Abstract
Background
Descending necrotizing mediastinitis (DNM) is one of the most virulent forms of mediastinitis. The main causes of high mortality in DNM are believed to stem from difficulty and delay in the diagnosis. Fast and accurate identification of pathogens is important for the treatment of these patients. Metagenomics next-generation sequencing (mNGS) is a powerful tool to identify all kinds of pathogens, especially for rare and complex infections.
Case presentation
A 64-year-old male patient was admitted to the intensive care unit (ICU) with unconsciousness, dyspnea, and swelling in the mandible and neck. Computed tomography (CT) scan results combined with clinical laboratory examination indicated DNM. Vancomycin and imipenem were used, and vacuum sealing drainage was applied for debridement and drainage of the infected area. The positive mNGS results of drainage fluid confirmed the presence of mixed infection caused by Streptococcus anginosus, Prevotella oris, and several other anaerobes. The antibiotics were adjusted to piperacillin/tazobactam and tinidazole according to the mNGS results and antimicrobial susceptibility testing of cultured pathogens. After 11 days of antibiotic therapy, the infection symptoms of the neck and mediastinum improved, and the patient was transferred out of the ICU on the 26th day after negative result of drainage fluid culture.
Conclusion
This case suggested that mNGS is a promising technology for precise and fast pathogens identification with high sensitivity, which may guide the diagnosis of infectious diseases in the future trend.
Similar content being viewed by others
Background
Descending necrotizing mediastinitis (DNM) is a type of pyogenic mediastinitis, the infections usually have a fulminant course leading to sepsis and even death. Dental infection is a common cause of DNM, followed by retropharyngeal abscesses, peritonsillar abscesses, traumatic endotracheal intubation, trauma, cervical lymphadenitis, and osteomyelitis. Criteria for diagnosis of DNM are: (1) clinical manifestations of oropharyngeal infection; (2) characteristic roentgenographic features of mediastinitis; (3) documentation of mediastinitis during surgery or postmortem examination; (4) establishment of a relationship between oropharyngeal infection and subsequent necrotizing mediastinitis [1].The mortality of DNM has been reported to be 40%, which is approximately treble the risk of septic shock [2, 3]. However, prompt diagnosis, early aggressive incision, sufficient drainage, targeted antibiotic therapy combined with intensive care unit (ICU) management could significantly reduce the mortality to less than 20% [4]. Herein, we report a case in which mNGS was applied to identify the pathogens of DNM caused by odontogenic infection.
Case presentation
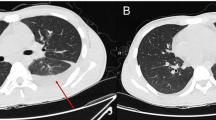
A 61-year-old male patient with a history of hypertension was admitted to the ICU, with loss of consciousness and dyspnea. He had a toothache 15 days ago, and got some non-steroidal anti-inflammatory drugs in local pharmacy. However, the condition was not improved and mandible and neck begun to swell 10 days ago, and the situation deteriorated in the following days until he was admitted. The patient’s vitals were as follows: radial artery blood pressure was 112/77 mmHg, axillary temperature was 36.8℃, heart rate was 90 bpm, oxygen concentration was 45% with high flow oxygen inhalation, and blood oxygen saturation was 99%. Lung breath was clear, with no audible dry or wet rales. Blood test results: the leukocyte count was 4.05 × 109 /L with high percentage of neutrophils and low percentage of lymphocyte (90.4% and 4.3%, respectively), C-reactive protein (CRP) was > 200 mg/L, and procalcitonin (PCT) was 9.32 ng/ml (Table 1). CT scan showed gas-like images in the left submandibular tissues and neck, cervical lymph node enlargement, obvious pleural effusion, and normal lungs (Fig. 1A–D). Clinical examinations and CT results indicated the presence of infection with aerogenic bacteria.
CT images of the patient at day 0, day 5 and day 11 of hospitalization.A–D The CT images of the patient at the time of admission, the mandibular space and mediastinum of the patient has obvious gas shadow, there is pleural effusion, and the lung image is normal; E–H The CT images of the patient 5 days after admission and treatment, the mandibular space and mediastinal gas after drainage treatment reduced, but the infection of the neck and mediastinum did not change significantly, the amount of pleural effusion increased compared with previous, and the lungs appeared as strips of increased density which indicating lung infection; I–L The CT images of the patient 11 days after admission, the submandibular space and mediastinal gas decreased, neck and mediastinal infections improved, lung infections improved, and pleural effusion decreased
On the first day of hospitalization, the patient's blood oxygen saturation dropped to 80%, he was treated with tracheal intubation and mechanical ventilation, vacuum sealing drainage (VSD) was also applied for the debridement and drainage of the infected area. Symptomatic treatment was also conducted, such as nutritional support and maintenance of water-electrolyte balance. Vancomycin (VAN) was treated for suspected gram-positive cocci infection. On the second day of hospitalization, the inflammatory indexes were still high with wet rales in both lungs. Therefore, imipenem (IPM) was administered to treat gram-negative bacteria infection. However, on the third hospital day the temperature rose to 38.3℃, indomethacin suppository was administered rectally to bring down a fever. Drainage fluid from the lesions and blood were drawn to culture for microbes, an aliquot of the drainage fluid was also sent to the clinical laboratory for mNGS. On the 5th day of hospitalization, the patient’s temperature returned to normal, but the percentage of neutrophils, CRP, and PCT levels were still high (87%, > 200 mg/L, 9.77 ng/mL, respectively). A second CT scan showed that the gas content in the tissues and extent of neck inflammation were reduced, but inflammatory lesions had appeared in the lungs, with increased bilateral pleural effusion volume (Fig. 1E–H). The result of mNGS revealed mixed infection by Streptococcus anginous, Prevotella oris, Prevotella denticola, Peptostreptococcus stomatis, Fusobacterium nucleatum, and Alloprevotella tannerae (Table 2). Thus, the antibiotic therapy was adjusted to piperacillin/tazobactam (TZP) and tinidazole (TNZ) based on positive results of mNGS.
On the 6th day of hospital stay, Streptococcus anginous was retrieved by culture of the drainage fluid, without any anaerobic bacteria detected. The isolated strain was sensitive to TZP (Table 3) according to antimicrobial susceptibility testing (AST) results. Hence, TZP and TNZ were continued for the treatment. The interpretive criterion of AST was established according to the latest edition of performance standards for antimicrobial susceptibility testing updated by the Clinical and Laboratory Standards Institute. Bronchoalveolar lavage was performed through fiberoptic bronchoscopy to remove secretions of respiratory, and bronchoalveolar lavage fluid (BALF) was sent to the microbiology laboratory for culture. On the 7th day of hospital stay, ultrasound-guided thoracic puncture was performed to drain pleural effusion fluids. On the 8th day of hospital stay, A methicillin-resistant coagulase-negative (MRSCN) Staphylococcus hominis strain was identified by blood cultures, and VAN was added to the antibiotic regiments according to the AST result (Table 3). On the 9th day a strain of Pseudomonas aeruginosa which sensitive to TZP was recovered from BALF by culture, thus change in antibiotics was not necessary (Fig. 2, Table 3). The patient’s general condition was improved and the mechanical ventilation was switched to high-flow oxygen inhalation. On the 11th day of hospital stay, the percentage of neutrophils returned to normal, CRP and PCT levels were significantly lower than before, indicating reduction of the infection. A third CT scan showed that the gas content in the inferior space and mediastinum cavity decreased, the infection symptoms of the neck and mediastinum relieved, the lung infection decreased, and the amount of pleural effusion was less extensively than before (Fig. 1I–L). Based on the patient’s improved clinical conditions and previous AST results, the antibiotic treatment was changed to Levaquin (LEV) and TZP. The patient was discharged out of ICU after obtaining a negative result of the drainage fluid bacterial culture (Fig. 2).
Discussion and conclusion
Acute purulent mediastinitis is a fatal condition which occurs after an esophageal perforation or that develops as a complication of odontogenic infection[5]. Infections in the posterior lower teeth are more likely to progress to DNM due to their drainage into the submandibular space, located near the retropharyngeal and lateral pharyngeal spaces[6]. Infection of the oropharyngeal cavity can rapidly spread along the fascial planes into the mediastinum space, where DNM develops. Because of the high lethality and rapid progression of DNM, prompt diagnosis, appropriate and adequate surgical drainage, in addition to supportive antibiotic therapy, combined with intensive medical care, are determinants for the success of the treatment and recovery [4]. CT reportedly contributes to early diagnosis of mediastinitis, owing to the capability of identifying the presence and extension of the disease, providing accurate information about the location and extent of the infection, guiding the management approach, and monitoring the drainage process [7, 8]. CT plays an important role in the diagnosis and assessment of therapeutic effectiveness. Another important factor that affects the clinical outcome of the patient is the type of microorganism that leading to the infection. As previously reported, DNM often comes from polymicrobial infection, and the most frequent microorganisms isolated are of the genus Streptococcus as well as anaerobic microbial bacteria of the genera Prevotella, Peptostreptococcus, Bacteroides, and Fusobacterium. Other pathogens isolated from DNM patients including bacteria belonging to the genera Staphylococcus, Pesudomonas, Escherichia, Enterobacter, Acinetobacter, Enterococcus, etc. [9, 10]. As reported in a literature review, a total of 156 microorganisms were isolated from 55 patients (61.8%), no pathogens can be isolated in approximately 40% of the cases of DNM [11]. The reason for this may be the complexity of the microorganisms and limitations of traditional microbial detection methods, such as morphological detection, culture, biochemical detection, and serotyping. These methods can only identify one or several specific pathogens, but cannot identify unknown or rare pathogenic microorganisms.
mNGS is a versatile technology which can identify pathogens more rapidly and precisely than traditional methods, and can even provide new insights into disease transmission, virulence, and antimicrobial resistance. Compared to traditional detection methods which can only detect certain targeted pathogens, mNGS is a shotgun sequencing method of RNA and DNA from clinical samples, where all DNA or RNA of the sample to be tested are mixed and sequenced, and the data is then compared with the pathogen database to obtain classification information of the pathogens. This method can detect tens of thousands of pathogens in a run within 48 h. The pathogen profiles include almost all viruses, bacteria, fungi, and parasites that can infect patients [12, 13].The details of materials and methods for mNGS was provided as supplement material (Additional file 1). Since 2013, professor Charles Chiu first applied mNGS to diagnose the encephalopathy caused by Leptospira infection in a 14-year-old boy [14], this method plays an increasingly important role in pathogen identification. As reported in previous studies, mNGS is more sensitive than culture, and is especially useful in the diagnosis of tuberculosis, fungi, viral and anaerobic bacteria infections [15]. This method is particularly important for the diagnosis of serious clinical infections, and intractable cases caused by complex pathogens. In this case, Streptococcus anginosus was cultured from drainage fluid, while other anaerobic pathogens were sterile by culture. However, mNGS retrieved all the pathogens presented in the infected tissues in a short time. The number of total reads obtained from the patient’s drainage fluid was 58,184,734, of which 407,293 reads were of microbial origin. Prevotella oris and Streptococcus anginosus were identified as the top two predominant pathogens, taking up 0.13% (78,656 reads) and 0.05% (28,322 reads) of the total sequence reads, and 19.31% and 6.95% of the total bacterial reads, respectively.
DNM is a serious infectious condition with aggressive and proliferative behavior which could be life-threatening. Prompt diagnosis, early surgical drainage, proper antibiotic therapy, and multidisciplinary collaborations are beneficial. Application of mNGS for pathogen detection makes it possible to detect all the microorganisms quickly, which is important for the adjustment of antibiotics. The wide application of mNGS would contribute to the characterization of pathogen profiles in DNM, which is still poorly characterized, since in almost 40% of the cases no pathogens can be detected by traditional methods. We believe that, with the progress of technological innovation and the decline of sequencing costs, mNGS will become a routine detection method for pathogenic microorganisms, and a growing number of patients will benefit from it.
Availability of data and materials
The datasets analyzed during the current study are not publicly available due to patient privacy concerns but are available from the corresponding author on reasonable request.
Abbreviations
- DNM:
-
Descending necrotizing mediastinitis
- mNGS:
-
Metagenomics next generation sequencing
- CT:
-
Computed tomographic
- AST:
-
Antimicrobial susceptibility testing
- VSD:
-
Vacuum Sealing Drainage,
- CRP:
-
C reaction protein
- PCT:
-
Procalcitonin
- BALF:
-
Bronchoalveolar lavage fluid
- ME:
-
Mediastinal emphysema
- PE:
-
Pleural effusion
- IMP:
-
Imipenem, VAN: vancomycin
- TNZ:
-
Tinidazole
- TZP:
-
Piperacillin/tazobactam
- LEV:
-
Levofloxacin
- N%:
-
Percentage of neutrophils
- L%:
-
Percentage of lymphocyte
References
Estera A. Descending necrotizing mediastinitis. Surg Gynecol Obstetr. 1983. Doi: https://doi.org/10.1016/S1079-2104(00)70121-1.
Freeman RK, Vallières E, Verrier ED, Karmy-Jones R, Wood DE. Descending necrotizing mediastinitis: an analysis of the effects of serial surgical debridement on patient mortality. J Thorac Cardiovasc Surg. 2000;119(2):260–7. https://doi.org/10.1016/S0022-5223(00)70181-4.
Sarna T, Sengupta T, Miloro M, Kolokythas A. Cervical Necrotizing fasciitis with descending mediastinitis: literature review and case report. J Oral Maxillofac Surg Off J Am Assoc Oral Maxillofac Surg. 2012;70(6):1342–50. https://doi.org/10.1016/j.joms.2011.05.007.
Mihos P, Potaris K, Gakidis I, Papadakis D, Rallis G. Management of descending necrotizing mediastinitis. J Oral Maxillofac Surg. 2004;62(8):966–72. https://doi.org/10.1016/j.joms.2003.08.039.
Sakamoto H, Aoki T, Kise Y, Watanabe D, Sasaki J. Descending necrotizing mediastinitis due to odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2000;89(4):412–9.
Cai XY, Zhang WJ, Zhang ZY, Yang C, Zhou LN, Chen ZM. Cervical infection with descending mediastinitis: a review of six cases. Int J Oral Maxillofac Surg. 2006;35(11):1021–5. https://doi.org/10.1016/j.ijom.2006.06.021.
Scaglione M, Pinto A, Romano S, Giovine S, Sparano A, Romano L. Determining optimum management of descending necrotizing mediastinitis with CT; experience with 32 cases. Emerg Radiol. 2005;11(5):275–80. https://doi.org/10.1007/s10140-005-0422-3.
Marty-Ané C-H, Berthet JP, Alric P, Pegis JD, Rouvière P, Mary H. Management of descending necrotizing mediastinitis: an aggressive treatment for an aggressive disease—ScienceDirect. Ann Thorac Surg. 1999;68(1):212–7. https://doi.org/10.1016/S0003-4975(99)00453-1.
Pa Stene B, Cassir N, Tankel J, Einav S, Fournier PE, Thomas P, et al. Mediastinitis in the intensive care unit patient: a narrative review. Clin Microbiol Infect. 2020;26(1):26–34. https://doi.org/10.1016/j.cmi.2019.07.005.
Brook I. Current management of upper respiratory tract and head and neck infections. Eur Arch Otorhinolaryngol. 2009;266(3):315–23. https://doi.org/10.1007/s00405-008-0849-8.
Mfe A, et al. Mediastinitis of odontogenic origin. A serious complication with 80 years of history. Br J Oral Maxillofac Surg. 2020. Doi: https://doi.org/10.1016/j.bjoms.2020.09.004.
Schlaberg R, Chiu CY, Miller S, Procop GW, Weinstock G, Committee PP, et al. Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch Pathol Lab Med. 2017;141(6):776. https://doi.org/10.5858/arpa.2016-0539-RA.
Westblade LF, Belkum AV, Grundhoff A, Weinstock GM, Pamer EG, Pallen MJ, et al. Role of clinicogenomics in infectious disease diagnostics and public health microbiology. J Clin Microbiol. 2016;54(7):1686–93. https://doi.org/10.1128/JCM.02664-15.
Seroogy CM, Naccache SN, Somasekar S, Miller S, Biagtan M, Buckley RH, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. N Engl J Med. 2014;370(25):2408–17. https://doi.org/10.1056/NEJMoa1401268.
Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin WT, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(S2):231–40. https://doi.org/10.1093/cid/ciy693.
Acknowledgements
Not applicable.
Funding
The work was supported by the corresponding author of this paper. The article-processing and language editing services of this study were supported by the Medicine and Health Science Technology Development Plan of the Shandong Province of China (Grant Number 2016WS0217).
Author information
Authors and Affiliations
Contributions
DJ was major contributor in drafting the manuscript, CXS, FJJ and PF participated in the diagnosis and treatment of the patient, ZCC and ZQG participated in revising the manuscript critically, YZQ participated in revising the manuscript critically and contributed to conception. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As this manuscript is a case report of clinical care provided to a patient, and it does not include any of the patient’s personal identifiers, ethics approval by the institutional review board was not required.
Consent for publication
The patient provided written informed consent for the publication of the data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Materials and methods for mNGS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Duan, J., Zhang, C., Che, X. et al. Detection of aerobe–anaerobe mixed infection by metagenomic next-generation sequencing in an adult suffering from descending necrotizing mediastinitis. BMC Infect Dis 21, 905 (2021). https://doi.org/10.1186/s12879-021-06624-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06624-4