Abstract
Background
Mucormycosis is a rare but serious opportunistic fungal infection that occurs in immunocompromised individuals, especially those with diabetic ketoacidosis. Presently, early diagnosis of the disease remains a challenge for clinicians.
Case presentation
The patient, a 68-year-old woman with type 2 diabetes mellitus, was admitted with paroxic sharp pain in the left upper abdomen. CT imaging revealed a patchy hypodense shadow of the spleen with wedge-shaped changes. The patient was not considered early for fungal infection. The diagnosis of spleen mucormycosis was not confirmed until pathological biopsy after splenectomy. After surgery, blood glucose level was controlled, acidosis was corrected, and antifungal therapy was effective.
Conclusions
We report here, for the first time ever, a case of isolated splenic mucormycosis secondary to diabetic ketoacidosis that was diagnosed and treated with antifungal drugs and splenectomy. Following splenectomy, the presence of splenic mucormycosis was confirmed when characteristic mycelia were observed in a tissue biopsy. As the location of any fungal infection is extremely relevant for treatment options and prognoses, early diagnosis and clinical intervention can greatly affect outcomes and prognoses for patients.
Similar content being viewed by others
Background
Mucormycosis is a rare invasive fungal infection that occurs mainly as a secondary condition in individuals with other underlying diseases (particularly diabetic ketoacidosis) that cause immune deficiency [1]. Based on the infected organ and clinical presentation, mucormycosis is usually divided into six clinical forms, namely, rhinocerebral, pulmonary, cutaneous, gastrointestinal, disseminated, and miscellaneous. Rhinocerebral mucormycosis is the most common form that occurs in diabetes patients with ketoacidosis; this form of mucormycosis rarely progresses to pulmonary or disseminated forms [2]. Mucormycosis is extremely rare, and isolated cases occurring in the spleen are rarer. Since the disease is extremely aggressive with a high mortality rate, early diagnosis and treatment are crucial for patient survival. This article provides some interesting points, which may be of value for the future clinical management of mucormycosis.
Case presentation
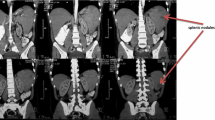
A 68-year-old woman with type 2 diabetes mellitus with poor glycaemic control was admitted with paroxic sharp pain in the left upper abdomen. She had a history of diabetes for 8 years and took metformin orally to control blood glucose; her blood glucose levels were stable. However, in the past six months, the fasting blood glucose was between 8 and 10 mmol/L, and the blood glucose at 2 h postprandial was between 12 and 18 mmol/L. There was no history of autoimmune diseases or long-term use of immunosuppressive drugs such as glucocorticoids. Physical examination showed tenderness in left upper abdomen, flat and soft abdomen, no gastrointestinal type and peristaltic wave, no varicose veins in the abdominal wall, no rebound tenderness, and no palpable liver and spleen. Laboratory analyses revealed that her blood glucose level was 32.83 mmol/L, C-reactive protein was 45.683 mg/L, HbA1c was 14.43%, urinary ketone bodies (++), and coagulation function tests reported the following: fibrinogen level 6.36 g/L, activated partial thromboplastin time 22.50 s, D-dimer level 2.28 mg/L, and fibrin degradation products 6.10 mg/L. Arterial blood gas analysis revealed a state of metabolic acidosis: pH 6.80, partial pressure of carbon dioxide in arterial blood (PaCO2) < 10 mmHg, bicarbonate (HCO3) < 10 mmol/L, and base excess − 33.8. Laboratory findings suggested hyperglycaemia with glycosuria and ketoacidosis. An abdominal computed tomography (CT) imaging revealed a patchy hypodense shadow of the spleen with wedge-shaped changes (Fig. 1). After admission, the patient was diagnosed as having diabetic ketoacidosis combined with spleen infarction, which was life-threatening, based on clinical characteristics and auxiliary examination.
Histopathological examination shows a large amount of coagulated necrotic tissue containing neutrophils, lymphocytes, eosinophils, multinucleated giant cells, and necrotic tissue (A1 H&E stain ×50; A2 H&E stain ×200). Folded, wrinkled, or swollen broad tubular hyphae are clearly observed under high magnification. Disordered hyphae rarely show branching; a few are visible as right-angled branches detached from the lateral wall, and the rest are seen as transversely cut round, oval, and polygonal unbranched cystic hyphae (B1 H&E stain ×200; B2 H&E stain ×400). Massive mycelial invasion is seen in the necrotic area of the spleen and in the blood vessels, with neutrophil infiltration both inside and outside the vessels, leading to fungal vasculitis (C1 H&E stain ×200; C2 H&E stain ×400). Periodic acid Schiff (PAS) stain shows the mycelium with pink walls (D1 PAS stain ×200; D2 PAS stain ×400). Grocott methylamine silver (GMS) stain shows the mycelium with brown–black walls (E1 GMS stain ×200; E2 GMS stain ×400)
Therefore, emergency splenectomy was performed, and routine spleen histopathology showed a large amount of coagulated necrotic tissue containing neutrophils, lymphocytes, eosinophils, multinucleated giant cells, and necrotic tissue (Fig. 2A1,2). Broadly tubular hyphae that were folded, wrinkled, or swollen (10–15 μm) were clearly observed under high magnification. The disordered hyphae rarely branched, though a few were seen to branch at right angles separating from the lateral wall. Transversely cut, round, elliptical, or polygonal unbranched cystic hyphae were seen in other parts of the tissue (Fig. 2B1,2). Massive mycelial invasion was seen in the necrotic areas of the spleen and in the blood vessels, where mycelial and neutrophil infiltration leading to fungal vasculitis was also observed (Fig. 2C1,2). Grocott methylamine-silver (GMS) (Fig. 2D1,2) and periodate-Schiff (PAS) (Fig. 2E1,2) were used to stain the fungal walls purplish-red and brownish-black, respectively, and the results clearly revealed structural details of the walls of the fungal hyphae.
After surgery with rehydration correct dehydration, acidosis and electrolyte disorder, antibiotics to prevent infection, intravenous antifungal therapy (one 2-week course of amphotericin B in liposome form, approx. 5 mg/kg). After 12 h of intravenous infusion of insulin (blood glucose was measured every hour), levels of blood ketone body and urine ketone body turned negative for three consecutive times, the intravenous infusion of insulin was stopped, and the blood glucose was controlled by insulin pump, with close monitoring via blood gas analysis until the acid-base balance was achieved.
After discharge, metformin was prescribed regularly to control blood glucose. The patient was followed up for 2 years without relapse.
Discussion
Mucormycosis is a life-threatening and angio-invasive fungal infection that often occurs secondary to diabetic ketoacidosis. Thus far, no specific clinical symptoms and ancillary tests have been identified for this condition, which makes the clinical diagnosis of mucormycosis very difficult.
The occurrence of isolated splenic mucormycosis is extremely rare, as till now, only five cases of this condition have been reported [3,4,5,6,7] (Table 1). The underlying conditions in these cases were human immunodeficiency virus infection, multiple myeloma, aplastic anaemia, acute lymphoblastic leukaemia, and lung transplant.
As of now, no cases of isolated splenic mucormycosis secondary to diabetic ketoacidosis have been reported. In Roden’s review of 929 cases of mucormycosis [8], diabetes was the primary risk factor (36%), with the most common sites of infection being the nasal sinuses, orbit, and brain. Patients with diabetes have low immune function, and the phagocytosis and chemotaxis abilities of neutrophils and macrophages are limited, enabling fungal germination and mycelium growth. Iron reuptake by transferrin and ferritin is also reduced, which will increase free iron and stimulate rapid mucosal proliferation [9]. Usually, mucormycosis spores are known to enter the body through respiratory inhalation, gastrointestinal ingestion, contaminated wounds caused by skin loss, intravenous infusion, etc. [2, 10]. The route of infection in isolated splenic mucormycosis is controversial with some suggesting that it may be blood-borne [11]; however, the specific underlying mechanism has not been clearly identified.
The clinical manifestations of splenic mucormycosis are nonspecific [12] and may present as pain in the upper left part of the abdomen, occasionally radiating to the left shoulder, and may be accompanied by common symptoms of infection, such as fever. Other symptoms may include gastrointestinal symptoms such as nausea and vomiting, with pressure-induced pain in the upper left part of the abdomen on examination. Low-density images of the spleen, which may be patchy or wedge-shaped, are also commonly seen in abdominal CTs, suggesting splenic infarction [13].
As of now, fungal cultures, histopathological biopsies and molecular biology-based tools may be used to confirm a diagnosis for mucormycosis. The fungal culture is highly specific, but low sensitivity results in false negatives in more than half the cases of mucormycosis infections. Histopathological biopsies can identify fungal infections more clearly, while also allowing visualisation of vascular invasion by the fungus [14].
An important hallmark of mucormycosis is vascular invasion and extensive tissue infarction, with the infected organ/area often showing intense granulomatous inflammation (with epithelioid cells, multinucleated giant cells, lymphocytes, and necrotic tissue) [15]. The infected areas also show massive neutrophil and eosinophil infiltration, interstitial fibrosis, and granulation tissue formation, sometimes with the classic Splendore-Hoeppli phenomenon [16].
The fungal hyphae are wide (5–20 μm), thin-walled, and irregularly ribboned with little separation (pauciseptate), and branch irregularly at no fixed angle; in cross-sections, the fungus appears cystic, and spores are rarely seen [15]. Haematoxylin and eosin staining of the mycelia reveals only the cell wall with no internal structures; however, GMS and PAS, which stain the fungal cell walls purplish-red and brownish-black, respectively, are better at revealing fungal wall structures [15]. New molecular biology-based methods can also confirm the presence of mucormycosis but are not yet widely used in clinical practice; more advanced non-invasive tests for this condition have yet to be developed [15].
In this case, the lungs, nose, brain, or skin were found to be free of mucormycosis. Since the spleen is an intra-abdominal parenchymal organ, the diagnosis of mucormycosis splenicum was confirmed mainly through histopathological biopsy. Splenic mucormycosis is mainly diagnosed by comparing biopsies of infected tissue with other splenic tissue infected by other fungal agents such as Aspergillus and Candida; Candida hyphae are thin (2–4 μm in diameter) and often bead-like [18], whereas Aspergillus hyphae are moderately and uniformly thick (5–10 μm in diameter) and usually exhibit acute angular branching [19].
In this case, the splenic mucormycosis was treated through surgical splenectomy and administration of an antifungal agent (amphotericin B), along with symptomatic treatment (acid correction, rehydration, etc.). However, some characteristics of the patients might have also influenced the treatment decision; for example, the patient in this case was older and had a history of diabetes for many years. During the treatment, a large amount of fluid rehydration is likely to induce acute left heart failure, which is life-threatening [20]. Since mucormycosis is associated with high mortality rates, early and definitive diagnosis and clinical intervention can directly affect prognosis [13].
In conclusion, we report here, the first known case of isolated splenic mucormycosis occurring secondary to diabetic ketoacidosis. The diagnosis was confirmed by microscopy of the splenic biopsy tissue, in which characteristic mycelia were observed. The patient was treated with an antifungal agent (amphotericin B) after surgically removing the spleen, and significant improvement was seen. This case serves as an important reference for the early diagnosis and treatment of isolated splenic mucormycosis associated with diabetic ketoacidosis.
Data availability
All the data regarding the findings are available within the manuscript.
Abbreviations
- CT:
-
Computed tomography
- PAS:
-
Periodicacid–Schiff
- GMS:
-
Gomori methenamine silver
References
Rammaert B, Lanternier F, Poirée S, et al. Diabetes and mucormycosis: a complex interplay. Diabetes Metab. 2012;38(3):193–204.
Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–69.
Teira R, Trinidad JM, Eizaguirre B, et al. Zygomycosis of the spleen in a patient with the acquired immunodeficiency syndrome. Mycoses. 1993;36(11–12):437–9.
Pastor E, Esperanza R, Grau E. Splenic mucormycosis. Haematologica. 1999;84(4):375.
Nevitt PC, Das Narla L, Hingsbergen EA. Mucormycosis resulting in a pseudoaneurysm in the spleen. Pediatr Radiol. 2001;31(2):115–6.
O’Connor C, Farrell C, Fabre A, et al. Near-fatal mucormycosis post-double lung transplant presenting as uncontrolled upper gastrointestinal haemorrhage. Med Mycol Case Rep. 2018;21(3):30–3.
Sharma SK, Balasubramanian P, Radotra B, et al. Isolated splenic mucormycosis in a case of aplastic anaemia. BMJ Case Rep. 2018;2018(2):bcr2017223243.
Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–53.
Beiglboeck FM, Theofilou NE, Fuchs MD, et al. Managing mucormycosis in diabetic patients: A case report with critical review of the literature. Oral Dis. 2022;28(3):568–76.
Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi (Basel). 2020;6(4):1.
Gupta V, Singh SK, Kakkar N, et al. Splenic and renal mucormycosis in a healthy host: successful management by aggressive treatment. Trop Gastroenterol. 2010;31(1):57–8.
Schneider M, Kobayashi K, Uldry E, et al. Rhizomucor hepatosplenic abscesses in a patient with renal and pancreatic transplantation. Ann R Coll Surg Engl. 2021;103(4):e131-5.
Hammami F, Koubaa M, Chakroun A, et al. Survival of an immuno-competent patient from splenic and gastric mucormycosis-case report and review of the literature. J Mycol Med. 2021;31(4):101174.
Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405-21.
Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24(2):247–80.
Mamlok V, Cowan WT Jr, Schnadig V. Unusual histopathology of mucormycosis in acute myelogenous leukemia. Am J Clin Pathol. 1987;88(1):117–20.
Kung VL, Chernock RD, Burnham CD. Diagnostic accuracy of fungal identification in histopathology and cytopathology specimens. Eur J Clin Microbiol Infect Dis. 2018;37(1):157–65.
Zhang D, Li X, Zhang J, et al. Characteristics of invasive pulmonary fungal diseases diagnosed by pathological examination. Can J Infect Dis Med Microbiol. 2021;2021(10):5944518.
Love GL. Zygomycosis. In: Pritt BS, Procop GW, editors. Pathology of Infectious Diseases. Philadelphia: Elsevier/Saunders; 2015. p. 491–515.
Keller U. Diabetic ketoacidosis: current views on pathogenesis and treatment. Diabetologia. 1986;29(2):71–7.
Acknowledgements
The authors would like to thank Bulletedits (bulletedits.cn) for English language editing.
Funding
This study was supported by Guizhou Provincial Science and Technology Projects (grant No. Qiankehejichu[2020]1Y429, Qiankehezhicheng[2022]Yiban182, Qiankehejichu-ZK[2022]Yiban662), Science and Technology Projects of Guizhou Health and Health Committee (grant No. gzwjkj2020-1-175), science grant of affiliated Hospital of Zunyi Medical University (grant No. yuanzi(2017)14).
Author information
Authors and Affiliations
Contributions
Resources: SL, YL, Writing-original draft: SL. Writing-review and editing: SL, XH, JJW. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report was approved by the Ethics Committee of Affiliated Hospital of Zunyi Medical University. Written informed consent was obtained from the patient for publication of this clinical case report.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Luo, S., Huang, X., Li, Y. et al. Isolated splenic mucormycosis secondary to diabetic ketoacidosis: a case report. BMC Infect Dis 22, 596 (2022). https://doi.org/10.1186/s12879-022-07564-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07564-3