Abstract
Background
Tanzania ranks as the fourth country in the world with respect to the number of sickle cell disease (SCD) births; it is also endemic to the human immunodeficiency virus (HIV) and the hepatitis B virus (HBV). This study was done to determine the prevalence of HIV and HBV infections among SCD patients in Dar es Salaam, Tanzania.
Methods
A multicenter hospital-based descriptive cross sectional study was carried out among participants aged ≥ 16 years with a proven diagnosis of SCD. Socio-demographic and clinical data were recorded. Blood samples were drawn for HIV and HBV diagnosis. All categorical variables were summarized into frequencies.
Results
There were 185/325 (56.9 %) females. The mean age (SD) was 23.0 ± 7.5 years. The prevalence of HIV was 1.8 %; the prevalence of HBV was 1.2 %.
Conclusions
The prevalence of both HIV and HBV in SCD patients is no greater than in the general population of Dar es Salaam or Tanzania. For associations, a large study would be needed. From a detailed blood transfusion history of SCD patients we found no evidence that HIV or HBV infection was transmitted through blood transfusion.
Similar content being viewed by others
Background
It is estimated that 300,000 children are born annually in the world with sickle cell disease (SCD). An estimated 75 % of SCD affected children live in sub-Saharan Africa [1, 2]. Tanzania is ranked as the fourth country globally with the highest number of SCD births after Nigeria, Democratic Republic of Congo and India [2, 3].
Improvements in the care of SCD have increased the life span of these patients. Thus, there are now more SCD children who grow into adulthood and face other health risks, including infections. Human immunodeficiency virus (HIV) and hepatitis B virus (HBV) infections are transmissible diseases that may add a burden to SCD patients. It is estimated that nearly one adult in every 25 is living with HIV in Sub-Saharan Africa, that thus accounts for nearly two-thirds of the people living with HIV worldwide [4]. In 2017 Dar es Salaam had a 4.7 % prevalence of HIV infection while the prevalence in Tanzania as a country was 5.1 % [5]. Globally, HBV prevalence was estimated to be 3.5 % in 2015 resulting in an estimated 887,000 hepatitis B-related deaths, mostly from complications such as cirrhosis and hepatocellular carcinoma. Africa and Western Pacific regions accounted for 68 % of those infected with HBV [6]. Tanzania is among the countries with the highest HBV infection rates. A prevalence of 6 % was reported in 1998 among the general population of the largest city in the country, Dar es Salaam [7]. Another study carried out in 2004–2005 among blood donors in the same city revealed a prevalence of 8.8 % for HBV and 3.8 % for HIV infection [8].
HIV and HBV have similar modes of transmission: therefore, co-infection is common [9, 10]; and HIV co-infected patients have an increased risk of developing chronic HBV [11]. HIV infection of an SCD patient may pose special challenges in antiretroviral therapy (ART): as anemia is a contra-indication for certain drugs, as illustrated by a case report from Kenya [12].
SCD has been thought to be protective against acquiring HIV [13, 14], and it may slow down the progression of HIV disease [15] through host factors such as functional asplenia, or the use of hydroxyurea, that may be synergistic with some reverse transcriptase inhibitors [16, 17]. On the other hand, SCD patients with HIV infection tend to have more complicated hospitalization than those without HIV infection [18]. SCD patients are highly susceptible to infection with encapsulated organisms, and this susceptibility may be increased by concomitant HIV infection [19, 20].
Blood transfusion has been reported to be a risk factor for HIV acquisition in SCD patients in studies conducted in some African countries such as Democratic Republic of Congo [21], Nigeria [22] and Cameroon [23]. Unsafe blood transfusion is also a risk factor for HBV infection. In the USA, however, it was found that among adults with SCD the frequency of HIV was 1.5 %, compared to 3.3 % in African–Americans without SCD, suggesting that blood transfusion was not a risk factor.
This study was conducted to investigate the burden of HIV and HBV among SCD patients receiving SCD care in Dar es Salaam, Tanzania. This study was previously presented as an abstract at the 7th MUHAS Scientific Conference on June 27–28, 2019.
Methods
Design and setting of the study
A cross sectional multisite hospital-based study was conducted from August 2018 to January 2019 among sickle cell patients attending clinics in Dar es Salaam. Dar es Salaam is the largest city in Tanzania and has five municipal districts and an estimated population of 4,364,541 as per the 2012 National census [24]. The study participants were obtained from four study sites, one being a tertiary hospital (Muhimbili National Hospital) and three regional referral hospitals (Amana, Temeke and Mwananyamala). Sickle cell patients at Muhimbili National Hospital (MNH) received care in the department of Haematology and blood transfusion while those in the three regional hospitals received care in departments of internal medicine. All clinics ran once a week and received an average of 15 adult patients per week.
Study population
All consenting SCD patients aged 16 years or older who attended sickle cell clinics in Dar es Salaam were consecutively enrolled in the study. None of the patients was excluded.
Data collection procedure
A structured interview was done with all patients who consented to participate in the study. Independent variables collected were socio-demographic characteristics including age, sex, marital status, level of education, residency and occupation. Other risks of interests were condom misuse, multiple sexual partners, tattooing, piercing, intravenous drug abuse, history of blood transfusion, hospital admission, surgery, tooth extraction, sexual vulnerability behaviours such as alcohol use and forced sexual intercourse. The value of the last measured hemoglobin (HB) level was obtained from the SCD card. The HB was considered valid if it was done within 1 month of data collection.
Four milliliters of blood was drawn aseptically from a vein on the cubital fossa and put into an empty sterile red stoppered vacutainer for HIV and HBV screening. At the study sites samples were stored in cool boxes before being transferred to MUHAS Hematology Clinical and Research Laboratory at the end of each clinic day. Upon reaching the laboratory, samples were centrifuged, sera taken and tested for HBV surface antigen and HIV serology. HIV diagnosis was in accordance with Tanzanian algorithm [25] whereby SD Bioline HIV-1/2 3.0 (Standard Diagnostics, Inc, Republic of Korea) was done first. Negative results underwent no additional testing. Positive samples were retested using Determine™ hiv-1/2 (Abbots Laboratories,
Illinois, USA). Samples reactive to both tests were considered positive for IgG anti HIV antibodies. Positive tests By SD Bioline that tested negative by Determine™ hiv-1/2 were subjected to a third test, Uni-Gold™ HIV (Trinity Biotech Plc, Bray, Co. Wicklow, Ireland) and were considered negative if they tested negative or positive if they tested positive by Uni-Gold™.
Hepatitis B surface antigen was detected by the Onsite HBsAg rapid test by CTK Biotech, Inc, San Diego, USA. The test is a lateral flow chromatographic immunoassay for the qualitative detection of HBsAg in human serum or plasma. The test has a sensitivity and specificity of approximately 100 % and 100 % respectively when performed according to the instructions of the manufacturer. Negative results underwent no additional testing. Positive results were confirmed by ELISA using Murex HBsAg version 3 test kits manufactured by DiaSorin S.p.A, United Kingdom.
Statistical analysis
Data analysis was done using SPSS software version 20.0. Categorical variables were summarized into frequencies and percentage. Continuous variables were summarized into mean and standard deviation. No associations were calculated between variables and HIV or HBV infections due to small numbers of patients with these infections.
Results
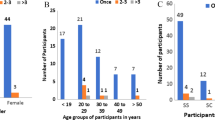
We tested 185 (56.9 %) females and 140 males with SCD (see Table 1). The mean age (± SD) was 23.0 ± 7.5 years, ranging from 16 to 52 years; 71.1 % were in the age group < 26 years. The majority (85.5 %) of the subjects had never married; 72.3 % had secondary school level education; 91.1 % had previous hospital admission, and the most frequent reason for admission in 56.1 % was a vaso-occlusive crisis. Only 18.8 % subjects were using hydroxyurea (HU) (Table 1). The mean recorded hemoglobin level (± SD) for the 325 study subjects was 7.4 (± 1.6) g/dl, (not shown in Table 1).
Of the 325 study participants, 6 (1.8 %; 95 % CI: 0.7–4.0 %) were HIV-infected; 4 (1.2 %; 95 % CI: 0.3–3.1 %) were infected with HBV. None of the study participants was HIV and HBV co-infected (not shown in Table 1).
All HIV and HBV-infected patients had been hospitalized and had received blood transfusion: the same was true for most of those who were not infected. Of the HIV infected patients none was on HU treatment; of the HBV-infected patients 2 were on HU. Of the 6 HIV-infected patients, 3 were sexually active; 3 had multiple sexual partners, and 2 of them used condoms. Of the 4 HBV-infected patients 3 were sexually active, and 3 reported a history of multiple sexual partners; only one reported using condom during the last sexual act, (Table 1).
Discussion
We have studied an adult population with a mean age of 23.0 ± 7.5 years, with a balanced gender ratio. Compared to previous surveys, it is interesting that a majority had secondary or higher-level education, thanks to the government policy to build secondary schools in each ward in Tanzania (2010), and following the introduction of free secondary education in 2015. The patient population we have studied have clearly benefited from these policies.
In this study the HIV prevalence of 1.8 % was lower than that reported in 2016–2017 in the general population of Dar es Salaam, which was 4.7 % [26]. The HIV prevalence was also lower than that observed in a similar study among SCD children in Tanzania, that was 4.4 % (unpublished data by Kamaria Kassim et al.). In studies carried out in other African countries endemic for HIV, frequencies have been higher: 5.6 % in Cameroon, 11.3 % in the Democratic Republic of Congo and 5.0 % in Togo [21, 23]: these studies had included both children and adults, whereas ours was on adults only. It is possible that some patients may have died during childhood, especially if they had perinatally acquired infection. On the other hand, our data may reflect the gradual decline in HIV prevalence that has been observed in Tanzania over recent years.
The prevalence of HBV in the present study was higher than that observed in children with SCD in the year 2010, which was 0.6 % (Kamaria Kassim et al., unpublished data). The difference could be due to cumulative exposure to the hepatitis B virus during life time, thus reflecting increasing prevalence with increasing age. Most important, vaccination against Hepatitis B was introduced in the Extended Programme for Immunization (EPI) in Tanzania in 2002, and most of our participants were born before then. On the other hand, our findings are similar to those in SCD patients in Brazil (3.1 %) [27]; in contrast, the HBV prevalence was as high as 10 % among SCD patients in the Democratic Republic of Congo (29).
Although co-infection with both HIV and HBV has been observed in Tanzania among blood donors [8] and adult HIV patients [30], we have not found it in our SCD patients, as in a similar study from Nigeria [29]. This is not surprising since, from the prevalence rates reported above, the a priori probability of co-infection can be estimated at 0.022 % (about 2 in 10,000); in addition, we must consider that this combination might increase mortality in patients with SCD.
Blood transfusion can of course tramsmit blood borne infections, if blood units are not properly screened. In our entire patient population 75 % had received at least one blood transfusion: this included all 6 HIV-infected and all 4 HBV infected patients. However, out of a total of 243 transfused patients, 233 (95.9 %) remained HIV-negative and HBV-negative. This is rather re-assuring: it indicates that the screenings that are obligatory for blood donors are reliable.
Study limitation
The present study was not powered to determine associations between the studied variables and the HIV or HBV viral infection.
Conclusion
The findings of this study suggest that the prevalence of HIV and HBV infection among SCD patients aged 16 years or older is no higher than the prevalence in the general population of Dar es Salaam. For the few SCD patients who are either HIV or HBV infected we do not know the source of infection; there is no reason to suspect that they had been infected via blood transfusion.
Availability of data and materials
The data set for this study can be found from the corresponding author upon reasonable request.
Abbreviations
- ART:
-
Antiretroviral therapy
- ELISA:
-
Enzyme linked immunosorbant assay
- EPI:
-
Extended Programme for Immunization
- HB:
-
Hemoglobin
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HIV:
-
Human immunodeficiency syndrome
- MHN:
-
Muhimbili National Hospital
- MUHAS:
-
Muhimbili University of Health and Allied Sciences
- SCD:
-
Sickle cell disease
- SD:
-
Standard deviation
- SPSS:
-
Statistical package for social sciences
- USA:
-
United States of America
References
Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–51.
Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, et al. Mortality in sickle cell anemia in africa: a prospective cohort study in Tanzania. PLoS One. 2011;6(2):e14699.
Makani J, Soka D, Rwezaula S, Krag M, Mghamba J, Ramaiya K, et al. Health policy for sickle cell disease in Africa: experience from Tanzania on interventions to reduce under-five mortality. Trop Med Int Health. 2015;20(2):184–7.
World Health Organization. Global Health Observatory (GHO) data: Prevalence of HIV among adults aged 15–49. 2018. https://www.who.int/gho/hiv/epidemic_status/prevalence_text/en/. Accessed 29 Dec 2020.
Tanzania Ministry of Health Community Development, gender elderly and children. Tanzania HIV Impact Survey (THIS) 2017. Vol. 1, Report. 2017. https://phia.icap.columbia.edu/wp-content/uploads/2017/11/Tanzania_SummarySheet_A4.English.v19.pdf
World Health Organization. Global hepatitis report, 2017. Global Hepatitis Programme. Who. 2017. 68 p. https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf?sequence=1&isAllowed=y%0Ahttps://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/%0Ahttp://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455eng.pdf
Miller WC, Shao JF, Weaver DJ, Shimokura GH, Paul DA, Lallinger GJ. Seroprevalence of viral hepatitis in Tanzanian adults. Trop Med Int Health. 1998;3(9):757–63.
Matee MI, Sciences A, Magesa P, Hospital MN, Lyamuya E. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis infections among blood donors at the Muhimbili National Hospital in Dar Es Salaam, Tanzania. BMC Public Health. 2006;6:21. https://doi.org/10.1186/1471-2458-6-21.
Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–67.
Thio CL. Hepatitis B and human immunodeficiency virus coinfection. Hepatology. 2009;49(SUPPL. 5).S138-45
Levy V, Grant RM. HIV/AIDS: antiretroviral therapy for hepatitis B virus–HIV–coinfected patients: promises and pitfalls. Clin Infect Dis. 2006;43(7):904–10.
Odera EB, Kwobah C, Stone G, Some F, Vreeman RC. Sickle cell disease and HIV. J Int Assoc Provid AIDS Care. 2014;13(2):113–6.
Oleske JM. Potential protective effect of sickle cell gene allele on HIV infection. Acad J Pediatr Neonatol. 2017;2(5):1–3.
Nouraie M, Nekhai S, Gordfile:///C:/Users/User/Desktop/MY DISSERTATION/sexuality and scd.pdfeuk VR. Sickle cell disease is associated with decreased HIV but higher HBV and HCV comorbidities in US hospital discharge records: a cross-sectional study. Sex Transm Infect. 2012;88(7):528–33.
Owusu ED a., Visser BJ, Nagel IM, Mens PF, Grobusch MP. The interaction between sickle cell disease and HIV infection: a systematic review. Clin Infect Dis. 2014;60(4):612–26.
Lori F, Kelly LM, Foli A, Lisziewicz J. Safety of hydroxyurea in the treatment of HIV infection. Expert Opin Drug Saf. 2004;3(4):279–88.
Lisziewicz J, Foli A, Wainberg M, Lori F. Hydroxyurea in the treatment of HIV infection: clinical efficacy and safety concerns. Drug Saf. 2003;26(9):605–24.
Kourtis AP, Bansil P, Johnson C, Meikle SF, Posner SF, Jamieson DJ. Children with sickle cell disease and human immunodeficiency virus-1 infection. Pediatr Infect Dis J. 2007;26(5):406–10.
Koffi KG, Sawadogo D, Meite M, Nanho DC, Tanoh ES, Attia AK, et al. Reduced levels of T-cell subsets CD4 + and CD8 + in homozygous sickle cell anaemia patients with splenic defects. Hematol J. 2003;4(5):363–5.
Caribbean HIV/AIDS Regional Training Network (CHART). HIV and sickle cell disease. http://www.chartcaribbean.org/careofplwa/pdfdcouments/subpdf/SectionIII/HIVandSickleCellDisease.pdf.
Tshilolo LM, Mukendi RK, Wembonyama SO. Blood transfusion rate in congolese patients with sickle cell anemia. Indian J Pediatr. 2007;74(8):735–8.
Ubesie A, Emodi I, Ikefuna A, Ilechukwu G, Ilechukwu G. Prevalence of human immunodeficiency virus transmission among transfused children with sickle cell anemia in Enugu Nigeria. Ann Med Health Sci Res. 2012;2(2):109–13.
Ngo Sack F, Eboumbou C, Ngouadjeu E, Zouhaïratou H, Mbanya D. Prevalence of HIV seropositivity among sickle cell disease patients at the Yaoundé Central Hospital. Health Sci Dis. 2013;14(3):3–7.
National Bureau of Statistics. 2012 Population and housing census. Zanzibar; 2013. http://www.tzdpg.or.tz/fileadmin/documents/dpg_internal/dpg_working_groups_clusters/cluster_2/water/WSDP/Background_information/2012_Census_General_Report.pdf
Tanzania National AIDS Control Program (NACP). Guidelines for HIV testing and counselling in clinical settings. online. 2007. http://www.who.int/hiv/topics/vct/TZ_PITC-Guidelines_finaledit_July2007.pdf.
National Bureau of Statistics. Tanzania HIV Impact Survey (THIS) 2016–2017. Tanzania HIV Impact Survey (THIS) 2016–2017. 2018. https://phia.icap.columbia.edu/wp-content/uploads/2019/06/FINAL_THIS-2016-2017_Final-Report__06.21.19_for-web_TS.pdf
Françoise NS, Carole E, Evelyne N, Haman Z, Dora M. Prevalence of HIV seropositivity among sickle cell disease patients at the Yaoundé Central Hospital. Health Sci Dis. 2013;14(3):3–7.
Neto JPM, Lyra IM, Reis MG, Goncalves MS. The association of infection and clinical severity in sickle cell anaemia patients. Trans R Soc Trop Med Hyg. 2011;105(3):121–6. https://doi.org/10.1016/j.trstmh.2010.11.007.
Tshilolo LM, Mukendi RK, Wembonyama SO. Blood transfusion rate in congolese patients with sickle cell anemia. Indian J Pediatr. 2007;74(8):735–8.
Kilonzo SB, Gunda DW, Kashasha F, Mpondo BC. Liver fibrosis and Hepatitis B coinfection among ART Naive HIV-infected patients at a tertiary level hospital in Northwestern Tanzania: a cross-sectional study. J Trop Med. 2017;2017.
Eberechukwu L, Ide Y, Babatunde S, Health C, Hospital HT, Harcourt P. Hepatitis B, C and human immunodeficiency virus (HIV) co-infection in nigerian children with sickle cell anaemia. Niger Heal J. 2015;15(1):18–23.
Acknowledgements
We are grateful to financial support from Mr. Godbless Mariki, without which this study would have been impossible. We are thankful to Professor Julie Makani and the Sickle Cell Disease team at Muhimbili University of Health and Allied Sciences (MUHAS) Hematology laboratory for their support on laboratory analyses. We acknowledge data analysis guidance from Dr. Candida Moshiro of the MUHAS Department of Biostatistics.
Funding
We are grateful to Mr. Godbless Mariki for funding the research. Mr. Mariki had no role in the design of the study, data collection, analysis, interpretation of data or writing the manuscript.
Author information
Authors and Affiliations
Contributions
This study was conceived by IM, GS and LL. Data collection, data entry and cleaning was done by IM and supervised by GS and LL. Analysis was done by IM and GS. The manuscript was written by GS and LL. All authors read and approved the final manuscript.
Authors’ information
Dr. Grace Shayo is a Senior lecturer and clinical instructor in the Department of Internal Medicine of the Muhimbili University of Health and Allied Sciences, Dr. Irene Makundi is a specialist in Internal medicine at Muhimbili National hospital. Dr. Lucio Luzzatto was a visiting Professor of hematology at the Muhimbili University of Health and Allied Sciences.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from the Muhimbili University of Health and Allied Sciences (MUHAS), Research and Publication Ethical Committee. Permission to conduct the study was obtained from the administration of MNH, Temeke, Mwananyamala and Amana hospitals. All patients provided a written informed consent. Patients diagnosed with HIV and/or HBV were referred to respective clinics for treatment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shayo, G., Makundi, I. & Luzzatto, L. The prevalence of human immunodeficiency and of hepatitis B viral infections is not increased in patients with sickle cell disease in Tanzania. BMC Infect Dis 21, 1028 (2021). https://doi.org/10.1186/s12879-021-06726-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06726-z