Abstract
Background
Acute fibrinous and organizing pneumonia (AFOP) is a rare lung condition that is associated with acute lung injury. Its etiology may be idiopathic or secondary to a series of conditions, including immune-related diseases, unclassified connective tissue diseases, hematopoietic stem cell transplantation, infections, hematological diseases and drug induced lung toxicity. We report for the first time a case of AFOP complicated with hemophagocytic lymphohistiocytosis (HLH) caused by chronic active Epstein-Barr virus (CAEBV) infection.
Case presentation
A 64-year-old man was admitted with a complaint of fever and dyspnea for 2 weeks. The patient presented with elevated serum aminotransferase levels, splenomegaly, progressive decrease of red blood cells and platelets, hyperferritinemia, hypofibrinogenemia, and elevated of Soluble interleukin-2 receptor (sCD25). His chest computed tomography (CT) scan revealed multiple patchy consolidation in both lungs and multiple lymphadenopathy in the mediastinum and hilum. The serology for antibodies of VCA-IgG was positive, EBV-DNA in peripheral blood was elevated, and EBV nucleic acid was detected in the alveolar lavage fluid. Histopathology of the lung tissue showed a dominant of intra-alveolar fibrin and organizing pneumonia. Hemophagocytic cells was found in the bone marrow smear and biopsy. EBV-DNA was detected in lung tissue and bone marrow using in situ hybridization with an EBV-encoded RNA (EBER) probe. After 50 days of hospitalization, he was improved in lung and hemogram.
Conclusion
We report a case of AFOP with HLH caused by CAEBV in an immunocompetent adult, suggesting that AFOP may be a rare but serious complication caused by CAEBV, and glucocorticoid therapy may improve short-term prognosis.
Similar content being viewed by others
Background
Acute fibrinous and organizing pneumonia (AFOP) is a rare lung condition that is associated with acute lung injury. Its etiology may be idiopathic or secondary to a series of conditions, including immune-related diseases, unclassified connective tissue diseases, hematopoietic stem cell transplantation, infections (bacteria, fungi, viruses, etc.), hematological diseases and drug induced lung toxicity [1]. Many viral infections, including H1N1 [2], HIV [3] and coronavirus [4] have been reported to be associated with AFOP. Epstein-Barr virus (EBV) is one of the most common viruses in the human body without causing disease symptoms. In recent years, the development of molecular, biological and immunological detection methods makes it possible to detect viral specific genes in affected tissues. The highly sensitive and specific detection methods for EBV have enabled investigators to delineate the role of the EBV in various diseases of unknown origin [5, 6]. We report for the first time to our knowledge a case of AFOP complicated with hemophagocytic lymphohistiocytosis (HLH) caused by chronic active EBV (CAEVB) infection. Both the genomic and traditional examination approaches were used in the diagnosis of the reported case.
Case presentation
In September 25, 2018, a 64-year-old man was admitted into our hospital with a complaint of fever, dry cough and dyspnea for 2 weeks while the patient was on antibiotic treatment for pneumonia. In July 6, 2016, the patient was hospitalized due to fever and multiple lymphadenopathy. At that time, an EBV-DNA load of 2.8*104 copies/ml was detected in the patient’s plasma. After 2 weeks of hospitalization, the enlarged lymph nodes subsided and the patient was improved without specific treatment. The patient also had a history of type 2 diabetes mellitus.
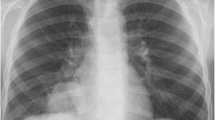
On admission, his temperature was 39.4 °C; respiration rate was 30/min; blood pressure was 153/76 mmHg; pulse rate was 102/min; SpO2% was 88% with 45% high-flow nasal oxygen, PaO2/FiO2 was 135 mmHg; and wheezing was present in both lungs. Blood tests (Table 1) yielded a leukocyte count of 8.42 × 109/L, hemoglobin level of 129 g/L, platelet count of 212 × 109/L, procalcitonin level of 0.56 ng/ml, erythrocyte sedimentation rate of 39/mm, C-reactive protein level of 17.4 mg/dl, alanine aminotransferase (ALT) level of 93 IU/L, and aspartate aminotransferase (AST) level of 186 IU/L. The LDH of pleural fluid was 925 mmol/L. Serology for antibodies of VCA-IgG was 87.34RU/ml (< 16RU/ml), VCA-IgM was 0.08S/CO (< 0.8S/CO), VCA-IgA was 0.13S/CO (< 0.8S/CO) and EBV-DNA load in plasma was 3.27 × 104 copies/ml. His chest computed tomography (CT) scan showed multiple patchy opacities with consolidation in both lungs, marked lesions in the left lung, and multiple lymphadenopathy in the mediastinum and hilum (Fig. 1). EBV nucleic acid in the bronchoalveolar lavage fluid (BALF) was positive by polymerase chain reaction (PCR). Other pathogenic microbials were not detected in the blood, urine, pleural effusion, or BALF. Piperacillin/tazobactam and levofloxacin were used as empirical antibiotics.
In 10 days after admission, the patient was till suffered fever and transferred to an intensive care unit where an intubation was required because of severe hypoxia. Meanwhile, a chest CT scan showed significant exacerbation (Fig. 2), and blood tests (Table 1) found a leukocyte count of 3.5 × 109/L, hemoglobin level of 87 g/L, platelet count of 32 × 109/L, fibrinogen of 1.47 g/L, triglyceride of 1.75mmo/L, serum ferritin level of > 15000 ng/ml, and soluble IL-2Ra (sCD25) level of 15185 pg/ml. Ultrasound showed splenomegaly with a length of 13 cm. In addition, a bone marrow examination identified hemophagocytosis. EBV DNA was detected in bone marrow using in situ hybridization with an EBV-encoded RNA (EBER) probe (Fig. 3). We used the SCOP 1 microscopy of Zeiss and MShot Image Analysis System was used to capture the microscopy images. Histopathology examination of a piece of lung tissue through percutaneous needle biopsy showed a dominant of intra-alveolar fibrin and organizing pneumonia, which suggested AFOP. In addition, EBERs were detected in the lung tissue (Fig. 4).
A Lung biopsy tissue showed a large number of fibrin were filled in the alveolar cavity, accompanied by acute inflammatory cells. There were no hyaline membranes or pulmonary edema. (HE, × 40). B Myofibroblasts proliferated in some alveolar cavities, showing the change of organic pneumonia. (HE, × 200). C EBV-positive activated lymphocytes in pulmonary interstitium. (In situ hybridization EBERs, × 200)
Based on the above diagnoses, methylprednisolone at a dosage of 120 mg/d was administered for 5 days, followed by dexamethasone at a dosage of 10 mg/m2/d for 10 days and immunoglobulin at 0.4 g/Kg/d for 5 days. The fever that lasted for one month finally improved after methylprednisolone. Sulfanilamide and ganciclovir were used as prophylactic for fungal and virus. Two weeks later, the ventilator was able to be removed from this patient. In 50 days after admission, lung lesions were absorbed (Fig. 5), EBV-DNA load in plasma decreased to 2.2 × 102 copies/ml, and the patient was discharged.
Discussion and conclusions
This is the first case report of AFOP with HLH caused by CAEBV to our knowledge. HLH is a group of clinical syndromes characterized by multiple factors causing cytokine cascade release and tissue cell proliferation accompanied by phagocytosis of various hematopoietic cells. HLH is often secondary to infection, tumors and/or autoimmune diseases. Infection-related HLH is common, of which EB virus infection accounts for the majority. Tumor-related HLH is also common, especially in hematological malignancies such as lymphoma [7]. This patient had fever, splenomegaly, cytopenia, hypofibrinogenemia, hemophagocytic cells found in the bone marrow, significantly elevated serum ferritin and sCD25, meeting 7 of the 8 diagnostic criteria [8]. Therefore, the HLH diagnosis is clear.
The main clinical manifestation of AFOP is dyspnea, which may be accompanied by fever and cough. It is acutely or sub-acutely onset. Most acute cases develop respiratory failure rapidly, requiring mechanical ventilation treatment. The imaging manifestations of AFOP are mostly nodules and solid changes with unclear boundary in both lungs, mainly in the basal part and/or along the peribronchial vascular distribution [9]. Histopathological features include large amount of cellulose deposition in alveolar cavity accompanied by organic loose connective tissue, lack of typical transparent membrane formation in diffuse alveolar injury, no granuloma, and no obvious eosinophil infiltration [10]. The clinical symptoms, imaging characteristics and histopathological features of our patient were consistent with those of AFOP, so the diagnosis of AFOP was clear. AFOP can be secondary to viral infection, bacterial infection, connective tissue diseases and so on. This patient had subacute onset, and the formation of AFOP may be related to the acute exacerbation of CAEBV. The overall prognosis of AFOP patients is poor, and the mortality rate can be as high as 50%. Some cases respond well to glucocorticoids and immunosuppressive agents in the acute phase, but relapse in the course of hormone reduction, and their long-term prognosis is still poor [9]. Our patient was treated with methylprednisolone before taking HLH into account. Glucocorticoids dosage was then given according to the HLH treatment guideline.
EBV is usually a latent infection in the human body, and some patients may develop into chronic active infection [11]. T-cell Chronic active Epstein-Barr virus tends to predominate in Asia, while B-cell CAEBV is frequently documented in Western regions. The differential diagnosis between CAEBV and hemophagocytic lymphohistiocytosis (EBV-HLH) is extremely challenging from clinical and pathologic points of view. Guidelines for classification between EBV-HLH and CAEBV are not clear and the two definitions are used interchangeably by various authors [12,13,14]. The diagnostic criteria of CAEBV include: (1) Persistent or recurrent IM-like symptom including fever, swelling of lymph nodes, and hepatosplenomegaly; (2) Unusual pattern of anti-EBV antibodies with raised anti-VCA and anti-EA, and/or detection of increased EBV genomes in affected tissues, including the peripheral blood; (3) Chronic illness which cannot be explained by other known disease processes at diagnosis [15]. This patient had recurrent fever with lymphadenopathy at both the current episode and 2 years ago, significantly elevated EBV-DNA in peripheral blood, and EBERs in bone marrow and lung tissue. So, CAEBV diagnosis was clear. CABEV can lead to life-threatening complications, including HLH, DIC, liver failure, coronary artery aneurysm, central nervous system involvement, myocarditis, lymphoma and hematological malignancies [5, 16]. Few cases involving the lungs have been reported. Pulmonary diseases associated with EBV infection reported in the literature include hilar/mediastinal lymph node enlargement, pleural effusion and interstitial pneumonia [17]. CAEBV infection with pulmonary parenchymal involvement is rare in immunocompetent patients. Joo EJ et al. reported a case of interstitial pneumonia caused by CAEBV [18]. In that case, a 28 year old female was admitted because of fever and bilateral pleural effusion. Her clinical features are similar to those of our patient, including pancytopenia, liver dysfunction, splenomegaly, and multiple lymphadenopathy. Unlike our patient’s multiple patchy opacities with consolidation in both lungs, her chest CT showed diffuse interstitial lesions. She was also admitted to the ICU because of severe respiratory failure. Her lung histopathology showed alveolar septal lymphocyte infiltration. Jacek Roliński et al. reported a case of CAEBV complicated with interstitial pneumonia, which was considered to be associated with infectious diseases [19].
In conclusion, we report a case of AFOP with HLH caused by CAEBV in an immunocompetent adult, suggesting that AFOP may be a rare but serious complication caused by CAEBV, and glucocorticoid therapy may improve short-term prognosis.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AFOP:
-
Acute fibrinous and organizing pneumonia
- EBV:
-
Epstein-Barr virus
- HLH:
-
Hemophagocytic lymphohistiocytosis
- CAEBV:
-
Chronic active EBV
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- CT:
-
Computed tomography
- BALF:
-
Bronchoalveolar lavage fluid
- EBER:
-
EBV-encoded RNA
References
Gomes R, Padrao E, Dabo H, Soares Pires F, Mota P, Melo N, Jesus JM, Cunha R, Guimaraes S, Souto Moura C, et al. Acute fibrinous and organizing pneumonia: a report of 13 cases in a tertiary university hospital. Medicine. 2016;95(27):e4073.
Otto C, Huzly D, Kemna L, Huttel A, Benk C, Rieg S, Ploenes T, Werner M, Kayser G. Acute fibrinous and organizing pneumonia associated with influenza A/H1N1 pneumonia after lung transplantation. BMC Pulm Med. 2013;13:30.
Heo JY, Song JY, Noh JY, Yong HS, Cheong HJ, Kim WJ. Acute fibrinous and organizing pneumonia in a patient with HIV infection and Pneumocystis jiroveci pneumonia. Respirology (Carlton, Vic). 2010;15(8):1259–61.
Cincotta DR, Sebire NJ, Lim E, Peters MJ. Fatal acute fibrinous and organizing pneumonia in an infant: the histopathologic variability of acute respiratory distress syndrome. Pediatric Crit Care Med. 2007;8(4):378–82.
Okano M. Haematological associations of Epstein-Barr virus infection. Bailliere’s Best Practice & Res Clin Haematol. 2000;13(2):199–214.
Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol. 2015;33:787–821.
Ramachandran S, Zaidi F, Aggarwal A, Gera R. Recent advances in diagnostic and therapeutic guidelines for primary and secondary hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2017;64:53–7.
Henter JI, Horne A, Arico M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–31.
Kim JY, Doo KW, Jang HJ. Acute fibrinous and organizing pneumonia: imaging features, pathologic correlation, and brief literature review(). Radiol Case Rep. 2018;13(4):867–70.
Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126(9):1064–70.
Rickinson AB. Chronic, symptomatic Epstein-Barr virus infections. Immunol Today. 1986;7(1):13–4.
Kimura H, Hoshino Y, Kanegane H, Tsuge I, Okamura T, Kawa K, Morishima T. Clinical and virologic characteristics of chronic active Epstein-Barr virus infection. Blood. 2001;98(2):280–6.
Kimura H, Ito Y, Kawabe S, Gotoh K, Takahashi Y, Kojima S, Naoe T, Esaki S, Kikuta A, Sawada A, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119(3):673–86.
Paik JH, Choe JY, Kim H, Lee JO, Kang HJ, Shin HY, Lee DS, Heo DS, Kim CW, Cho KH, et al. Clinicopathological categorization of Epstein-Barr virus-positive T/NK-cell lymphoproliferative disease: an analysis of 42 cases with an emphasis on prognostic implications. Leuk Lymphoma. 2017;58(1):53–63.
Okano M, Kawa K, Kimura H, Yachie A, Wakiguchi H, Maeda A, Imai S, Ohga S, Kanegane H, Tsuchiya S, et al. Proposed guidelines for diagnosing chronic active Epstein-Barr virus infection. Am J Hematol. 2005;80(1):64–9.
Kimura H. Pathogenesis of chronic active Epstein-Barr virus infection: is this an infectious disease, lymphoproliferative disorder, or immunodeficiency? Rev Med Virol. 2006;16(4):251–61.
Ankermann T, Claviez A, Wagner HJ, Krams M, Riedel F. Chronic interstitial lung disease with lung fibrosis in a girl: uncommon sequelae of Epstein-Barr virus infection. Pediatr Pulmonol. 2003;35(3):234–8.
Joo EJ, Ha YE, Jung DS, Cheong HS, Wi YM, Song JH, Peck KR. An adult case of chronic active Epstein-Barr virus infection with interstitial pneumonitis. Korean J Intern Med. 2011;26(4):466–9.
Roliński J, Grywalska E, Pyzik A, Dzik M, Opoka-Winiarska V, Surdacka A, Maj M, Burdan F, Pirożyński M, Grabarczyk P, et al. Interferon alpha as antiviral therapy in chronic active Epstein-Barr virus disease with interstitial pneumonia—case report. BMC Infect Dis. 2018;18(1):190.
Acknowledgements
Not applicable.
Funding
This work was supported, in part, by the National Natural Science Foundation of China (Grant NO. 81870072), the National Key Research and Development Program of China (Grant NO. 2016YFC1304300) and the CAMS Innovation Fund for Medical Sciences (No.2018-I2M-1-003). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
XJW prepared the initial manuscript draft and revised it. KJW, YYG, YC and WQW assisted in data management. DRZ and QYZ designed and supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, X., Wang, K., Gao, Y. et al. Acute fibrinous and organizing pneumonia complicated with hemophagocytic lymphohistiocytosis caused by chronic active Epstein-Barr virus infection: a case report. BMC Infect Dis 21, 1207 (2021). https://doi.org/10.1186/s12879-021-06868-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06868-0