Abstract
Purpose
To find pharmacokinetic/pharmacodynamic parameters of vancomycin associated with the optimal outcome of severe infection due to Enterococcus species.
Methods
We retrospectively reviewed enterococcal bacteremia cases treated with vancomycin from January 2015 to December 2020. The primary outcome was 30-day mortality. We calculated cutoff values of the ratio of vancomycin area under the concentration–time curve over 24 h to the minimum inhibitory concentration (AUC24/MIC) and trough concentration (Ctrough) during the initial 72 h of treatment. The optimal cutoff value was determined using the Youden index. Binary variables created based on these cutoffs were further assessed using multivariable analysis.
Results
A total of 65 patients were included. The majority (87.7%) had solid or hematologic malignancies. Thirty-day mortality and nephrotoxicity occurred in nine (13.4%) and 14 (21.5%) patients, respectively. Both vancomycin AUC24/MIC and Ctrough showed fair performance in predicting 30-day mortality (AUC of receiver-operator curve for AUC24/MIC, 0.712; 95% confidence interval [CI] 0.539–0.886; AUC for Ctrough, 0.760; 95% CI 0.627–0.892; pairwise AUC comparison: p = 0.570). Ctrough ≥ 13.94 μg/mL, but not AUC24/MIC ≥ 504, had a significant association with 30-day mortality after adjusting for confounders (odds ratio, 8.40; 95% CI 1.60–86.62; p = 0.010).
Conclusion
Mean Ctrough ≥ 13.94 μg/mL during the initial 72 h was associated with higher 30-day mortality in enterococcal bacteremia. Further studies are warranted to elucidate optimal pharmacokinetic targets for enterococcal bacteremia.
Similar content being viewed by others
Introduction
Enterococcus spp. are some of the common causes of nosocomial infections, and their significance has increased in the last several decades [1, 2]. Enterococcal infections are often treated with vancomycin when isolates are resistant to penicillin but susceptible to glycopeptides. Patients most affected by invasive enterococcal infections are those with multiple comorbidities. These patients are also more susceptible to nephrotoxicity [3]. Therefore, it is crucial to optimize vancomycin treatment for invasive enterococcal infections. Data regarding optimal pharmacokinetic/pharmacodynamic (PK/PD) parameters of vancomycin for serious infections caused by gram-positive organisms other than methicillin-resistant Staphylococcus aureus (MRSA) are scarce. Guidelines for therapeutic monitoring of vancomycin by the American Society of Health-System Pharmacists (ASHP) have confined their recommendations of its use to serious MRSA infections [4].
There are three small retrospective studies regarding this topic. One of these studies only assessed trough concentrations, a surrogate marker of the area under the curve (AUC) of the concentration–time curve [5,6,7]. A recently published larger retrospective study was limited by the inclusion of a considerable number of non-severe infections [8]. A prospective study from China also attempted to provide insights into this matter [9]. This study included both staphylococcal and enterococcal infections. However, the latter comprised only 23.19% of total infections, making it difficult to generalize the study results to enterococcal infections. Therefore, we aimed to determine the PK/PD parameters associated with the optimal clinical outcomes of enterococcal bacteremia.
Methods
Study population
We retrospectively reviewed all cases of enterococcal bacteremia from January 2015 to December 2020 at the Samsung Medical Center, a 1950-bed tertiary referral center in South Korea. Patients with enterococcal bacteremia who received intravenous vancomycin for an initial 72 h or longer were included. Patients whose isolates were resistant to vancomycin, who did not have vancomycin concentrations measured, received concurrent antimicrobial which is active against the isolated enterococci, were receiving renal replacement therapy, had polymicrobial bacteremia, or for whom treatment was considered futile due to underlying disease were excluded. If a patient had more than one episode of enterococcal bacteremia within the study period, only bacteremic episodes with a minimum of a 1-year interval since the last episode were included.
Study design and definitions
We selected two PK/PD parameters: the ratio of vancomycin area under the concentration–time curve over 24 h to the minimum inhibitory concentration (AUC24/MIC) and the trough concentration (Ctrough). The primary outcome was 30-day all-cause mortality. Secondary outcomes were clinical failure at the end of treatment (EOT), the incidence of nephrotoxicity, and 90-day recurrence. Clinical failure was defined as any of the following: presence of fever, hemodynamic instability, or death. Cutoff values of the PK/PD parameters for prediction of the primary outcome were determined from the receiver operating characteristic (ROC) curve. The study subjects were then divided based on these cutoff values. Baseline clinical characteristics and clinical outcome measures were compared between groups. Finally, independent risk factors for 30-day mortality were elucidated using a logistic regression model, including the PK/PD parameters.
Patients were considered immunocompromised if they had been on steroids equivalent to ≥ 20 mg/day of prednisolone for ≥ 3 weeks, received other immunosuppressants in the absence of active malignancy, or received a solid organ or hematopoietic stem cell transplant. Primary bacteremia was defined as bloodstream infection without an identifiable source of infection. The day of the first positive blood culture was defined as day 1. Acute kidney injury (AKI) [10], chronic kidney disease [11], Charlson comorbidity index (CCI) [12] and the Pitt bacteremia score [13] were defined as per previous studies. Creatinine clearance (CLCr) was calculated using the Cockcroft–Gault formula (14). In line with previous literature, nephrotoxicity was defined as an increase of > 0.5 mg/dL or a ≥ 50% increase in serum creatinine over baseline or a decrease in calculated CLCr of 50% from baseline on 2 consecutive days [4].
Microbiologic and pharmacokinetic/pharmacodynamic analysis
Species identification and antimicrobial susceptibility testing were performed using the VITEK 2 system (bioMérieux, Marcy-l’Etoile, France). MICs were then confirmed by the broth microdilution (BMD) method according to the Clinical and Laboratory Standards Institute guidelines [15, 16].
The institutional protocol states that vancomycin is infused over an hour if the dose is ≤ 1 g or 2 h if > 1 g. Blood samples were drawn immediately before the administration of the fourth dose for Ctrough calculation. Since there is no established PK target for enterococcal bacteremia, clinicians adjusted vancomycin doses at their discretion, guided by the Ctrough. Initial doses were given at 12-h intervals. The dosing interval was then adjusted according to the trough concentrations. From April 2017, loading doses of 25 mg/kg were administered to patients with hematologic malignancies or critical illnesses. The serum vancomycin concentration was measured using a fluorescence polarization immunoassay (COBAS INTEGRA Vancomycin, Roche, Germany).
AUC24 was calculated using the Bayesian method with the APK© software (version 3.5.28, RxKinetics, Plattsburg, MO). As previous studies suggested that the vancomycin AUC/MIC be optimized early in the course of infection [17,18,19], AUC24/MIC and Ctrough during the initial 72 h of treatment were chosen as measures of drug exposure. The average AUC24/MIC and Ctrough values during the initial 72 h were calculated as arithmetic means.
Statistical analysis
Cutoff values of AUC24/MIC and Ctrough were determined using the Youden index [20]. Continuous variables were compared using Student’s t-test or the Mann–Whitney U test. Categorical variables were compared using χ2 or Fisher’s exact test. Variables with a p-value < 0.1 were further examined by logistic regression with backward selection. Firth’s penalized-likelihood logistic regression was performed to resolve the complete separation of some variables (e.g., no one in the treatment or control group experienced the outcome event) [21]. Statistical significance was defined as a two-sided p-value < 0.05. All statistical analyses were executed by R (version 4.1.1, R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
A total of 65 patients were included in this study. The majority of the study population were elderly men with normal renal function and a high burden of comorbidity (Table 1). The most common cause of bacteremia was intraabdominal infection (50.8%), followed by primary bacteremia (35.4%). The median Pitt score was 0 (interquartile range [IQR], 0–2), and 3.1% of the population required ICU care at the onset of bacteremia. Thirty-day mortality and nephrotoxicity occurred in nine (13.8%) and 14 (21.5%) patients, respectively. The median time to the incidence of nephrotoxicity was 11 days (IQR, 7–15).
Sixty-two (95.4%) of the isolates were Enterococcus faecium, and 84.6% of isolates had MIC values measured by a BMD of 1 μg/mL. A considerable discrepancy was noted between the MIC measured by automated BMD (MICautoBMD) and manual BMD (MICBMD). Among the 53 isolates with MICautoBMD ≤ 0.5 μg/mL, 46 (86.8%) had a MICBMD of 1 μg/mL. In addition, two (25%) out of eight isolates with a MICautoBMD of 1 μg/mL had an MICBMD of 2 μg/mL. The MICBMD was used to calculate the AUC24/MIC throughout the study. The median value of average AUC24/MIC during the initial 72 h was 579 (IQR, 453–676), and the mean Ctrough was 13.71 μg/mL (standard deviation, 4.48). The average AUC24/MIC and Ctrough values showed a moderate correlation (Spearman’s rank correlation coefficient = 0.518, p < 0.001).
Determining PK/PD parameters for predicting clinical outcomes
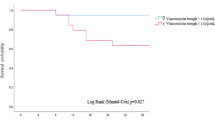
The distribution of 30-day mortality across vancomycin AUC24/MIC and Ctrough during the initial 72 h is presented in Fig. 1. No patient with an average AUC24/MIC < 500 died within 30 days after the onset of bacteremia. In addition, all patients with an average vancomycin Ctrough < 10 μg/mL survived by 30 days.
The cutoff value of the average AUC24/MIC during the initial 72 h was 504 for prediction of 30-day mortality. The AUC of the ROC curve was 0.712 (95% confidence interval [CI] 0.539–0.886; Fig. 2). The cutoff value of the average Ctrough during the initial 72 h was 13.94 μg/mL and that of the AUC was 0.760 (95% CI 0.627–0.892). As expected from the distribution presented in Fig. 1, these cutoff values were notable for their high sensitivity (1.000) and low specificity (0.379). There was no significant difference between AUC24/MIC and Ctrough in terms of predictive performance (pairwise comparison of AUC curves: p = 0.570).
When the study population was divided into two groups based on each cutoff value, there was no difference in baseline characteristics between the groups. This was except for a higher proportion of urinary tract infections and lower CLCr in the Ctrough ≥ 13.94 μg/mL group (Additional file 1: Tables S1 and S2).
Thirty-day mortality occurred exclusively in the high AUC24/MIC group (0.0% vs. 20.9%, p = 0.023; Table 2). Nephrotoxicity occurred more frequently in patients with Ctrough ≥ 13.94 μg/mL (8.1% vs. 39.3%, p = 0.022). The proportion of patients who experienced clinical failure at EOT or 90-day recurrence did not differ between the two groups.
Even though short-term mortality is a preferred measure of treatment outcome due to its objectivity, one may argue that mortality after enterococcal bacteremia cannot be attributed to the infection in considerable cases. Therefore, we attempted to build another prediction model for clinical failure at EOT. However, the models had no ability to predict clinical failure, as the 95% CI of the AUC of each ROC curve included 0.5 (for AUC24/MIC, AUC 0.536 [95% CI 0.387–0.685]; for Ctrough, AUC 0.561 [95% CI 0.397–0.724]).
Risk factors for 30-day mortality
Patients with higher average AUC24/MIC and Ctrough and those with higher white blood cell counts were more likely to die within 30 days after bacteremia (Table 3). Diabetes and AKI at the onset of bacteremia were marginally associated with 30-day mortality. Among these characteristics, vancomycin Ctrough ≥ 13.94 μg/mL was the only statistically significant risk factor for mortality in a multivariable model (OR 8.40, 95% CI 1.60–86.62, p = 0.010).
Discussion
We found that both vancomycin AUC24/MIC and Ctrough during the initial 72 h of treatment had fair performance in predicting 30-day mortality. However, when AUC24/MIC and Ctrough were transformed into binary variables with each cutoff value and analyzed in the regression model, only Ctrough ≥ 13.94 μg/mL was an independent risk factor for 30-day mortality in patients with enterococcal bacteremia.
The literature suggests that the risk of nephrotoxicity increases as a function of Ctrough or AUC [22,23,24], which is in accordance with our results. In our study, a higher Ctrough was significantly associated with both 30-day mortality and nephrotoxicity, but not with clinical failure. This may be explained by a higher burden of comorbidity and a higher incidence of both AKI at the onset of bacteremia and nephrotoxicity in those who died within 30 days. Although these variables turned out to be statistically insignificant after multivariable analysis, it is possible that we did not find a significant association due to the small population size.
Three studies demonstrated the benefit of higher vancomycin exposure in the treatment of enterococcal infections, all of which were notably different from our study [5, 7, 8]. In studies by Jumah et al. [5] and Sohn et al. [7], the severity of infection measured by the proportion of ICU patients at baseline, who might benefit from higher drug exposure, was higher than that of our population (14% and 37.8%, respectively, compared to 3.1% in our study). Moreover, those studies included larger proportions of infection with Enterococcus faecalis (36.8% and 29.7%, respectively, compared to 4.5% in our study), the majority of which are susceptible to penicillin. Although the proportion of concurrent antibiotic use did not differ between the survivor and non-survivor groups in those studies, there might have been an unadjusted difference in the use of antibiotics that are active against enterococci. Considering the low Pitt bacteremia score and low percentage of ICU admissions in our study, avoiding an unnecessarily high Ctrough might be justifiable when treating infections in non-severe conditions. Katip et al. restricted their study population to ampicillin-resistant enterococcal infections and reported that vancomycin AUC24/MIC ≥ 400 during the initial 72 h of treatment was associated with a higher likelihood of a better clinical outcome, which was a composite of the resolution of signs and symptoms related to enterococcal infection [8]. However, the adjusted hazard ratio for nephrotoxicity was 3.95 (95% CI 1.09–14.47). This might be quite high considering that the study population largely comprised patients with non-severe infections (non-bacteremic urinary tract infections, 70.2%; non-bacteremic wound infections, 12.5%).
Ctrough has been used as a surrogate marker for AUC/MIC because it is difficult to estimate the AUC using conventional methods. The new ASHP guidelines updated in 2020 no longer recommend Ctrough-based monitoring for serious MRSA infections [4]. However, they acknowledged that there is insufficient evidence to apply the same recommendation to non-MRSA infections. In addition to our study, Nakakura et al. [6] and Sohn et al. [7] found a significant association between Ctrough and mortality. Considering this, clinicians who practice in settings where AUC24/MIC are not routinely obtained may benefit from monitoring Ctrough when treating enterococcal bacteremia.
Our study has several strengths. First, by measuring MICs with BMD, the reliability of AUC24/MIC was higher than in previous studies, all of which did not confirm MIC by BMD or the E-test except Jumah et al. [5]. Second, we excluded cases in which antibiotics other than vancomycin with in vitro activity against enterococci were used.
However, this study also has important limitations. First, there are inherent limitations from the retrospective nature of the study. We used logistic regression to mitigate confounding; however, the possibility of unobserved bias could not be excluded. Furthermore, there might have been variability in how vancomycin dosage was adjusted after the measurement of Ctrough, which is difficult to control in a retrospective study and could have affected treatment outcomes. Second, the composition of the patients included in our study may limit the generalizability of the results. Patients with chronic kidney disease were unintentionally excluded due to the exclusion criteria unrelated to renal function. Also, most of the patients had either solid or hematological malignancies. Third, intraabdominal infection was the focus of bacteremia in about half of the study population. Considering the often polymicrobial nature of intraabdominal infections, antimicrobial treatment other than vancomycin might have affected the clinical outcomes. However, we excluded the patients with polymicrobial bacteremia to minimize such impact. Fourth, we calculated AUC24/MIC with a single concentration (Ctrough) since peak concentrations had not been measured. However, while larger studies should verify it, Neely et al. reported that trough-only data could generate reliable AUC estimates [25]. Fifth, there are limitations in the outcome measures. Although mortality is generally the most objective indicator, it may not fully reflect the clinical outcome when infection-attributable mortality is low. Clinical failure, one of the secondary outcomes, included some subjective variables. However, higher Ctrough was consistently not associated with better clinical outcomes in various measures. Last, there is a risk of overfitting. The study dataset was used both for derivation and application of the ROC curves. Furthermore, there is a sampling error in the selection of thresholds. The result from this study needs external validation in future studies. Our results call for a prospective study with a larger population to determine PK parameters to optimize outcomes in enterococcal bacteremia.
In conclusion, monitoring Ctrough during the initial 72 h may be useful as a pharmacokinetic target for the treatment of enterococcal bacteremia. Avoiding high Ctrough in this setting may prove beneficial for the prevention of nephrotoxicity without compromising treatment efficacy.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to institutional regulations on restrictions on disclosure of health information, but are available from the corresponding author on reasonable request.
Code availability
The datasets generated and/or analyzed during the current study are not publicly available due to institutional regulations on restrictions on disclosure of health information, but are available from the corresponding author on reasonable request.
References
Kang CI, Song JH, Chung DR, Peck KR, Yeom JS, Son JS, et al. Bloodstream infections in adult patients with cancer: clinical features and pathogenic significance of Staphylococcus aureus bacteremia. Support Care Cancer. 2012;20(10):2371–8.
Zhou X, Willems RJL, Friedrich AW, Rossen JWA, Bathoorn E. Enterococcus faecium: from microbiological insights to practical recommendations for infection control and diagnostics. Antimicrob Resist Infect Control. 2020;9(1):130.
Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10(4):266–78.
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–64.
Jumah MTB, Vasoo S, Menon SR, De PP, Neely M, Teng CB. Pharmacokinetic/pharmacodynamic determinants of vancomycin efficacy in enterococcal bacteremia. Antimicrob Agents Chemother. 2018;62(3):e01602-17.
Nakakura I, Sakakura K, Imanishi K, Sako R, Yamazaki K. Association between vancomycin pharmacokinetic/pharmacodynamic parameters, patient characteristics, and mortality in patients with bacteremia caused by vancomycin-susceptible Enterococcus faecium: a single-center retrospective study. J Pharm Health Care Sci. 2019;5:8.
Sohn Y, Rim JH, Cho Y, Hyun J, Baek Y, Kim M, et al. Association of vancomycin trough concentration on the treatment outcome of patients with bacteremia caused by Enterococcus species. BMC Infect Dis. 2021;21(1):1099.
Katip W, Oberdorfer P. A monocentric retrospective study of AUC/MIC ratio of vancomycin associated with clinical outcomes and nephrotoxicity in patients with Enterococcal infections. Pharmaceutics. 2021;13(9):1378.
Liang X, Fan Y, Yang M, Zhang J, Wu J, Yu J, et al. A prospective multicenter clinical observational study on vancomycin efficiency and safety with therapeutic drug monitoring. Clin Infect Dis. 2018;67(2):S249–55.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84.
Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013;3(1):19–62.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Chow JW, Yu VL. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents. 1999;11(1):7–12.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Performance Standards for Antimicrobial Susceptibility Testing. 31th ed. CLSI supplement M100. Wayne: Clinical & Laboratory Standards Institute; 2020.
Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 11th ed. Clinical and Laboratory Standards Institute; 2018.
Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, O’Sullivan MV, et al. Vancomycin AUC/MIC ratio and 30-day mortality in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2013;57(4):1654–63.
Casapao AM, Lodise TP, Davis SL, Claeys KC, Kullar R, Levine DP, et al. Association between vancomycin day 1 exposure profile and outcomes among patients with methicillin-resistant Staphylococcus aureus infective endocarditis. Antimicrob Agents Chemother. 2015;59(6):2978–85.
Chattaweelarp T, Changpradub D, Punyawudho B, Thunyaharn S, Santimaleeworagun W. Is early monitoring better? Impact of early vancomycin exposure on treatment outcomes and nephrotoxicity in patients with methicillin-resistant Staphylococcus aureus infections. Antibiotics (Basel). 2020;9(10):672.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5.
Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80(1):27–38.
Chavada R, Ghosh N, Sandaradura I, Maley M, Van Hal SJ. Establishment of an AUC (0–24) threshold for nephrotoxicity is a step towards individualized vancomycin dosing for methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2017;61(5):e02535-16.
Zasowski EJ, Murray KP, Trinh TD, Finch NA, Pogue JM, Mynatt RP, et al. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2018;62(1):e01684-17.
van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57(2):734–44.
Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58(1):309–16.
Acknowledgements
An abstract containing part of this study was presented at the 5th Interscience Conference of Infection and Chemotherapy (ICIC 2021) hosted by the Korean Society of Infectious Diseases.
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization: EN and KH; Formal analysis: EN and SYW; Investigation: EN and HMK; Methodology: EN and KH; Resources: HMK, HJH, H-DP, and NYL; Supervision: YMS, HJP, and KH; Writing—original draft: EN and KH; Writing—review and editing: EN, KH, J-HK, SYC, C-IK, DRC, and KRP. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No: 2021-09-032) and carried out in accordance with relevant guidelines and regulations.
The requirement of informed consent was waived by the Institutional Review Board of Samsung Medical Center (IRB No: 2021-09-032).
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Comparison of groups divided based on vancomycin AUC24/MIC cutoff. Table S2. Comparison of groups divided based on vancomycin trough concentration cutoff
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nham, E., Huh, K., Sohn, Y.M. et al. Pharmacokinetic/pharmacodynamic parameters of vancomycin for predicting clinical outcome of enterococcal bacteremia. BMC Infect Dis 22, 686 (2022). https://doi.org/10.1186/s12879-022-07668-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07668-w