Abstract
Background
Coronavirus disease 2019 (COVID-19) is associated with a high mortality rate, especially in patients with severe illness. We conducted a systematic review and meta-analysis to assess the potential predictors of mortality in patients with COVID-19.
Methods
PubMed, EMBASE, the Cochrane Library, and three electronic Chinese databases were searched from December 1, 2019 to April 29, 2020. Eligible studies reporting potential predictors of mortality in patients with COVID-19 were identified. Unadjusted prognostic effect estimates were pooled using the random-effects model if data from at least two studies were available. Adjusted prognostic effect estimates were presented by qualitative analysis.
Results
Thirty-six observational studies were identified, of which 27 were included in the meta-analysis. A total of 106 potential risk factors were tested, and the following important predictors were associated with mortality: advanced age, male sex, current smoking status, preexisting comorbidities (especially chronic kidney, respiratory, and cardio-cerebrovascular diseases), symptoms of dyspnea, complications during hospitalization, corticosteroid therapy and a severe condition. Additionally, a series of abnormal laboratory biomarkers of hematologic parameters, hepatorenal function, inflammation, coagulation, and cardiovascular injury were also associated with fatal outcome.
Conclusion
We identified predictors of mortality in patients with COVID-19. These findings could help healthcare providers take appropriate measures and improve clinical outcomes in such patients.
Similar content being viewed by others
Background
Coronavirus disease 2019 (COVID-19) is a severe emerging infection caused by a novel coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2). In December 2019, the first case of COVID-19 infection was reported in Wuhan, China [1]. Since then, the disease has spread rapidly around the world within a short period of time. As of May 9, 2020, there were more than 3.85 million laboratory-confirmed cases of COVID-19 and 265 thousand deaths, with a case fatality rate of 6.9% [2].
To help better understand factors associated with an increased risk of mortality, increasing prognostic studies have been published [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. However, most of the studies included relatively small sample sizes and presented inconsistent findings. To obtain an adequate number of cases for precision estimation of the correlations between predictors and a fatal outcome, we conducted a systematic review and meta-analysis to identify and summarize predictors of mortality in patients with COVID-19 infection.
Methods
This study was conducted in accordance with the guidelines for systematic review and meta-analysis of prognostic factors [39] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [40].
Literature search
PubMed, EMBASE, the Cochrane Library, and three electronic Chinese databases (Chinese National Knowledge Infrastructure, Wanfang, and VIP databases) were searched from December 1, 2019 to April 29, 2020. For those not familiar with Chinese electronic databases, details of these databases are presented in Additional file 1. The search terms were “(coronavirus OR SARS-CoV-2 OR COVID-19 OR 2019-nCoV) AND (mortality OR survivor OR death OR fatality OR deceased)”. To identify additional eligible studies, reference lists of included studies and relevant review articles were scanned. No language restriction was imposed.
Inclusion and exclusion criteria
Two reviewers independently reviewed and selected studies for inclusion. Randomized controlled trials and observational studies reporting potential predictors of mortality in patients with COVID-19 infection were included in the meta-analysis. The exclusion criteria were as follows: (1) reviews, case reports, editorials, and conference abstracts, (2) studies for which mortality data were not available, (3) studies with small sample sizes (≤ 30 participants or ≤ 10 nonsurvivors), and (4) studies that included only pregnant women or children.
Data extraction and quality assessment
The following data were extracted from each study by two authors independently: author, design, setting, patient recruitment period, location, sample size, patient characteristics, severity of illness, mortality rate, and potential risk factors. Any disagreement was resolved by consensus. The quality of the included studies was assessed using the Quality in Prognostic Factor Studies (QUIPS) tool. This tool is composed of six items: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, and statistical analysis and reporting [41].
Statistical analysis
The pooled unadjusted estimates for each predictor were performed if data from at least two studies were available. The results as risk ratios (RRs) for dichotomous data and weighted mean differences (WMDs) for continuous outcomes were presented, both with 95% confidence intervals (CIs). For studies that presented data as medians and interquartile ranges, we calculated the means and standard deviations based on the formulas used by Wan et al. [42]. To provide conservative pooling estimates, we applied a random-effects model in all analyses. Heterogeneity was assessed using the I2 statistic, and a value of > 50% was considered significant heterogeneity [43]. In the presence of significant heterogeneity, sensitivity analyses were performed to assess the stability of the results by omitting the largest (or smallest) study. We also calculated the fixed-effects model for additional sensitivity analyses. Publication bias was examined by Egger’s test. All statistical analyses were performed using RevMan version 5.3 (the Cochrane Collaboration) and Stata 15.0 software (StataCorp, College Station, Texas, USA). Pooled adjusted estimates for predictors were not available because only few original studies reported adjusted data, different types of effect measures were used (such as odds ratios and hazard ratios), and potential overlap of the patients existed between the included studies. Therefore, we presented adjusted data using qualitative analysis.
Results
Literature search
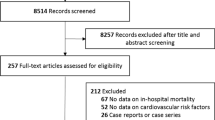
The search process identified 1832 publications in the initial search. After screening, we included 36 studies in the systematic review [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38], of which 27 were included in the meta-analysis [3, 5, 6, 9, 11,12,13, 15, 16, 19,20,21,22,23,24,25,26, 28,29,30,31,32,33,34,35,36, 38]. The literature selection process is shown in Fig. 1. We noted that several included studies were from the same hospital and had overlapping patient recruitment periods. To avoid potential patient overlap, we extracted only the data from the studies with the largest sample size for each evaluated predictor if multidata were available from the same hospital.
The characteristics of the studies included in the systematic review are presented in Table 1. The sample size in each study ranged from 52 to 5688, and the mortality rate ranged from 3.1 to 61.5%. All the studies were from China with the exception of two studies conducted in the USA [22, 24] and one study conducted in Italy [11]. The mean ages of the patients ranged from 47 to 70 years, and the proportion of female patients ranged from 18.0 to 60.2%. The quality assessment of the included studies is presented in Table S1. The item showing greatest potential risk of bias was study confounding. The quality of some studies was also sub-optimal with respect to the remaining five items.
Meta-analyses of unadjusted estimates
We conducted meta-analyses of unadjusted estimates for 106 potential predictors of mortality in patients with COVID-19 (Table 2 and Fig. 2). All individual forest plots and further details are presented in Table S2-S107 and Figure S1-S106 in Additional file 2.
Compared with survivors, the mean age of the deceased patients was significantly higher (WMD, 13.9; 95% CI, 8.95 to 18.9). Advanced age (age ≥ 60–70 years) was associated with a significant increase in the risk of mortality (RR, 2.31; 95% CI, 1.99 to 2.67). Male sex was also correlated with a higher death rate than female sex (RR, 1.30; 95% CI, 1.17 to 1.44).
Patients with any comorbidity had a 2.85-fold higher risk of mortality than patients without comorbidities (RR, 2.85; 95% CI, 1.47 to 5.51). The highest mortality risk was observed in patients with chronic kidney disease (RR, 8.37; 95% CI, 3.94 to 17.8), followed by cerebrovascular disease (RR, 7.66; 95% CI, 3.87 to 15.2), chronic respiratory disease (RR, 3.84; 95% CI, 1.81 to 8.16), cardiovascular disease (RR, 3.16; 95% CI, 2.19 to 4.56), diabetes mellitus (RR, 2.21; 95% CI, 1.37 to 3.56) and hypertension (RR, 2.11; 95% CI, 1.49 to 2.99). However, other comorbidities were not associated with survival after pooled analyses.
Symptoms of dyspnea (RR, 1.98; 95% CI, 1.70 to 2.30) were more common in the nonsurvivor group than in the survivor group. No associations between the remaining clinical symptoms and mortality were observed. Antiviral agents and immunoglobulin therapies were not associated with mortality. However, patients who received glucocorticoids (RR, 1.79; 95% CI, 1.25 to 2.55) or antibiotics (RR, 1.20; 95% CI, 1.02 to 1.40) were more likely to die than those who did not.
The values of the following laboratory parameters were significantly higher in the deceased patients than in survivors: white blood cells (WBCs), neutrophils (NEUs), total bilirubin (TBIL), aspartate aminotransferase (AST), creatinine (Cr), blood urea nitrogen (BUN), urea, prothrombin time (PT), D-dimer, C-reactive protein (CRP), procalcitonin (PCT), ferritin, lactate dehydrogenase (LDH), creatine kinase (CK), the erythrocyte sedimentation rate (ESR), creatine kinase-MB (CK-MB), N-terminal pro-brain natriuretic peptide (NT-proBNP), hypersensitive cardiac troponin I (hs-cTnI), myoglobin, cystatin C, and interleukin-6 (IL-6). The lymphocyte (LYM), monocyte (MON), platelet (PLT), albumin (ALB), CD3+, CD4+, and CD8+ cell counts were significantly lower in the nonsurvivors than in the survivors. Moreover, the following abnormal laboratory parameters were more prevalent in the nonsurvivor group than in the survivor group: WBCs ≥10 × 109/L, LYMs < 0.8 × 109/L, PLTs < 125 × 109/L, alanine aminotransferase (ALT) > 40 U/L, AST > 40 U/L, Cr ≥133 μmol/L, PCT ≥0.5 ng/mL (or ≥ 0.1 ng/mL and ≥ 0.05 ng/mL) and D-dimer ≥1.0 μmol/L.
Patients suffering from any comorbidity had a significantly increased risk of mortality, with RRs ranging from 2.59 to 12.6. The highest mortality risk was associated with shock (RR, 12.6; 95% CI, 1.25 to 127.1), followed by superinfection (RR, 9.78; 95% CI, 2.05 to 46.7), acute kidney injury (AKI, RR, 9.64; 95% CI, 6.01 to 15.4), acute cardiac injury (RR, 8.22; 95% CI, 4.95 to 13.7), acute respiratory distress syndrome (ARDS, RR, 6.82; 95% CI, 2.56 to 18.2), arrhythmia (RR, 4.86; 95% CI, 1.24 to 19.0), heart failure (RR, 4.18; 95% CI, 2.37 to 7.36), acute liver injury (RR, 3.78; 95% CI, 1.18 to 12.1) and sepsis (RR, 2.59; 95% CI, 2.11 to 3.17).
Oxygen treatment was applied more often in nonsurvivors than in survivors (high flow nasal cannula, RR, 10.6; 95% CI, 5.97 to 18.8; noninvasive ventilation, RR, 5.12; 95% CI, 3.98 to 6.57; invasive ventilation, RR, 29.3; 95% CI, 21.5 to 39.9). Renal replacement therapy was also observed to be more prevalent in the deceased patients than in the surviving patients (RR, 53.5; 95% CI, 22.4 to 127.3). No correlation between the use of extracorporeal membrane oxygenation (ECMO) and mortality was observed.
A significant difference between nonsurvivors and survivors was also observed for the following variables: respiratory rate, heart rate, respiratory rate > 30 (or 24) breaths per min, partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), ratio of partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2), bilateral pneumonia, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, and Sequential Organ Failure Assessment (SOFA) score.
Significant heterogeneity was observed in the analyses of 54 tested predictors. Sensitivity analyses did not change the conclusions about the most tested variables, whereas the results for the following 14 tested predictors were inconsistent: preexisting malignancy, symptoms of anorexia, antiviral therapy, immunoglobulin therapy, CRP ≥10 mg/L, shock, acute liver injury, MON and PLT count, activated partial thromboplastin time (APTT), γ-glutamyl transpeptidase, PaO2, SpO2, and IL-6 (Additional file 2: Table S108-S109). No significant publication bias was observed for any risk factors except for two predictors, current smoking status and antibiotic therapy (Table 2 and Fig. 2).
Qualitative analysis of adjusted estimates
Adjusted data regarding mortality due to COVID-19 infection were available in 16 studies [4, 5, 7, 9, 10, 12, 17,18,19, 21, 26, 28, 29, 31, 37, 38] (Table 3). A multivariate Cox regression model was used in ten studies, and a multivariate logistic regression model was applied in six studies. Demographic characteristics highlighted as predictors for increased risk of mortality were advanced age [4, 9, 18, 26, 28, 29, 38], male sex [5, 17] and presence of a comorbidity [4, 5, 9, 12, 18, 21, 29, 31], such as cardio-cerebrovascular disease [4, 9, 29], chronic obstructive pulmonary disease (COPD) [12, 29], diabetes [12], hypertension [12], and malignancy [12]. In two studies, the symptom of dyspnea was correlated with a greater risk of death [4, 31].
Decreased PLTs [26, 31] and increased WBCs (≥10 × 109/L) [17, 31], NEUs (≥6 × 109/L) [31], neutrophil-to-lymphocyte ratio [19], PCT (> 0.5 ng/mL) [4], CRP (≥27.8 mg/L) [31], AST (> 40 U/L) [4], hs-cTnI (≥0.05 ng/mL) [9], Cr [5, 7], BUN [7], NT-proBNP [10] and D-dimer [26, 37, 38] were identified as risk factors for mortality. Moreover, CD3+ CD8+ T cells ≤75/μL [9] and the presence of proteinuria and hematuria [7] were also correlated with an increased mortality rate.
Patients suffering from AKI [7], ARDS [29], cardiac injury [17, 28], or hyperglycemia [17] during hospitalization had an increased risk for mortality. Other identified risk factors include shortness of breath [5], SpO2 ≤ 90% [31], days from symptom onset to hospitalization [18], severe disease [21], SOFA score [38], and high-dose corticosteroid therapy [17].
Discussion
To the best of our knowledge, this is the most comprehensive systematic review and meta-analysis evaluating predictors of mortality in patients with COVID-19 conducted to date. Important risk factors associated with an increased fatality rate included older age, male sex, current smoking, baseline comorbidities (especially chronic kidney, respiratory, and cardio-cerebrovascular diseases), symptoms of dyspnea, complications during hospitalization, corticosteroid therapy and a severe condition. Additionally, a series of abnormal biomarkers of hematologic parameters (especially WBC, NEU, and LYM counts), hepatorenal function (especially Cr, BUN, and AST), inflammation (especially PCT, CRP, ferritin, and the ESR), coagulation (especially D-dimer and PT), and cardiovascular injury (especially hs-cTnI and NT-proBNP) were also associated with fatal outcomes.
Similar to the two previous emergences of coronavirus diseases, severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), the outbreak of COVID-19 has posed great challenges for public health. Although most COVID-19 cases are mild, patients with severe conditions may quickly progress to ARDS, multiple organ failure and even death. The present study identified predictors of mortality that clinicians and other healthcare providers can consider when discussing the expected prognosis of patients with COVID-19 and thus take appropriate measures.
Advanced age has been identified as an independent risk factor for mortality in SARS [44, 45] and MERS [46, 47]. Our meta-analysis confirmed that older age was also correlated with an increased mortality rate in patients with COVID-19. Several factors might contribute to this mortality risk, including age-related physiological changes, impaired immune function, and preexisting illnesses. In the present meta-analysis, articles that only enrolled children were excluded. Although available studies show that the mortality rate due to COVID-19 in children is relatively low [48, 49], healthcare providers and parents are concerned about the health of children. Future prognostic studies focusing on children with COVID-19 are warranted.
The present meta-analysis found that patients who are current smokers were 2.95 times more likely to die than nonsmokers. Upregulation of angiotensin-converting enzyme 2 (ACE2) expression in airways might explain the increased risk of death in current smokers with COVID-19 infection [50]. The pooled analysis indicated that male sex was associated with a 30% increased risk of mortality among patients with COVID-19. Although the factors accounting for the sex difference in the incidence of death remain unknown, we suggest that smoking might be one of the contributing factors. Of note, the included studies were primarily from China, where the proportion of adult men who smoke (> 50%) is much higher than that the proportion of adult women who smoke (< 3%) [51]. It is possible that the sex differences in the survival rate are due to the different prevalence of smoking in the two sexes. The sex difference in the risk of mortality among patients with COVID-19 may also be related to differential ACE2 expression in males and females related to the X chromosome [52, 53].
Accumulated evidence has shown that COVID-19 infection is more likely to occur in patients with preexisting conditions than in those without preexisting conditions [54]. Similar to SARS [45, 55] and MERS patients [56], preexisting conditions were also found to have an important effect on the prognosis in COVID-19 patients. Our pooled analysis showed that the risk of mortality in COVID-19 patients with any comorbidity was 2.85 times higher than that in those without preexisting conditions. Patients with chronic kidney disease, cerebrovascular disease, respiratory disease, cardiovascular disease, diabetes mellitus or hypertension had approximately 8-fold, 8-fold, 4-fold, 3-fold, 2-fold, and 2-fold higher risks of mortality, respectively, than individuals without these conditions. Considering that these comorbidities are predictors of poor outcomes, optimum control of these conditions may be beneficial for the management of COVID-19. A recent large-sample study investigated the correlation of blood glucose control and outcomes in COVID-19 patients with diabetes [57]. The results indicated that patients with well-controlled blood glucose who maintain glycemic variability within 3.9 to 10.0 mmol/L had significantly improved survival compared to patients with poorly controlled blood glucose [57]. Further studies are still urgently needed to achieve a comprehensive understanding of how specific comorbidities exacerbate COVID-19 disease severity so as to improve clinical outcomes of the disease through precision-targeted management.
The symptoms of COVID-19 infection are nonspecific. Patients with COVID-19 can present with fever, cough, muscle aches, fatigue, headache, gastrointestinal symptoms, and dyspnea [58]. Of these symptoms, dyspnea was significantly associated with an increased mortality rate in the pooled analysis, corroborating the findings of two studies [4, 31] that demonstrated that dyspnea was correlated with a higher mortality rate even after adjustment for age, sex, and other confounding factors. As dyspnea can be easily observed in clinical practice, it may be a valuable predictor to help identify individuals who are at high risk for fatal outcomes and may need additional attention. Additionally, blood gas analysis may be a useful tool to determine the severity of dyspnea. Decreased SpO2 may reflect severe dyspnea, indicating an increased mortality risk, as shown in the present meta-analysis.
Dramatically reduced LYM levels as well as CD3, CD4, and CD8 cell counts in the deceased patients suggests that SARS-CoV-2 may act on T lymphocytes, and viral replication contributes to the destruction of T lymphocytes, decreasing immune function. Not surprisingly, patients with poor immune function were more likely to suffer from acute infection than those with normal immune function, and patients suffering from acute infection were more likely to die than those without this complication. In the present meta-analysis, a positive correlation between the PCT level or WBC count and mortality was observed, indicating that an increased WBC count (≥10 × 109/L) may be a useful predictor and that PCT-guided antibiotic therapy might be beneficial in COVID-19 infection.
Compared with that in survivors, serum concentrations of Cr were higher in patients in the deceased group, indicating worse kidney function, although the mean values remained within the normal range. A single-cell transcriptome analysis indicated that the cytopathic effects of SARS-CoV-2 on podocytes and proximal straight tubule cells may contribute to the development of AKI in patients with COVID-19 [59]. Increased baseline Cr or BUN, peak Cr > 133 μmol/L, and the presence of hematuria, hematuria or AKI were identified as independent predictors of in-hospital mortality in a large-sample prospective study evaluating 701 COVID-19 cases [7]. In the present meta-analysis, the development of AKI and increased SCr (> 133 μmol/L) were associated with an approximately 9.6-fold and 3.6-fold increased risk of mortality among patients with COVID-19, respectively. Considering the great impact of renal damage on prognosis, close monitoring of renal function-related parameters is required.
Regarding markers of liver injury, the pooled analysis demonstrated statistically higher levels of AST and TBIL in nonsurvivors than in survivors, and patients with increased AST (> 40 U/L) were approximately twice as likely to die as patients with normal AST values. Although increased ALT (> 40 U/L) was also correlated with an increased risk of death, no significant difference in the mean levels of ALT between nonsurvivors and survivors was observed in our meta-analysis. In a large-sample longitudinal study that evaluated 5771 patients with COVID-19 infection, elevation of AST was correlated with the highest mortality risk compared to other markers of liver injury such as ALT, TBIL and alkaline phosphatase. In addition, the elevation of AST occurred before the elevation of ALT [60]. These findings indicate that AST may be a better liver injury marker for predicting clinical outcomes than the other markers, and frequent monitoring of AST and early detection of liver injury are suggested. Decreased albumin (< 35 g/L) has been identified as an independent predictor of severe infection requiring intensive care unit (ICU) admission in MERS infection [61]. In our study, serum albumin was also significantly lower in the deceased patients than in the surviving patients, indicating that malnutrition might contribute to the adverse outcome of COVID-19 infection and that nutritional support may be beneficial in the management of this disease.
Cytokine storm, also known as hypercytokinemia, refers to the excessive and uncontrolled release of pro-inflammatory cytokines. Huang et al. [62] found markedly higher plasma levels of cytokines in COVID-19 patients requiring ICU admission than in those not treated in the ICU, indicating that cytokines are correlated with disease severity. In the present meta-analysis, numerous inflammatory biomarkers (ESR, CRP, PCT, ferritin, and IL-6) were higher in deceased patients than in survivors, providing further evidence for the presence of a cytokine storm that can contribute to the fatal outcome of COVID-19 patients. Mehta et al. [63] suggested that each COVID-19 patient with a severe condition should be screened for hyperinflammation considering laboratory trends (increasing ferritin, decreasing PLTs or ESR) and HScore [64].
Cardiovascular complications of COVID-19, such as cardiac injury, heart failure, and arrhythmia, were more prevalent in patients who died than in patients who survived. Among them, cardiac injury was correlated with the highest mortality risk and has been widely studied. The possible mechanisms of cardiac injury caused by COVID-19 may involve cardiac stress due to respiratory failure and hypoxemia, direct myocardial infection by the virus, and indirect damage from the systemic inflammatory response [65]. In the present meta-analysis, several indicators of cardiovascular injury, such as hs-cTnI, NT-proBNP, CK-MB and myoglobin, were significantly higher in patients in nonsurvivor group than in those in the survivor group. Increased hs-cTnI and NT-proBNP among COVID-19 patients have been identified as independent risk factors for mortality even after adjustment for age and other confounding factors [9, 10]. Therefore, we suggest that frequent measurement of hs-cTnI and NT-proBNP should be required in the management of COVID-19, especially for patients with preexisting cardio-cerebrovascular disease.
Coagulation dysfunction is common in patients with COVID-19. We identified significantly lower PLTs in patients with a fatal outcome, and thrombocytopenia (PLT < 125 × 109/L) was correlated with a 4.65-fold increased risk of mortality. Increased D-dimer and prolonged PT were also observed more frequently in nonsurvivors than in survivors. These findings indicated that excessive activation of the coagulation cascade and PLTs existed in the progression of COVID-19 infection. The underlying mechanisms of activated coagulation remain unclear but may be due to inflammatory responses induced by SARS-CoV-2 [66]. Coagulation screening, especially the determination of D-dimer and PLT levels, has been suggested.
At present, effective pharmacological interventions for the treatment of COVID-19 are still limited. Antiviral agents have been widely applied in the management of COVID-19 infection. However, there was no significant difference between nonsurvivors and survivors regarding antiviral therapy efficacy. Regarding antiviral therapy, the latest COVID-19 Treatment Guidelines recommended the use of remdesivir in hospitalized COVID-19 cases requiring oxygen support [67]. Corticosteroid treatment for COVID-19 infection is a hot topic. A recently published meta-analysis investigated the impact of corticosteroids on the outcomes of patients with coronavirus infections, including SARS, MERS, and COVID-19 [68]. The results demonstrated that the use of corticosteroids significantly delayed the clearance of the virus and did not improve the survival rate, shorten the hospitalization time, or reduce the ICU admission and mechanical ventilation rate. In the present meta-analysis, we found that corticosteroid treatment was correlated with an elevated risk of mortality in COVID-19 patients, with a pooled RR of 1.79 (95% CI 1.25 to 2.55). Current evidence and our findings further support the recommendations by the Infectious Diseases Society of America (IDSA) denouncing the use of corticosteroids in patients with COVID-19 pneumonia [69]. However, a subset analysis of 84 patients with ARDS secondary to COVID-19 infection found an improved survival rate in patients who received methylprednisolone, indicating that corticosteroid therapy might be beneficial for COVID-19 patients who develop ARDS. One randomized controlled trial performed in the UK (RECOVERY trial) showed that the application of dexamethasone improved survival of severe COVID-19 patients who required respiratory support [70]. However, no survival benefit of dexamethasone was observed in mild COVID-19 cases [70]. Currently, the use of dexamethasone is recommended for severe COVID-19 patients requiring respiratory support, but not for non-hospitalized patients, mild to moderate cases, or hospitalized patients who did not receive oxygen support [67]. It is likely that the beneficial effect of corticosteroid treatment for COVID-19 infection is dependent on correct selection of the timing of administration, dose, and patient.
Patients who have indications for organ supportive care, such as the need for invasive mechanical ventilation and renal replacement therapy, tend to be sicker than other patients, and this could explain the increased death rate among those patients. Other indicators of severe conditions, such as APACHE II and SOFA scores, can also be used to predict the prognosis of COVID-19 infection. Probably due to small sample size, the effect of ECMO support on the mortality rate of COVID-19 patients was not statistically significant.
The present study has several strengths. First, the current study was performed based on recent guidelines for the systematic review and meta-analysis of prognostic factors. Second, we made our best effort to avoid potential patient overlap by checking the settings and patient recruitment periods. Third, many potential predictors of mortality in COVID-19 patients were tested.
The present study also had several limitations and should be interpreted cautiously. First, only unadjusted prognostic effect estimates were pooled because only a few of the original studies reported adjusted data, different types of effect measures were used (such as odds ratios and hazard ratios), and there was potential overlap of the patients in the included studies. It is possible that the unadjusted estimates of several factors may become nonsignificant after adjustment. Second, the present meta-analysis included substantial heterogeneity in some tested factors that could be explained by differences in patient populations and in the severity of the disease. Third, although a total of 27 studies were included in the meta-analysis, the number of studies available for analysis of each predictor was insufficient to allow meaningful subgroup analyses.
Conclusions
The present meta-analysis provides evidence of correlations between important prognostic factors and survival in patients with COVID-19. Clinicians and other healthcare providers should consider these factors when discussing the expected prognosis of COVID-19 patients and take appropriate measures accordingly. Further studies are required to provide a better understanding of the pathophysiological mechanisms of the association between these predictors and COVID-19 infection.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- AKI:
-
Acute kidney injury
- ALB:
-
Albumin
- ALT:
-
Alanine aminotransferase
- APACHE II:
-
Acute Physiology and Chronic Health Evaluation II
- APTT:
-
Activated partial thromboplastin time
- ARDS:
-
Acute respiratory distress syndrome
- AST:
-
Aspartate aminotransferase
- BUN:
-
Blood urea nitrogen
- CIs:
-
Confidence intervals
- CK:
-
Creatine kinase
- CK-MB:
-
Creatine kinase-MB
- COPD:
-
Chronic obstructive pulmonary disease
- COVID-19:
-
Coronavirus disease 2019
- Cr:
-
Creatinine
- CRP:
-
C-reactive protein
- ECMO:
-
Extracorporeal membrane oxygenation
- ESR:
-
Erythrocyte sedimentation rate
- hs-cTnI:
-
Hypersensitive cardiac troponin I
- ICU:
-
Intensive care unit
- IDSA:
-
Infectious Diseases Society of America
- IL-6:
-
Interleukin-6
- LDH:
-
Lactate dehydrogenase
- LYM:
-
Lymphocyte
- MERS:
-
Middle East respiratory syndrome
- MON:
-
Monocyte
- NEUs:
-
Neutrophils
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- PaCO2 :
-
Partial pressure of carbon dioxide
- PaO2 :
-
Partial pressure of oxygen
- PaO2/FiO2 :
-
Ratio of partial pressure of oxygen to fraction of inspired oxygen
- PCT:
-
Procalcitonin
- PLT:
-
Platelet
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PT:
-
Prothrombin time
- QUIPS:
-
Quality in Prognostic Factor Studies
- RRs:
-
Risk ratios
- SOFA:
-
Sequential Organ Failure Assessment
- SARS:
-
Severe acute respiratory syndrome
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TBIL:
-
Total bilirubin
- WBCs:
-
White blood cells
- WMDs:
-
Weighted mean differences
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33. https://doi.org/10.1056/NEJMoa2001017.
World Health Organization. Coronavirus disease (COVID-2019) situation reports. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 9 May 2020.
Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):748–55. https://doi.org/10.1093/cid/ciaa243.
Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158(1):97–105. https://doi.org/10.1016/j.chest.2020.04.010.
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. https://doi.org/10.1136/bmj.m1091.
Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75(9):1788–95. https://doi.org/10.1093/gerona/glaa089.
Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–38. https://doi.org/10.1016/j.kint.2020.03.005.
Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133:1261–7. https://doi.org/10.1097/CM9.0000000000000824.
Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. https://doi.org/10.1183/13993003.00524-2020.
Gao L, Jiang D, Wen XS, Cheng XC, Sun M, He B, et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21(1):83. https://doi.org/10.1186/s12931-020-01352-w.
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. Italy JAMA. 2020;323(16):1574–81. https://doi.org/10.1001/jama.2020.5394.
Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. https://doi.org/10.1183/13993003.00547-2020.
Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–8. https://doi.org/10.1001/jamacardio.2020.1017.
He XW, Lai JS, Cheng J, Wang MW, Liu YJ, Xiao ZC, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 patients. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(6):456–60. https://doi.org/10.3760/cma.j.cn112148-20200228-00137.
Hu H, Yao N, Qiu Y. Comparing rapid scoring systems in mortality prediction of critical ill patients with novel coronavirus disease. Acad Emerg Med. 2020;27(6):461–8. https://doi.org/10.1111/acem.13992.
Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935–41. https://doi.org/10.1111/dom.14057.
Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–8. https://doi.org/10.1016/j.jaci.2020.04.006.
Liang WH, Guan WJ, Li CC, Li YM, Liang HR, Zhao Y, et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): a nationwide analysis of China. Eur Respir J. 2020;55(6):2000562. https://doi.org/10.1183/13993003.00562-2020.
Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Inf Secur. 2020;81(1):e6–e12. https://doi.org/10.1016/j.jinf.2020.04.002.
Liu Y, Sun W, Guo Y, Chen L, Zhang L, Zhao S, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31(4):490–6. https://doi.org/10.1080/09537104.2020.1754383.
Luo M, Jiang B, Hong X, Yang Q, Zhou X, Lv Q, et al. Analysis of influencing factors of death in patients with COVID-19. Chin Tradit Herb Drugs. 2020;51:1450–4. https://doi.org/10.7501/j.issn.0253-2670.2020.06.010.
Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol. 2020;31(8):1088–9. https://doi.org/10.1016/j.annonc.2020.04.006.
Peng YD, Meng K, Guan HQ, Leng L, Zhu RR, Wang BY, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48(6):450–5. https://doi.org/10.3760/cma.j.cn112148-20200220-00105.
Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–9. https://doi.org/10.1001/jama.2020.6775.
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–10. https://doi.org/10.1001/jamacardio.2020.0950.
Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–9. https://doi.org/10.1111/jth.14817.
Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7. https://doi.org/10.1111/jth.14768.
Wang L, He WB, Yu XM, Liu HF, Zhou WJ, Jiang H. Prognostic value of myocardial injury in patients with COVID-19. Zhonghua Yan Ke Za Zhi. 2020;56(0):E009. https://doi.org/10.3760/cma.j.cn112148-20200313-00202.
Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Inf Secur. 2020;80(6):639–45. https://doi.org/10.1016/j.jinf.2020.03.019.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43. https://doi.org/10.1001/jamainternmed.2020.0994.
Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95(6):1138–47. https://doi.org/10.1016/j.mayocp.2020.04.006.
Xu B, Fan CY, Wang AL, Zou YL, Yu YH, He C, et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81(1):e51–60. https://doi.org/10.1016/j.jinf.2020.04.012.
Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18(6):1469–72. https://doi.org/10.1111/jth.14848.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. https://doi.org/10.1016/S2213-2600(20)30079-5.
Yao Q, Wang P, Wang X, Qie G, Meng M, Tong X, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020;130:390–9. https://doi.org/10.20452/pamw.15312.
Zhang J, Wang X, Jia X, Li J, Hu K, Chen G, et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–72. https://doi.org/10.1016/j.cmi.2020.04.012.
Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–9. https://doi.org/10.1111/jth.14859.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3.
Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. https://doi.org/10.1136/bmj.k4597.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–6. https://doi.org/10.7326/0003-4819-158-4-201302190-00009.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. https://doi.org/10.1186/1471-2288-14-135.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186.
Hu X, Deng Y, Wang J, Li H, Li M, Lu Z. Short term outcome and risk factors for mortality in adults with critical severe acute respiratory syndrome (SARS). J Huazhong Univ Sci Technolog Med Sci. 2004;24(5):514–7. https://doi.org/10.1007/BF02831124.
Chan JW, Ng CK, Chan YH, Mok TY, Lee S, Chu SY, et al. Short term outcome and risk factors for adverse clinical outcomes in adults with severe acute respiratory syndrome (SARS). Thorax. 2003;58(8):686–9. https://doi.org/10.1136/thorax.58.8.686.
Alburikan KA, Abuelizz HA. Identifying factors and target preventive therapies for Middle East respiratory syndrome sucsibtable patients. Saudi Pharm J. 2020;28(2):161–4. https://doi.org/10.1016/j.jsps.2019.11.016.
Ahmed AE. The predictors of 3- and 30-day mortality in 660 MERS-CoV patients. BMC Infect Dis. 2017;17(1):615. https://doi.org/10.1186/s12879-017-2712-2.
Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. https://doi.org/10.1136/bmj.m3249.
Jeng MJ. Coronavirus disease 2019 in children: current status. J Chin Med Assoc. 2020;83(6):527–33. https://doi.org/10.1097/JCMA.0000000000000323.
Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55(5):2000688. https://doi.org/10.1183/13993003.00688-2020.
Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–40. https://doi.org/10.1001/jamacardio.2020.1286.
Sama IE, Voors AA. Men more vulnerable to COVID-19: explained by ACE2 on the X chromosome? Eur Heart J. 2020;41(32):3096. https://doi.org/10.1093/eurheartj/ehaa526.
Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251(3):228–48. https://doi.org/10.1002/path.5471.
Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12:6049–57. https://doi.org/10.18632/aging.103000.
Yang JK, Feng Y, Yuan MY, Yuan SY, Fu HJ, Wu BY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med. 2006;23(6):623–8. https://doi.org/10.1111/j.1464-5491.2006.01861.x.
Banik GR, Alqahtani AS, Booy R, Rashid H. Risk factors for severity and mortality in patients with MERS-CoV: analysis of publicly available data from Saudi Arabia. Virol Sin. 2016;31(1):81–4. https://doi.org/10.1007/s12250-015-3679-z.
Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–77. https://doi.org/10.1016/j.cmet.2020.04.021.
Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92(10):1902–14. https://doi.org/10.1002/jmv.25884.
Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46(6):1114–6. https://doi.org/10.1007/s00134-020-06026-1.
Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72(2):389–98. https://doi.org/10.1002/hep.31301.
Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–6. https://doi.org/10.1016/j.ijid.2014.09.003.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–4. https://doi.org/10.1016/S0140-6736(20)30628-0.
Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–20. https://doi.org/10.1002/art.38690.
Akhmerov A, Marbán E. COVID-19 and the heart. Circ Res. 2020;126(10):1443–55. https://doi.org/10.1161/CIRCRESAHA.120.317055.
Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–40. https://doi.org/10.1182/blood.2020006000.
National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2021. Available at: https://www.covid19treatmentguidelines.nih.gov. Accessed 24 June 2021.
Li H, Chen C, Hu F, Wang J, Zhao Q, Gale RP, et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020;34(6):1503–11. https://doi.org/10.1038/s41375-020-0848-3.
Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, et al. Infectious Diseases Society of America Guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020:ciaa478. https://doi.org/10.1093/cid/ciaa478.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. https://doi.org/10.1056/NEJMoa2021436.
Acknowledgements
Not applicable.
Funding
This study was supported by the Hangzhou Health Science and Technology Planning Project (grant number: OO20190083).
Author information
Authors and Affiliations
Contributions
Conceived and designed the protocol: NL and CS. Execution of literature search: CS and LW. Execution of data extraction: JY and ZG. Execution of quality assessment: LW and JY. Data analysis and interpretation: CS, LW, JY, ZG, SW, JX, YX, QL, RX, and NL. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, C., Wang, L., Ye, J. et al. Predictors of mortality in patients with coronavirus disease 2019: a systematic review and meta-analysis. BMC Infect Dis 21, 663 (2021). https://doi.org/10.1186/s12879-021-06369-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06369-0