Abstract
Background
Pneumococcal vaccine immunizations may be responsible for alterations in serotype epidemiology within a region. This study investigated the pneumococcal carriage prevalence and the impact of the 13-valent pneumococcal conjugate vaccine (PCV-13) on circulating serotypes among healthy children in Northern Ghana.
Methods
This was a cross sectional study conducted in the Kassena-Nankana districts of Northern Ghana from November to December during the dry season of 2018. Nasopharyngeal swabs collected from 193 participants were cultured per standard microbiological protocols and pneumococcal isolates were serotyped using the latex agglutination technique and the capsular Quellung reaction test. We examined for any association between the demographic characteristics of study participants and pneumococcal carriage using chi-square test and logistic regression.
Results
Of the 193 participants that were enrolled the mean age was 8.6 years and 54.4% were females. The carriage rate among the participants was 32.6% (63/193), and twenty different serotypes were identified. These included both vaccine serotypes (VT), 35% (7/20) and non-vaccine serotypes (NVT), 65% (13/20). The predominant serotypes (34 and 11A), both of which were NVT, accounted for a prevalence of 12.8%. PCV-13 covered only 35% of serotypes identified whiles 40% of serotypes are covered by PPV 23.
Conclusion
Post-vaccination carriage of S. pneumoniae is high and is dominated by non-vaccine serotypes. There is therefore a need for the conduct of invasive pneumococcal disease surveillance (IPD) to find out if the high non-vaccine serotype carriage translates to disease. And in addition, a review of the currently used PCV-13 vaccine in the country would be considered relevant.
Similar content being viewed by others
Background
Streptococcus pneumoniae, is described as a pathobiont inhabiting the host nasopharynx as a commensal and causes several invasive and non-invasive diseases [1, 2]. High proportions of disease incidence have been associated with the pneumococcus in recent times, contributing to high levels of disease burden globally. Annually, an average of 800,000 deaths are reported among children due to invasive pneumococcal diseases with considerable proportions occurring in developing countries [3, 4]. Globally an estimated 50% of all pneumococcal deaths reported in 2015 occurred in developing countries [5].
Nasopharyngeal carriage of the pneumococcus may ultimately lead to severe disease conditions including septicaemia, meningitis, pneumonia, sinusitis and otitis media [6]. The pneumococcus exhibits a nonrandomized serotype distribution within the carriage reservoir and this is determined by variations in age, time and geographical jurisdictions [3]. Currently, up to 100 pneumococcal serotypes has been described [7], from which an average of 20 serotypes are reported to be commonly associated with juvenal invasive pneumococcal disease (IPD), namely 19A, 3, 7F, 23F, 11, 6A, 6B, 14, 8, 18C and 19F [2, 8].
Since the introduction of vaccines against pneumococcus, substantial health benefits have been realized despite suggestions of serotype replacement following implementation of these vaccines [9, 10]. Currently two main forms of pneumococcal vaccines are in existence namely pneumococcal polysaccharide vaccines (PPVs) which targets 23 antigenic types of the pneumococcus and pneumococcal conjugate vaccines (PCVs) which targets 10 and 13 antigenic serotypes [11,12,13]. Whereas PCVs offer better protection for children, eliciting herd protection, inducing effective immune bodies which are long lived, PPVs are known to confer protection specifically for the adult population and immune compromised individuals [14, 15]. Pneumococcal conjugate vaccines, though effective in inducing immune response across all age groups, cover a limited number of serotypes [16, 17].
Ghana introduced the 13-valent conjugate vaccines (PCV-13) into its Expanded Programme for Immunization (EPI) in 2012 for infant vaccinations at 6, 10 and 14 weeks of age [18]. Nonetheless, the targeted serotype range of the vaccine is quite limited. A previous study from Ghana prior to the introduction of the vaccine in 2012 indicated a vaccine serotype coverage of approximately 50% [19]. Limitation in serotype coverage could exert a vaccine selective pressure inducing evolution of non-vaccine serotypes [20]. Thus, a post vaccination upsurge in non-vaccine type serotypes (NVT) is likely to favour transmissions and emergence of NVT pneumococcal attacks.
Serotype replacement post pneumococcal vaccination deployment is thus a major issue of concern and may pose significant health challenges. This highlights the need for maintaining carriage surveillance in order to explore the dynamics in serotype distribution within the carriage reservoir and other post-vaccination impacts [21]. In Ghana, several post-vaccination pneumococcal surveillance studies have been carried out [22,23,24]. However, these studies were limited to southern Ghana and there is limit pneumococcal post-vaccination surveillance in the northern part of the country where pneumococcal outbreaks are relatively common. This study aimed at investigating the rate of nasopharyngeal carriage of S. pneumoniae and the serotypes circulating among school-aged children aged 5 to 12 years in the Kassena-Nankana districts of Ghana.
Methods
Study site
The study was conducted in the Kassena–Nankana Districts (KND) of Northern Ghana, which is predominantly characterized by arid climatic conditions with a population of 165,000 and population density of 91.5km2 [25]. The study area has a Health and Demographic Surveillance System (HDSS) which monitors the population dynamics of the area [25]. Fieldworkers visit all the households 2–3 times a year and register all demographic events such as births, deaths, marriages, in-migrations and out-migrations. The district has a rural setting except for those living in the town of Navrongo, which has a population of 20,000 with an average of 10 inhabitants living in a compound [26] (Fig. 1). The study area lies within the meningitis belt of sub-Saharan Africa where there are frequent outbreaks of meningitis during the dry seasons (between December and May) with seasonal occurrence of larger outbreaks reported every 8–12 years [27, 28]. In 2005, the district reported a serotype 1 pneumococcal meningitis outbreak resulting in a case fatality rate of 44.4% out of the 117 pneumococcal meningitis cases recorded during the outbreak season [26]. The current coverage of PCV-13 vaccination in Ghana is reportedly 97% [29].
Study design and data collection
This was a cross-sectional study carried out from November to December 2018 during the dry season. The study population involved children of both sexes, aged between 5 and 12 years. A multi-staged random sampling technique was adopted. First, we randomly sampled four communities within the study districts. In the second stage, we randomly sampled 50 eligible children comprising healthy children between 5 to 12 years whose parents have granted consent for participation from the communities using the database of the Navrongo HDSS which monitors the population dynamics of the area. Community engagement was undertaken to sensitize the selected communities on the aims and objectives of the project, all sampled children were then contacted through their parent to be part of the study. During data collection each household was visited, introductions were made, consent obtained. Data collection involved a four-member team comprising the investigator, field assistant, field worker and a consenter. Invitations to all selected participants were carried out a day prior to recruitments by the designated field worker and a parental informed consent process was conducted by a field consenter who ensured that parents were well informed about the survey.
A pre-piloted questionnaire (Supplemental file 1) was used to collect data on the demographic factors such as age, sex, and house-hold size as well as medical history and PCV-13 vaccination status of participants.
Sample size estimation
Sample size was determined using the sample size formula N = Zα 2 × P (1-P)/d2 as cited elsewhere [30]. With the assumption that the prevalence of pneumococcal carriage was 15% [31], a 95% confidence level and an accuracy rate of 5%, the sample size was estimated to be 196 participants. This was adjusted for a 5 % non-response rate resulting in a total sample size of 206 participants.
Specimen collection and processing
Nasopharyngeal specimens were collected according to the WHO working group standard method [32] for detecting carriage of S. pneumoniae. In brief, nylon flocked swabs were used to collect samples from participants and transported in skimmed milk tryptone glucose glycerol (STGG) transport medium under a cold chain to the lab within six hours after collection [33].
Samples were cultured on 5 mg/L gentamicin sheep blood agar and incubated at 37 °C for 24 h under 5% CO2 atmospheric conditions. A well isolated pure colony showing alpha hemolysis was subcultured on blood agar and gram stained. Biochemical testing comprising bile solubility testing and optochin susceptibility testing were used to confirm suspected isolates [33]. Pneumococcal isolates were stored in 10% glycerol Brain-Heart Infusion (BHI) broth and subsequently shipped on dry ice to Statens Serum Institut (SSI), Copenhagen, Denmark.
The serotyping of the isolates was perform as described by Slotved and colleagues [34]. Briefly, a latex agglutination test was performed by using the ImmuLex™ test (ImmuLex™ Pneumotest kit, SSI Diagnostica, Denmark). Ten microliters of an overnight culture in Todd Hewit broth (TH-broth, SSIDiagnostica, Denmark) was reacted with serotype specific antisera for observation of agglutination reactions and confirmed by the Quellung reaction using serotype specific antisera (SSIDiagnostica, Denmark) [34, 35]. Non-typeable strains were defined as isolates presenting no phenotypic detectable capsule [23].
Data analysis
Data was double entered and verified using EPI data 6.0 with built in consistency checks to control data output. Data were analyzed using Stata 13 for Windows. Categorical variables were summarized using proportions and continuous variables were presented as means with standard deviations or median with interquartile range. Descriptive analyses comprising computation of arithmetic means, frequencies and percentages were done on the study variables. Chi-square test was used to assess association between pneumococcal carriage and demographic characteristics. Bivariate and multiple logistic regression analyses were used to assess the relationship between pneumococcal carriage and other independent variables and results were presented as Odds Ratios (OR), p values and Confidence Intervals (95% CI). Statistical significance level was set at p-values less than 0.05. Permutations on vaccine serotype coverage of PCV-10, PCV-13 and PPV-23 on serotype distribution pattern were analyzed using the theoretical serotype coverage analytical method [36, 37].
Ethical standards
Ethical approvals for this study were obtained from the KNUST Committee on Human Research Publication and Ethics (CHRPE/AP/371/18) and the Navrongo Health Research Centre Institutional Review Board (NHRCIRB314). The study was conducted in accordance with the regulatory requirements which affords greater protection to the subject under the human protection enactment stipulated in the declaration of Helsinki. All protocol methods were performed per the guidelines of the scientific and ethical review boards from which ethical approvals were sought.
Results
Demographic characteristics of children recruited into the study
193 participants aged between 5 and 12 years were enrolled into the study. The mean and median ages of participants were 8.6 (± 2.3) and 8 years (IQR 7–11) respectively. 54.4% were females (Table 1), and the majority (40.4%) of the participants were 8–10 years of age. Nearly all (98.4%) of the children had received their childhood immunization but only 24.4% (47) had received the PCV-13 vaccine. There were 37 children (19.2%) who had respiratory symptoms and 3 children (1.6%) who had been exposed to antibiotics prior to sampling. Participants from households bigger than the mean household size of seven were in the majority (56.5%, 109/193) (Table 1).
Carriage prevalence
The overall prevalence of S. pneumoniae among study participants was 32.6% (95%Cl, 26.0–40.0). Carriage rates of 40% (95% CI, 28.6–52.6), 32.1% (95% CI, 22.5–43.4) and 24.0% (95%CI, 13.9–38.2) were respectively recorded among age categories 5–7 years, 8–10 years and 11–12 years. Male and female participants recorded carriage rates of 36.4% (95% CI, 2–47) and 29.5% (95% CI, 22–39). Moreover, carriage prevalence of 38.3, 37.8 and 33.3% were observed for participants who were administered PCV-13, had respiratory symptoms and were exposed to antibiotics. Carriage prevalence for participants above and below the average household size was 33.9 and 30.9% respectively (Table 1).
In a sub-group analysis of pneumococcal carriage prevalence among 44 children who were eligible to receive PCV-13 when it was introduced in 2012, 40 children who were immunized against the pneumococcus had a carriage rate of 35% (95% CI, 21.0–52.0) compared to a carriage rate of 75% (95% CI, 4.0–100.0) among PCV-13 unvaccinated children.
Pneumococcal serotype distribution and vaccine coverage
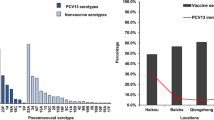
Polysaccharide capsular typing analysis was carried out on 38 out of the 63 pneumococcal isolates. This was due to lack of viability of 25.4% (16/63) of the harvested isolates and 14.3% (9/63) being non-typeable. Nonetheless, twenty (20) distinct serotypes were identified; among which serotypes 35 and 11A were most predominant, each with a prevalence of 12.8% (5/39). Moreover, other prevailing serotypes recorded respective prevalence rates of 7.7% (13, 17F, 18C, 6A), 5.1% (16F, 3, 19F) and 2.6% (10A, 14, 15A, 19B, 28F, 33B, 35B, 4, 40, 6B, 9A). Only one participant carried multiple serotypes (1.6%) (Fig. 2).
Serotype distribution varied among PCV-13 vaccinated and PCV-13 unvaccinated children. Our results showed that the most commonly occurring serotypes among PCV-13 vaccinated children were serotypes 18C, 34, 6C, 11A, recording a uniform prevalence rate of 15.38% whereas serotypes 11(15%), 17F (15%) and 34 (12%) were predominant among PCV-13 unvaccinated group (Fig. 2).
A vaccine serotype coverage analysis showed a uniform serotype coverage rate 35% for all PCVs (PCV-10 and PCV-13) and a coverage rate of 40% for PPV 23. Additionally, a serotype coverage of 25% was observed for all PCVs among children vaccinated against the pneumococcus and a coverage of 42% was observed among PCV-13 unvaccinated children. Moreover, serotype coverage of 13 and 58% were observed among PCV-13 vaccinated and PCV-13 unvaccinated children respectively for PPV 23 (Fig. 3).
Association between carriage and background factors
A bivariate and multivariate analysis performed to assess the association between carriage and other background factors (age, sex, PCV-13 vaccination, antibiotic exposure, respiratory symptoms and household size) did not show any statistically significant association between pneumococcal carriage and the background characteristics of the study participants (Table 2).
Discussion
The study investigated the nasopharyngeal carriage of S. pneumoniae among school children in the Kassena-Nankana districts of Northern Ghana. Though there was lack of association between pneumococcal carriage and background features, the rate of pneumococcal carriage, 32.6% (95%Cl, 26.0–40.0) observed among study participants was quite high. It was also observed that the majority (65%) of serotypes identified were non-vaccine type serotypes. Of notable concern was the observed high non-vaccine targeted strains in circulation. The persistently high rate of pneumococcal carriage despite various interventions implemented against the bacterium poses significant risk to the success of these interventions. The rate of pneumococcal carriage observed among the study participants conforms with findings of previous studies conducted pre and post implementation of PCV-13 which showed respective prevalence of 32% (95% CI 29–36%) to 39% (95% CI: 32.3 to 46.2) in 2013 and 2018 within the country [19, 36, 37]. However, carriage rates ranging between 27% (95% CI: 19.1 to 35.1) to 51% have been reported from other parts of the country, thus reflecting the variation in pneumococcal carriage prevalence per the different geographical locations [19, 23, 36, 37]. Relative to this study, a higher carriage rate of 54% (95% CI, 49–59%) was observed from a recent study post PCV-13 implementation in the country. This may be due to the lower age population (between 6 to 60 months) [23] which was investigated compared to the higher age group (5 to 12 years) in this study. Previous trends in carriage rates suggest that the pneumococcal carriage reservoir may be undergoing an epidemiological transition within the population. A pre-vaccination publication reported a higher rate of carriage in 2002 within the country followed by a drastic decline almost a decade after. Thus a carriage rate ranging from 51 to 32% was reported by the end of the pre-vaccination era [19, 38].
Recently published data on pneumococcal carriage prevalence post PCV-13 deployment within the country however showed no significant reduction in carriage rates among study participants. The study revealed a carriage rate of 54% (95% CI, 49–59%) among study participants from southern Ghana, reflecting the persistence in carriage despite vaccine deployments within the country [23].
Several countries have reported dramatic decline in the incidence of invasive pneumococcal disease (IPD) following implementation PCVs into their childhood immunization programme [39]. However, these immunization impacts are being supressed by the emergence of NVTs which are contributing to antimicrobial resistance developments translating in poor disease managements [12, 14].
In recent times carriage rates and disease incidents by VT have declined and a concurrent rise in in colonization by NVT post pneumococcal vaccination have become apparent globally [8, 40]. The 2016 meningitis outbreak in Ghana indicated that NVT 12F and 35 B were responsible for a proportion of cases reported during the outbreak season [41] whereas an increase in antibiotic-resistant NVTs, including serotype 19A, post-PCV 7 has been shown in other studies [13].
A comparison of PCV-13 serotype coverage among this study cohort showed a marked difference in prevalence between VT and NVT serotypes. A prevalence rate of 65% was observed for NVT serotypes relative to a prevalence rate of 35% for VT serotypes. In comparison with previous findings from Ghana in 2013, which reported a PCV-13 serotype coverage of 50%, the current study suggests a PCV-13 serotype coverage of 35% indicating a decline in PCV-13 serotype coverage. The observed drift in serotype distribution could be attributed to the replacement in serotypes by NVT serotypes which could be due to the effect of the currently used PCV-13 vaccine [42]. Reports from previous studies have suggested the role of genetic recombination in evolutions within the pneumococcal population including acquisition of antibiotic resistance and serotype-switching [43]. The upsurge in NVT within the pneumococcal reservoir with the increasing rates of antimicrobial resistance associated with these events [44] is of public health concern.
Earlier studies from Ghana and elsewhere in Africa have reported similar trends where a drastic decline in VT carriage was observed and the main attributable factor was vaccine driven [45]. Replacement serotypes may super evolve, evade herd immunity and drive NVT disease transmissions [46, 47].
We did not find any statistically significant association between carriage and the background characteristics of the children in the study. This could partly be attributed to the under power of the study, however, this study is important, in that it is the first of its kind to characterize the prevalence and serotype distribution of S. pneumoniae among carriers in the study area. This will serve as baseline data to guide future pneumococcal studies.
In this study, we could only serotype 38 (60.3%) out of the 63 isolates identified. This was because 16 (25.4%) were not viable and 9 (14.3%) were non-typeable. However, we compared the distribution of the background characteristics of the serotyped and non-serotyped groups, and found no statistically significant difference between them (Supplementary Table 1). Also, a prevalence rate of 15% [31] was used to estimate the sample size. However, subsequent studies have come out with varied prevalence, some of which are higher than the 15% prevalence that we used to estimate our sample size. We therefore recommend continuous surveillance to enhance comprehension on the dynamics of the pneumococcal carriage reservoir.
Conclusion
Pneumococcal carriage persists within the populace despite the introduction of PCV-13 vaccine into Ghana’s EPI programme. Replacement in serotypes due to the limited serotype coverage of the current vaccine may be of minimal benefit to the populace. This therefore requires a review of the existing PCV vaccine to ensure optimal protection against the pathogen and wellbeing of the populace.
Availability of data and materials
The datasets used for the study are available on request from the corresponding author (Deborah K. Narwortey, MPhil, Senior Research Scientist, Biomedical Department, Navrongo Health Research Centre, Email: narworteydeborah@gmail.com).
Abbreviations
- ATTC:
-
American type culture collection
- BHI:
-
Brain heart infusion
- MIC:
-
Minimum inhibitory concentration
- NHDSS:
-
Navrongo Health and Demographic Surveillance System
- NVT:
-
Non-vaccine types
- PCV-13:
-
13-Valent pneumococcal conjugate vaccine
- VT:
-
vaccine types
- PPV:
-
Pneumococcal polysaccharide vaccine
- STGG:
-
skim milk-tryptone-glucose-glycerin medium
References
Finland M. Forward to the second printing. In: Heffron R, editor. Pneumonia: with special reference to pneumococcal lobar pneumonia. Cambridge: Massachusetts Harvard Univ Press; 1978.
Bogaert D, De Groot RHP. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–54. https://doi.org/10.1016/S1473-3099(04)00938-7.
Johnson HL, Deloria-Knoll M, Levine OS. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7(10):e1000348. https://doi.org/10.1371/journal.pmed.1000348.
O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet [Internet]. 2009;374(9693):893–902. Available from:. https://doi.org/10.1016/S0140-6736(09)61204-6.
Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y,. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Heal [Internet]. 2018 Jul 1 [cited 2019 Jun 11];6(7):e744–e757. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29903376.
Kim L, Mcgee L, Tomczyk S, Beall B. Biological and Epidemiological Features of Antibiotic-Resistant Streptococcus pneumoniae in Pre- and Post-Conjugate Vaccine Eras: a United States Perspective. Clin Microbiol Rev. 2016;29(3):525–52. https://doi.org/10.1128/CMR.00058-15.
Ganaie F, Saad JS, McGee L, van Tonder AJ, Bentley SD, Lo SW. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. MBio. 2020;11(3):1–15.
Jauneikaite E, Mary Carnon Jefferies J, William Vere Churton N, Tzer Pin Lin R, Lloyd Hibberd M, Charles Clarke S et al. Genetic diversity of Streptococcus pneumoniae causing meningitis and sepsis in Singapore during the first year of PCV7 implementation. Emerg Microbes Infect. 2014;3(6):e39. https://doi.org/10.1038/emi.2014.37.
Singleton RJ, Hennessy TW, Bulkow LR. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297(16):1784–92. https://doi.org/10.1001/jama.297.16.1784.
Hanage WP. Serotype-specific problems associated with pneumococcal conjugate vaccination. Future Microbiol. 2008;3(1):23–30. https://doi.org/10.2217/17460913.3.1.23.
Ko KS, Baek JY, Song JH. Capsular gene sequences and genotypes of “serotype 6E” streptococcus pneumoniae isolates. J Clin Microbiol. 2013;51(10):3395–9. https://doi.org/10.1128/JCM.01645-13.
Lin FL, Vinogradov E, Deng C, Zeller S, Phelan L, Green BA,. Structure elucidation of capsular polysaccharides from Streptococcus pneumoniae serotype 33C, 33D, and revised structure of serotype 33B. Carbohydr Res [Internet]. 2014 Jan 13 [cited 2020 Jan 10];383:97–104. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0008621513003947?via%3Dihub
Moore MR, Gertz RE WR Jr. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197(7):1016–27. https://doi.org/10.1086/528996.
Selman S, Hayes D, HW PLA. Pneumococcal conjugate vaccine for young children. Manag Care. 2000;9(9):49–52 56–57.
WHO. Pneumococcal conjugate vaccine for childhood immunization – WHO position paper. Wkly Epidemiol Rec. 2007;82(12):93–104.
Trzciński K, Li Y, Weinberger DM, Thompson CM, Cordy D, Bessolo A, Malley R, Lipsitch M. Effect of Serotype on Pneumococcal Competition in a Mouse Colonization Model. mBio. 2015;6(5):e00902–15. https://doi.org/10.1128/mBio.00902-15.
Weinberger DM, Trzciński K, Lu YJ, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 2009;5(6):e1000476. https://doi.org/10.1371/journal.ppat.1000476.
WHO. World Health Organization. Ghana: WHO and UNICEF estimates of immunization coverage. 2018;
Dayie NT, Arhin RE, Newman MJ, Dalsgaard A, Bisgaard M, Frimodt-Møller N, et al. Penicillin resistance and serotype distribution of Streptococcus pneumoniaein Ghanaian children less than six years of age. BMC Infect Dis [Internet]. 2013 Dec 22 [cited 2019 Jun 11];13(1):490. Available from: https://doi.org/10.1186/1471-2334-13-490
Hanage WP. Serotype replacement in invasive pneumococcal disease: where do we go from here? J Infect Dis. 2007;196(9):1282–4. https://doi.org/10.1086/521630.
Knol MJ. Invasive pneumococcal disease 3 years after introduction of 10-valent pneumococcal conjugate vaccine, the Netherlands. Emerg Infect Dis. 2015;21(11):2040–4. https://doi.org/10.3201/eid2111.140780.
Dayie NTKD, Arhin RE, Newman MJ, Dalsgaard A, Bisgaard M, Frimodt-Møller N. Multidrug-resistant streptococcus pneumoniae isolates from healthy Ghanaian preschool children. Microb Drug Resist. 2015;21(6):636–42. https://doi.org/10.1089/mdr.2014.0314.
Dayie NTKD, Tettey EY, Newman MJ, Bannerman E, Donkor ES, Labi AK. Pneumococcal carriage among children under five in Accra, Ghana, five years after the introduction of pneumococcal conjugate vaccine. BMC Pediatr. 2019;19(1):316. https://doi.org/10.1186/s12887-019-1690-5.
Donkor ES, Annan JA, Badoe EV, Dayie NTKD, Labi A-K, Slotved H-C. Pneumococcal carriage among HIV infected children in Accra, Ghana. BMC Infect Dis [Internet]. 2017;17(1):133. Available from:. https://doi.org/10.1186/s12879-017-2224-0.
Oduro AR, Wak G, Azongo D, Debpuur C, Wontuo P, Kondayire F. Profile of the Navrongo health and demographic surveillance system. Int J Epidemiol. 2012;41(4):968–76. https://doi.org/10.1093/ije/dys111.
Leimkugel J, Forgor AA, Bastien Gagneux S, Pflü Ger V, Flierl C, Awine E. An Outbreak of Serotype 1 Streptococcus pneumoniae Meningitis in Northern Ghana with Features That Are Characteristic of Neisseria meningitidis meningitis epidemics. J Infect Dis. 2005;192(2):192–9. https://doi.org/10.1086/431151. Epub 2005 Jun 10.
Akweongo P, Dalaba MA, Hayden MH, Awine T, Nyaaba GN, Anaseba D,. The Economic Burden of Meningitis to Households in Kassena-Nankana District of Northern Ghana. Merler S, editor. PLoS One [Internet]. 2013 Nov 21 [cited 2018 Jan 8];8(11):e79880. Available from: https://doi.org/10.1371/journal.pone.0079880.
Opare JKL, Awoonor-williams JK, Odoom JK, Afari E, Oduro A, Awuni B. Bacterial Meningitis: A Review in the Upper East Region of Ghana. Int J Trop Dis Health. 2015;10(3):1–11. https://doi.org/10.9734/IJTDH/2015/19398.
WHO and UNICEF. Ghana : WHO and UNICEF estimates of immunization coverage : 2019 revision Ghana : WHO and UNICEF estimates of immunization coverage : 2019 revision. 2021;1–22.
Bokaeian M, Khazaei HA, Javadimehr M. Nasopharyngeal carriage, antibiotic resistance and serotype distribution of Streptococcus pneumoniae among healthy adolescents in Zahedan. Iran Red Crescent Med J. 2011;13(5):328–33.
Donkor ES, Newman MJ, Oliver-Commey J, Bannerman E, Dayie NTKD, Badoe E V. Invasive disease and paediatric carriage of Streptococcus pneumoniae in Ghana. Scand J Infect Dis [Internet]. 2010 [cited 2020 Jul 13];42(4):254–259. Available from: https://doi.org/10.3109/00365540903490000
Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine [Internet]. 2014 Dec 17;32(1):165–79 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24331112.
O’Brien KL, Nohynek N, World Health Organization Pneumococcal Vaccine Trials Carriage Working Group. World health organization pneumococcal vaccine trials carriage working group. Report from a WHO working group: standard method for detecting upper respiratory carriage of Strept. Pediatr Infect Dis J. 2003;22(2):133–40. https://doi.org/10.1097/01.inf.0000048676.93549.d1.
Slotved H, Sheppard CL, Dalby T, Van Der EA, Norman K, Morfeldt E. External Quality Assurance for Laboratory Identification and Capsular Typing of Streptococcus pneumoniae. Sci Rep [Internet]. 2017;(September):1–10. Available from:. https://doi.org/10.1038/s41598-017-13605-8.
Fjeldhøj S, Laursen RP, Larnkjær A, Mølgaard C, Fuursted K, Krogfelt KA,. Probiotics and carriage of Streptococcus pneumoniae serotypes in Danish children, a double-blind randomized controlled trial. Sci Rep [Internet]. 2018 Dec 1 [cited 2020 Jul 7];8(1):15258. Available from: www.nature.com/scientificreports
Dayie NTKD, Tetteh-Ocloo G, Labi A-K, Olayemi E, Slotved H-C, Lartey M, et al. Pneumococcal carriage among sickle cell disease patients in Accra, Ghana: Risk factors, serotypes and antibiotic resistance. PLoS ONE. 2018;13(11):e0206728. https://doi.org/10.1371/journal.pone.0206728.
The PLOS ONE Staff. Correction: Pneumococcal carriage among sickle cell disease patients in Accra, Ghana: Risk factors, serotypes and antibiotic resistance. PLoS ONE. 2019;14(1):e0211838. https://doi.org/10.1371/journal.pone.0211838.
Denno DM, Frimpong E, RWS MG. Nasopharyngeal carriage and susceptibility patterns of Streptococcus pneumoniae in Kumasi, Ghana. West Afr J Med. 2002;21(3):233–6.
Sharma D, Baughman WHA. Pneumococcal carriage and invasive disease in children before introduction of the 13-valent conjugate vaccine: comparison with the era before 7-valent conjugate vaccine. Pediatr Infect Dis J. 2013;32(2):e45–53. https://doi.org/10.1097/INF.0b013e3182788fdd.
Jansen AGSC, Rodenburg GD, van der Ende A, van Alphen L, Veenhoven RH, Spanjaard L, et al. Invasive pneumococcal disease among adults: associations among serotypes, disease characteristics, and outcome. Clin Infect Dis. 2009;49(2):e23–9. https://doi.org/10.1086/600045.
Kwambana-Adams BA, Asiedu-Bekoe F, Sarkodie B, Afreh OK, Kuma GK, Owusu-Okyere G. An outbreak of pneumococcal meningitis among older children (≥5 years) and adults after the implementation of an infant vaccination programme with the 13-valent pneumococcal conjugate vaccine in Ghana. BMC Infect Dis. 2016;16(1):575. https://doi.org/10.1186/s12879-016-1914-3.
Elston, J. W. T., Santaniello-Newton, A., Meigh , D. H., Allgar, V., Allison, T. Richardson, G., Meigh, R., Palmer, S.R., Barlow AG. Increasing incidence of invasive pneumococcal disease and pneumonia despite improved vaccination uptake : surveillance in Hull and East Yorkshire , UK , 2002–2009. Epidemiol Infect [Internet]. 2012;140:1252–1266. Available from: https://doi.org/10.1017/S0950268811001907.
Mostowy R, Croucher NJ, Hanage WP, Harris SR, Bentley S, Fraser C. Heterogeneity in the frequency and characteristics of homologous recombination in pneumococcal evolution. PLoS Genet. 2014;10(5):e1004300. https://doi.org/10.1371/journal.pgen.1004300.
Gherardi G, D’Ambrosio F, Visaggio D, Dicuonzo G, Del Grosso MPA. Serotype and clonal evolution of penicillin-nonsusceptible invasive Streptococcus pneumoniae in the 7-valent Pneumo- coccal conjugate vaccine era in Italy. Antimicrob Agents Chemother. 2012;56(9):4965–8. https://doi.org/10.1128/AAC.00830-12.
Kobayashi M, Conklin LM, Bigogo G, Jagero G, Hampton L, Fleming-Dutra KE. Pneumococcal carriage and antibiotic susceptibility patterns from two cross-sectional colonization surveys among children aged <5 years prior to the introduction of 10-valent pneumococcal conjugate vaccine - Kenya, 2009-2010. BMC Infect Dis [Internet]. 2017;17(1):1–12. Available from:. https://doi.org/10.1186/s12879-016-2103-0.
Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS. Incidence of pneumococcal disease due to non–pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196(9):1346–54. https://doi.org/10.1086/521626.
Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination [internet]. Vol. 378, the lancet. 2011 [cited 2020 mar 17]. p. 1962–1973. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21492929.
Acknowledgements
We wish to thank the director and the management of the Navrongo Health Research Centre for funding the project and the Staten’s Serum Institute for providing the platform for the serotyping analyses. We are also grateful to the biomedical team who contributed to the success of this work. Profound gratitude to all senior research officers for their significant contributions in facilitating the completion of the work.
Authors’ declaration
HCS participate in Pfizer supported projects, while the remaining authors have no declaration concerning this manuscript.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
DKN conceived and developed the protocol, carried out the lab and data analysis. ARO contributed to the design of the study, reviewed and contributed to manuscript write-up. AOO contributed to the design of the study, reviewed and contributed to manuscript write-up. HCS contributed to serotyping analysis and manuscript review. ESD contributed to the manuscript review. GA contributed to the statistical analysis and reviewed protocol. PW contributed to statistical data analysis. POA assisted with field sampling. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participants
The study was approved by the KNUST Committee on Human Research Publication and Ethics (CHRPE/AP/371/18) as well as from the Navrongo Health Research Centre Institutional Review Board (NHRCIRB314). Written informed consent was obtained from parents of participants prior to enrolment into the study.
Consent for publication
Not applicable.
Competing interests
The authors have declared no competing of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Narwortey, D.K., Owusu-Ofori, A., Slotved, HC. et al. Nasopharyngeal carriage of Streptococcus pneumoniae among healthy children in Kassena-Nankana districts of Northern Ghana. BMC Infect Dis 21, 661 (2021). https://doi.org/10.1186/s12879-021-06302-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06302-5