidity index as explanatory variables. Age and gender were normalized before fitting the models, meaning the variables were scaled to zero mean and unit variance. In order to account for potential non-linearity, age was entered as a quadratic polynomial. We report statistics for Elixhauser comorbidity index as well as its items. For the weighted Elixhauser comorbidity index, the Agency for Healthcare Research and Quality (AHRQ) algorithm was applied [20].
The analysis was carried out according to the principles outlined in the Declaration of Helsinki. Patient-related data were stored in an anonymized form. The local ethics committee (vote: AZ490/20-ek) and the Helios Kliniken GmbH data protection authority approved data use for this study. Due to the retrospective study of anonymized data informed consent was not obtained.
Results
Figure 1 displays trends in COVID-19 diagnoses since the onset of the global pandemic stratified by additionally encoded SARI. During each of the first four pandemic waves, a large proportion of inpatients with COVID-19 also had SARI (hereafter referred to as SARI+). The share of patients with present SARS-CoV-2 infection but without SARI (hereafter referred to as SARI-) mostly followed the course of the pandemic waves but case numbers remained significantly below those with SARI. During the Delta to Omicron transition phase, this relation changed. Case numbers of SARI- exceeded SARI + for the first time in the pandemic (Fig. 1, Delta to Omicron transition phase marked in green).
A total of 4,494 patients with inpatient treatment and laboratory confirmed COVID-19 diagnosis were identified in the period 2021-12-06 to 2022-02-06 (Table 1). Patient characteristics are shown in Supplemental Table 2. Proportion of SARI + cases decreased from 64.3% in the Delta dominance period to 30.6% in the Omicron dominance period (p < 0.01). Mean age [SD] in the total COVID-19 cohort decreased from 61.6 [22.2] to 47.8 [28.1] (p < 0.01). This was driven by a significant increase of patients in the age group ≤ 59 years. Patients in the SARI + cohort were older as compared to SARI- and mean age also decreased significantly throughout the observational period when the separate cohorts were analysed (Supplemental Table 2). Proportion of male patients decreased significantly for the total cohort (52.5–45.3%; p < 0.01). Regarding comorbidities, a significant reduction in the mean weighted Elixhauser comorbidity index could be observed for the total cohort with 8.2 [9.4] during Delta and 5.4 [8.7] during Omicron (p < 0.01) and the SARI- cohort (6.1 [8.9] to 4.1 [7.8]; p < 0.01) but not for SARI+ (9.4 [9.5] to 8.5 [9.8]; p = 0.22).
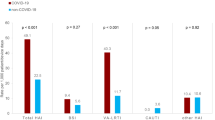
For all treatments and outcomes, odds were significantly reduced comparing the Delta and Omicron periods in the total COVID-19 cohort (Table 2). Adjusted odds ratios [95%CI] were 0.72 [0.57–0.91] for intensive care treatment (p = 0.005), 0.42 [0.31–0.57] for mechanical ventilation (p < 0.001) and 0.42 [0.32–0.56] for in-hospital mortality (p < 0.001). Raw in-hospital mortality rates were 16.3% for the Delta period, 12.2% for Delta to Omicron transition phase and 5.5% for the Omicron period. Considering the SARI + cohort, a largely comparable picture emerged with adjusted odds ratios comparing Delta and Omicron of 0.73 [0.54-1.00] for intensive care treatment (p = 0.05), 0.62 [0.44–0.87] for mechanical ventilation (p = 0.006) and 0.63 [0.45–0.87] for in-hospital mortality (p = 0.005). No significant risk reduction could be observed in the SARI- cohort for intensive care treatment and mechanical ventilation but for in-hospital mortality (p = 0.021). Length of stay, length of ICU stay as well as the duration of mechanical ventilation were reduced significantly in the total cohort comparing Delta and Omicron (Table 3), regarding length of stay and duration of mechanical ventilation also in the SARI + cohort.
Discussion
We present an analysis of 4,494 inpatients treated in 89 German hospitals with laboratory confirmed SARS-CoV-2 infection during the transition period of dominating virus variants Delta and Omicron. To the best of our knowledge, this is the first study comparing characteristics and outcomes of COVID-19 patients during the fourth and fifth pandemic wave in Germany with respect to the dominating VoC.
Our analysis yielded the following findings: Occurrence of SARI is common among COVID-19 patients which was most obvious during the first four pandemic waves. During the Delta to Omicron transition phase, case numbers of SARI- patients exceeded those of SARI+. We noticed a significant change in patient characteristics. In the phase of Omicron dominance, patients were younger, more likely to be female and characterized by a lower comorbidity burden. Patients infected during the Omicron dominance period compared to Delta were less likely to receive intensive care treatment, mechanical ventilation and were at significantly lower mortality risk in the total cohort as well as the SARI + cohort. This leads to the assumption that disease severity in Omicron might be lower. Concomitant occurrence of SARI in hospitalized patients seems to correlate with the severity of SARS-CoV-2 infections. SARI surveillance could therefore play a crucial role in pandemic monitoring. However, future studies are needed to confirm this observation.
Our results are in line with the findings by Nyberg et al. [15] and other previously mentioned studies [12,13,14, 17] as to the dominant SARS-CoV-2 variant Omicron appears to cause milder courses of infection. Importantly, Nyberg et al. performed stratifications by vaccination status and history of past infection which confirmed the reduction of disease severity even in unvaccinated subjects. As administrative data does not contain information about vaccination status, a similar stratification could not be executed within our analysis. Therefore, potential influence of an underlying population immunity which prevents severe courses of infection cannot be ruled out. The current pandemic situation in Hong Kong shows that Omicron can indeed lead to high case fatality rates in a rather non-immunized population due to low vaccination rates and low background immunity because of the so-called “Zero-COVID strategy” [24]. The effect of booster vaccinations, which offer 70% protection against hospitalisation and death as was reported Nyberg et al.[15], must also be highlighted as a large proportion of booster doses were administered during the Omicron period [14]. However, our observation that patients in the SARI + group had a comparable comorbidity burden during Omicron and Delta phases (as indicated by Elixhauser comorbidity index values) but still were at lower risk for adverse outcomes strengthens the assumption of a reduced disease severity. This is backed by the previously mentioned publication from Sievers et al. reporting of a reduced hospitalization risk also in unvaccinated patients [17] and a recent experimental study [25].
According to our data, Omicron affects a younger population, more frequently females and patients with lower comorbidity burden. A similar shift in patient characteristics was observed in previous studies investigating the clinical differences of Omicron and Delta – for example in a recent study by Bouzid et al. who analysed 1,716 patients presenting to 13 emergency departments in France [26]. In this analysis, prevalence of comorbidities (except obesity) did not differ between the groups, however, only a few distinct comorbidities were surveyed. With respect to outcomes, the findings were comparable to our analysis with reduced risk among Omicron patients for ICU treatment, mechanical ventilation and in-hospital death [26]. A possible explanation for the shift in demographic parameters, especially age, might be the proportion of booster vaccinations among people aged 60 + which is significantly higher in comparison to people in the age group 18–59 according to RKI data [27].
Leveraging existing national and international surveillance systems to monitor pandemic trends is of crucial importance as was also stated by the WHO and ECDC [28,29,30,31]. In Germany, an ICD-code-based SARI surveillance system (ICOSARI) closely linked to the RKI was introduced in 2017 [19]. In a recent preprint article, Tolksdorf et al. analysed hospitalization numbers and ICU treatments derived from ICOSARI in the first three pandemic waves, compared those with the German national notification system for SARS-CoV-2 infections and found a more accurate estimation of hospitalizations and ICU treatments utilizing ICOSARI data [30]. Monitoring trends of SARI-COVID cases has yet become an integral part of pandemic surveillance in Germany [16]. Our study further highlights, that this easily applicable system fully based on administrative data could play a central role in assessing disease severity.
Limitations
We acknowledge several limitations in connection with this study. First, some limitations must be attributed to administrative data in general as data quality depends on correct coding and data is stored for remuneration reasons. However, SARI surveillance in Germany utilizing claims data is a robust and valid approach as was mentioned previously [19]. Second, laboratory- confirmed COVID-19 cases were identified through ICD-10-codes and no data for viral genome sequencing was available which could have potentially biased the results. Thus, identification of Omicron and Delta cases were based on epidemiological data provided by the RKI [16, 32]. In addition, differentiation between BA.1 and BA.2 sub-lineages was not possible. Third, as outlined above, the effect of person-level vaccination status could not be assessed in this study as respective data was not available. Fourth, identification of COVID-19 cases is dependent on correct testing and cases might stay undetected. However, in Helios hospitals, reverse transcriptase polymerase chain reaction (RT-PCR) testing is mandatory for inpatients in addition to rapid antigen tests. Fifth, nosocomial COVID-19 cases were not excluded in the present analysis. The increased transmissibility of Omicron could have led to more incident infections with unknown impact on disease severity assessment [14]. Sixth, this analysis included all patients hospitalized within the observational period with a documented COVID-19 diagnosis. The reason for admission was therefore not considered which may have led to possible selection bias as, due to postponement of elective procedures, relatively more patients with severe diseases that require urgent treatment entered the healthcare during the fourth and fifth pandemic wave.
Conclusion
During the Omicron dominance period, numbers of SARI in hospitalized patients with COVID-19 were lower relative to Non-SARI cases. Analysis of timely trends revealed that this represents a unique finding during the pandemic’s course. Adjusted for age, gender and comorbidity burden, the risk for adverse hospital outcomes (ICU treatment, mechanical ventilation, and in-hospital mortality) was substantially decreased during Omicron dominance as compared to Delta. Patients affected by Omicron were younger, more likely to be female and the comorbidity burden was lower. Continuous ICD-code based SARI monitoring could contribute decisively to COVID-19 surveillance and evaluation of disease severity especially in the light of possible future virus variants.
Data availability
Helios Health and Helios Hospitals have strict rules regarding data sharing due to the fact that health claims data are a sensible data source and have ethical restrictions imposed due to concerns regarding privacy. Access to anonymized data that support the findings of this study are available on request from the Helios Health Institute / Leipzig Heart Institute (www.leipzig-heart.de). Please direct queries to the data protection officer (Email: info@leipzig-heart.de) and refer to study “Omicron dominance”).
Abbreviations
- CI:
-
Confidence interval
- COVID-19:
-
Coronavirus disease 2019
- ECDC:
-
European Centre for Disease Prevention and Control
- GLMM:
-
Generalized linear mixed models
- ICD-10-GM:
-
International Statistical Classification of Diseases and Related Health Problems Version 10, German Modification
- ICU:
-
Intensive care unit
- LMM:
-
Linear mixed models
- OPS:
-
Operation and Procedure Classification System
- RKI:
-
Robert-Koch-Institute
- SARI:
-
Severe acute respiratory infection
- SARI+:
-
COVID-19 with SARI
- SARI-:
-
COVID-19 without SARI
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- VoC:
-
Variant of Concern
- WHO:
-
World Health Organization
References
European Centre for Disease Prevention and Control (ECDC): SARS-CoV-2 variants of concern as of 11 March 2022 https://www.ecdc.europa.eu/en/covid-19/variants-concern [Accessed April 14, 2022].
World Health Organization (WHO): Tracking SARS-CoV-2 variants https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ [Accessed April 14, 2022].
World Health Organization (WHO): Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern [Accessed April 14, 2022].
Our World in Data: Share of SARS-CoV-2 sequences that are the omicron variant as of Mar 21, 2022 https://ourworldindata.org/grapher/covid-cases-omicron?region=Europe&country=GBR~FRA~BEL~DEU~ITA~ESP~USA~ZAF~BWA~AUS [Accessed April 14, 2022].
World Health Organization (WHO): Statement on Omicron sublineage BA.2 https://www.who.int/news/item/22-02-2022-statement-on-omicron-sublineage-ba.2 [Accessed April 14, 2022].
World Health Organization (WHO): Enhancing response to Omicron SARS-CoV-2 variant: Technical brief and priority actions for Member States https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states [Accessed April 14, 2022].
Robert-Koch-Institut (RKI): Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19) vom 24.03.2022 [https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2022-03-24.pdf?__blob=publicationFile] [Accessed April 14, 2022].
Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. The Lancet. 2021;398(10317):2126–8.
Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV‐2 Omicron variant. Journal of Medical Virology 2022.
Jung C, Kmiec D, Koepke L, Zech F, Jacob T, Sparrer Konstantin MJ, Kirchhoff F, Aguilar Hector C. Omicron: What Makes the Latest SARS-CoV-2 Variant of Concern So Concerning? Journal of Virology, 96(6):e02077-02021.
Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, Amoako DG, Everatt J, Bhiman JN, Scheepers C, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. The Lancet. 2022;399(10323):437–46.
Veneti L, Bøås H, Bråthen Kristoffersen A, Stålcrantz J, Bragstad K, Hungnes O, Storm ML, Aasand N, Rø G, Starrfelt J, et al: Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Eurosurveillance 2022, 27(4).
Ulloa AC, Buchan SA, Daneman N, Brown KA: Estimates of SARS-CoV-2 Omicron Variant Severity in Ontario, Canada. JAMA 2022.
Iuliano AD, Brunkard JM, Boehmer TK, Peterson E, Adjei S, Binder AM, Cobb S, Graff P, Hidalgo P, Panaggio MJ, et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods — United States, December 2020–January 2022. MMWR Morbidity and Mortality Weekly Report. 2022;71(4):146–52.
Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, Hinsley W, Bernal JL, Kall M, Bhatt S, et al: Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. The Lancet 2022.
Robert-Koch-Institut (RKI): Wochenberichte zu COVID-19 [https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenberichte_Tab.html;jsessionid=8286D2EA87630E86B19A3F1014BD10C8.internet051?nn=13490888] [Accessed April 14, 2022].
Sievers C, Zacher B, Ullrich A, Huska M, Fuchs S, Buda S, Haas W, Diercke M, An Der Heiden M, Kröger S. SARS-CoV-2 Omicron variants BA.1 and BA.2 both show similarly reduced disease severity of COVID-19 compared to Delta, Germany, 2021 to 2022. Eurosurveillance 2022, 27(22).
Fitzner J, Qasmieh S, Mounts AW, Alexander B, Besselaar T, Briand S, Brown C, Clark S, Dueger E, Gross D, et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ. 2018;96(2):122–8.
Buda S, Tolksdorf K, Schuler E, Kuhlen R, Haas W. Establishing an ICD-10 code based SARI-surveillance in Germany – description of the system and first results from five recent influenza seasons. BMC Public Health 2017, 17(1).
Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying Increased Risk of Readmission and In-hospital Mortality Using Hospital Administrative Data: The AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698–705.
Robert-Koch-Institut (RKI): Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19) vom 24.02.2022 [https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2022-02-24.pdf?__blob=publicationFile] [Accessed April 14 2022].
Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59(4):390–412.
Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;67(1):1–48.
Taylor L. Covid-19: Hong Kong reports world’s highest death rate as zero covid strategy fails. BMJ 2022:o707.
Halfmann PJ, Iida S, Iwatsuki-Horimoto K, Maemura T, Kiso M, Scheaffer SM, Darling TL, Joshi A, Loeber S, Singh G, et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature. 2022;603(7902):687–92.
Bouzid D, Visseaux B, Kassasseya C, Daoud A, Fémy F, Hermand C, Truchot J, Beaune S, Javaud N, Peyrony O, et al: Comparison of Patients Infected With Delta Versus Omicron COVID-19 Variants Presenting to Paris Emergency Departments. Annals of Internal Medicine 2022.
Robert-Koch-Institut (RKI): Digitales Impfquotenmonitoring zur COVID-19-Impfung https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Impfquoten-Tab.html [Accessed April 14, 2022].
European Centre for Disease Prevention and Control (ECDC): Strategies for the surveillance of COVID-19 https://www.ecdc.europa.eu/en/publications-data/strategies-surveillance-covid-19 [Accessed April 14, 2022].
World Health Organization (WHO): Operational considerations for COVID-19 surveillance using GISRS: interim guidance https://apps.who.int/iris/handle/10665/331589 [Accessed April 14, 2022].
Tolksdorf K, Haas W, Schuler E, Wieler LH, Schilling J, Hamouda O, Diercke M, Buda S. Syndromic surveillance for severe acute respiratory infections (SARI) enables valid estimation of COVID-19 hospitalization incidence and reveals underreporting of hospitalizations during pandemic peaks of three COVID-19 waves in Germany, 2020–2021. In.: Cold Spring Harbor Laboratory; 2022.
Klavs I, Serdt M, Učakar V, Grgič-Vitek M, Fafangel M, Mrzel M, Berlot L, Glavan U, Vrh M, Kustec T, et al: Enhanced national surveillance of severe acute respiratory infections (SARI) within COVID-19 surveillance, Slovenia, weeks 13 to 37 2021. Eurosurveillance 2021, 26(42).
Robert-Koch-Institut (RKI): Deutscher Elektronischer Sequenzdaten-Hub (DESH) https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/DESH/DESH.html [Accessed April 14, 2022].
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JL is the first author of this manuscript. VP, SH, SK, ES, RM, IN, MB, GH, RK, AB contributed substantially to the study design, data analysis and interpretation and revision of the manuscript and gave final approval for publishing.
Corresponding author
Ethics declarations
Guarantor statement
Mr. Johannes Leiner (JL) takes full responsibility for the content of the manuscript, including the data and analysis.
Consent for publication
All authors gave final approval for publication.
Competing interests
We declare no conflicts of interest associated with this publication.
Ethics approval and consent to participate
The analysis was carried out according to the principles outlined in the Declaration of Helsinki. Patient-related data were stored in a pseudonymized form. The Ethics Committee at the Medical Faculty of the University of Leipzig (vote: AZ490/20-ek) and the Helios Kliniken GmbH data protection authority approved data use for this study. Due to the retrospective study of anonymized data informed consent was not obtained (need for informed consent waived by the Ethics Committee at the Medical Faculty of the University of Leipzig).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Leiner, J., Pellissier, V., Hohenstein, S. et al. Characteristics and outcomes of COVID-19 patients during B.1.1.529 (Omicron) dominance compared to B.1.617.2 (Delta) in 89 German hospitals. BMC Infect Dis 22, 802 (2022). https://doi.org/10.1186/s12879-022-07781-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07781-w