Abstract
Background
Vaginal candidiasis is frequent in women of reproductive age. Accurate identification Candida provides helpful information for successful therapy and epidemiology study; however, there are very limited data from the Vietnam have been reported. This study was performed to determine the prevalence, species distribution of yeast causing vaginal discharge and antifungal susceptibility patterns of Candida albicans among symptomatic non-pregnant women of reproductive age.
Methods
Vaginal discharge samples were collected from 462 women of reproductive age in Hanoi, Vietnam between Sep 2019 and Oct 2020. Vaginal swabs from these patients were examined by direct microscopic examination (10% KOH). CHROMagar™ Candida medium and Sabouraud dextrose agar supplemented with chloramphenicol (0.5 g/l) were used to isolate yeast, and species identification was performed using morphological tests and molecular tools (PCR and sequencing). Antifungal susceptibility testing was determined according to the Clinical and Laboratory Standards Institute guidelines (M27-A3 and M27-S4).
Results
The prevalence of vaginal yeast colonization in non-pregnant women was 51.3% of 462 participants. Nine different yeast species were identified. Among these isolates, C. albicans (51.37%) was the most frequent, followed by C. parapsilosis (25.88%), C. glabrata (11.37%), C. tropicalis (4.31%), C. krusei (3.92%), C. africana (1.57%), Saccharomyces cerevisiae (0.78%), C. nivariensis (1 isolates, 0.39%), and C. lusitaniae (1 isolates, 0.39%), respectively. Among C. albicans, all 46 isolates were 100% susceptible to micafungin, caspofungin, and miconazole. The susceptibility rates to amphotericine B, 5-flucytosine, fluconazole, itraconazole and voriconazole were 95.65, 91.30, 91.30, 82.61 and 86.95%, respectively.
Conclusions
The prevalence of VVC among symptomatic non-pregnant women of reproductive age in Vietnam was higher than many parts of the world. The high frequency of non-albicans Candida species, which were often more resistant to antifungal agents, was a notable feature. Resistance rates of vaginal C. albicans isolates to antifungal agents was low. Our findings suggest that continued surveillance of changes in species distribution and susceptibility to antifungals should be routinely screened and treated.
Similar content being viewed by others
Introduction
Candida species are part of the normal flora of the genital tract. In healthy asymptomatic non-pregnant women, these yeasts have been found in 20–30% [1, 2]. Approximately 75% of all women experience at least one episode of vulvovaginal candidiasis (VVC) and 40–50% will have recurrent episodes during their lifetime [2, 3]. Epidemiology of vaginal Candida infection suggests that up to 50% of all women would have experienced two or more episodes of VVC by the age of 25 years. This age group with the onset of sexual activity is an important risk factor [4]. The risk factors are believed to be associated with increased rate of VVC including host-related factors such as hyper-estrogenic state (pregnancy, hormone replacement therapy), poorly controlled diabetes, immunodeficiency states, use of antibiotic, treatment with glucocorticoids and genetic predispositions and behavioral factors such as birth control pills, intrauterine device, spermicides and condoms and hygiene habits, tight-fit clothing and sexual behaviour [5].
Epidemiological surveys around the world have indicated that distribution of Candida species responsible for VVC in women varies widely among countries, regions and also the study population, and women with vaginal candidiasis are more susceptible to HIV [2, 6]. Traditionally, Candida albicans, which is responsible for 85–95% of Candida vaginal infections, is the predominant species [7,8,9,10]; however, the raising frequency of non-albicans Candida (NAC) species has reported worldwide, particularly C. glabrata, C. tropicalis, C. parapsilosis, C. krusei, C. dubliniensis, with C. glabrata as the predominant species [8,9,10]. There are many options for the treatment of uncomplicated VVC caused by C. albicans. A variety of short-course topical treatments are available, including polyenes, imidazoles, or ciclopirox olamine [11]. In that, the azole antifungals are most usually available in vaginal suppositories and creams [11, 12]. Therapy with the oral azoles, including fluconazole, itraconazole and miconazole, is also possible [11]. In contrast, complicated VVC requires a more prolonged course of therapy and is often difficult to achieve successful results, especially in the patient with VVC caused by NAC [13]. The epidemiological data from previous studies showed that NAC species are more common among complicated VVC than uncomplicated cases and NAC species are often more resistant to antifungal agents [9, 14, 15]. Therefore, identification and antifungal susceptibility testing are necessary for selection of appropriate antifungal therapy and performance of infection control methods to prevent the transmission of these infections [9, 13].
In recent years there have been many publications about identification and antifungal susceptibility of Candida causing VVC from different countries in the world as China [16], Iran [7, 17, 18], Japan [19], Lebanon [1], Ethiopia [20] and Italia [21], etc., but very limited data is available on the species distribution of Candida isolates in cases of VVC in Vietnam. The epidemiology of antifungal resistance among C. albicans in reproductive age women in Vietnam remains poorly reported. Therefore, the aim of this study was to determine the prevalence, species distribution of yeast causing vaginal discharge and antifungal susceptibility pattern of C. albicans among symptomatic non-pregnant women of reproductive age.
Methods
Study population
In current study, a cross-sectional design was conducted between Oct 2019 and Sep 2020. A total of 462 symptomatic non-pregnant women within the age range of 18–49 years with a clinical picture suggestive of VVC were selected from obstetrics and gynecology clinic at 103 Military Hospital (550 beds, Ha Dong district) in Hanoi city, Vietnam. Patients who were non-married, in pregnancy, outside the childbearing age period or using any systemic or topical antifungal therapy in the previous 2 weeks were excluded from the study.
Sampling, phenotypic identification of yeast species
The diagnosis of VVC was based on clinical appearance and culturing on CHROMagar™ Candida (CHROMagar Company, Paris, France) plates. A case of the VVC was defined as a patient with symptoms and signs include vulvar itching, vaginal discharge, and had positive results for Candida spp. [22]. Clinical samples were taken from each patient’s vagina by obstetrician and gynecologist with sterile cotton-tipped swab. After that, vaginal swabs were transported to the clinical mycology or microbiology laboratory within 2 h for isolation of yeasts. And then, all of vaginal swab specimens were subjected to direct 10% KOH smear examination as well as cultured on CHROMagar™ Candida plates, incubated at 35 °C for 4 days to determine the co-infection rate of yeast species and distinguish between C. albicans, C. tropicalis, C. glabrata and C. krusei and other yeast species according to the manufacturer’s instructions. All agar plates were evaluated for the yeast growth and colony color every day. The presence of yeasts on agar plates was confirmed by the presence of budding yeast in wet preparations with 0.85% saline. If yeast species were the same, it was considered to be a single isolate. All yeast isolates were initially subcultured on Sabouraud dextrose agar (SDA) (Merck, Germany) supplemented with 0.02% chloramphenicol and kept in tryptic soy broth medium (TSB, Himedia, India) containing 2.5% glucose, 3% peptone, and 20% glyceron at − 80 °C for further use. Germ tube tests in serum at 37 °C for 2–3 h was also used for the differentiation of C. albicans and NAC species.
Genomic DNA isolation
Genomic DNA of yeasts was extracted from isolates using a commercial DNA isolation kit (Cat.#51,304, QIAGEN, Germany) according to the manufacturer’s recommendations. After that quality and quantity of extracted DNA was estimated using a NanoDropTM 2000 Spectrophotometer at 260 nm (Thermo Fisher Scientific, USA). The purified DNA was maintained at − 20 °C until used in the PCR.
Molecular identification of C. albicans species complex
C. albicans species complex was identified by molecular techniques PCR using the specific primer pair of CR-f (5′-GCTACCACTTCAGAATCATCATC-3′) and CR-r (5′-GCACCTTCAGTCGTAGAGACG-30) (Integrated DNA Technologies, USA) for the hyphal wall protein 1 (HWP1) gene [23]. The amplified PCR products of HWP1 gene were used to distinguish C. albicans (941 bp), C. dubliniensis (569 bp) and C. africana (750 bp) [23]. PCR reaction were performed as previously described [23]. Reference strain used as a control was C. albicans (ATCC 90028).
Determination of NAC based on PCR-RFLP technique
NAC species were identified by PCR-RFLP assays using universal primers (Integrated DNA Technologies, USA) and restriction enzyme MspI as described by previous studies [24, 25]. Total volume of PCR reactions was 30 μl containing 3 μl of DNA solution, 15 μl 2X Master Mix (Cat.# M7505, Promega, USA), 0.75 μl of each primer (0.25 μM) and distilled water up to 30 μl. PCR amplification was carried out with Thermo Mastercycler Gradient (Thermo Fisher Scientific, USA). PCR reaction were carried out as described in previous study [24]. Restriction fragment length polymorphism (RFLP) assay was performed in a total volume of 20 μl containing 10 μl PCR product, 1 μl of MspI enzyme (BioLabs, England), 2 μl of 10X NEbuffer (BioLabs, England) and 7 μl of distilled water. According to the manufacturer’s recommendations, the mix tubes were incubated at 37 °C for 3 h, and then enzyme was completely inactivated by heating at 65 °C for 15 min. The amplified PCR and digestion products were analyzed on 2.0% agarose gels containing 0.5 μg/ml ethidium bromide in 1X TBE buffer for about 1.5 h at 90 V and visualized on UV illumination (UVP, Canada). The PCR products and digestion sizes were determined by a 100 bp size marker (Cleaver, UK). In addition, unknown isolates were subjected to direct DNA sequencing.
ITS rDNA region sequencing
Amplification of the ITS1 and ITS2 domains of the rRNA gene were performed with the primers ITS5 (5′-GGA AGT AAA AGT CGT AAC AAG − 3′) and NL4 (5′- GGT CCG TGT TTC AAG ACG G − 3′) as previously described [26]. The PCR products of forty strains of yeast were shipped to Apical Scientific Sdn. Bhd (Kembangan 43,300, Selangor, Malaysia) for purification and automatic bidirectional sequencing, using the same PCR primers. ABI 3130 Genetic Analyzer software (SeqScape 2.1) was used to read the sequences. Species identification was accurately confirmed by two-directional sequencing. In total there were 40 fungal cultures sequenced, and all sequences were deposited in GenBank under the accession number MW307689-MW307696, MW307701-MW307705, MW307707-MW307727, MW057251- MW057254, MW057257, and MW055675.
In vitro antifungal susceptibility test
C. albicans isolates were removed from the − 70 °C freezer and revived on a SDA plate at 35 °C for 24 h. Fungal suspensions in saline sulotion of isolates were made and the density of the suspension was adjusted at 0.5 McFarland standards at a wavelength of 530 nm, and diluted to 0.5 × 103 or 2.5 × 103 cells/ml using RPMI 1640 medium. The antifungal susceptibility tests with micafungin (MFG), caspofungin (CAS), amphotericin B (AMB), flucytosine (5-FC), fluconazole (FCZ), itraconazole (ICZ), voriconazole (VCZ), miconazole (MCZ) (Sigma-Aldrich, St. Louis, MO, USA) were performed for 46 C. albicans strains using broth microdilution method as described in the clinical and laboratory standard institute (CLSI) document M27-A3 [27]. These strains were selected from all patients who had at least two episode of VVC within a year. Stock solutions of the antifungal agents were prepared in the appropriate solvent according to the CLSI [27]. The final concentration ranges were between 0.015 and 16 μg/mL for micafungin; 0.03 and 16 μg/mL for caspofungin, amphotericin B and miconazole; 0.12 and 64 μg/mL for flucytosine; 0.12 and 64 μg/mL for fluconazole; 0.015 and 8 μg/mL for itraconazole and voriconazole. The minimum inhibitory concentration (MIC) values for all antifungal agents were read after 24 and 48 h of incubation at 35 °C. The MIC values of AMB were visually determined at the lowest concentration of agent that prevents any visible growth (100%). The MICs endpoints of the azole, echinocandins and flucytosine have been defined as the lowest drug concentration at which there is at least a 50% decrease in growth compared to that of the drug-free well. Species-specific clinical breakpoints for isolated Candida were classified according to the M27-S4 document [28]. Because breakpoint for miconazole was not defined yet in literature, it is indicated that C. albicans is susceptible and resistant at MIC ≤1 μg/mL and MIC ≥8 μg/mL, respectively [29, 30]. Resistance rate is the percentage of isolates resistant to a specific antifungal drug. For quality control, C. parapsilosis (ATCC 22019) and C. albicans (ATCC 90028) were chosen as reference strains.
Statistical analyses
All statistical calculations were done using IBM SPSS Statistics for Microsoft Windows, version 20.0 (IBM Corp., Armonk, NY, USA). The sequences of the ITS-1 and ITS-2 regions of yeasts were compared to the available data in the NCBI database, using BLAST guidelines (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Results
During the study period, the 462 vaginal samples were collected from vagina of patients with signs and symptoms of vaginal infection. Of them, 237 (51.30%) patients were diagnosed with VVC. Patients had a mean age of 35.51 ± 7.99 years (range, 18–49 years). Women between 30 and 39 years had the highest prevalence rates of VVC (54.67%), while in the age groups 18–29 and 40–49 the prevalence rates were 51.96 and 45.89%, respectively.
Of the 237 patients, 17 (7.17%) had more than one yeast species, i.e., seven patients were colonized by both C. albicans and C. parapsilosis; three patients were coinfected with C. albicans and C. glabrata; two patients had both C. albicans and C. tropicalis; one patient with C. albicans and C. krusei (Figure 1); one patient with C. krusei and C. glabrata; one patient with C. krusei and C. parapsilosis; and one patient with C. parapsilosis and C. lusitaniae. One patient had three different Candida species, including C. albicans, C. krusei and C. glabrata (Table 1). One isolate of each species recovered from the same patient was analyzed further.
A total of 255 yeast isolates were obtained from the 237 vaginal samples. Based on CHROMagar™ Candida medium, PCR, PCR-RFLP, and gene sequencing methods, nine yeast species were identified, the most common yeast was C. albicans (131 isolates, 51.37%) followed by C. parapsilosis (66 isolates, 25.88%), C. glabrata (29 isolates, 11.37%), C. tropicalis (11 isolates, 4.31%), C. krusei (10 isolates, 3.92%), C. africana (4 isolates, 1.57%), S. cerevisiae (2 isolates, 0.78%), C. nivariensis (1 isolate, 0.39%), and C. lusitaniae (1 isolate, 0.39%) (Fig. 2, Fig. 3 and Fig. 4).
Gel electrophoresis of C. albicans complex PCR products targeting the HWP1 gene. Lane 1: molecular size standard (100 bp DNA ladder); lanes 2: negative control; lane 3: positive control (C. albicans ATCC 90028); lanes 4, 5 and 7: strains SD106, SD107 and SD158 (C. albicans, 940 bp); lane 6: strain SD139 (C. africana, 700 bp)
The observed susceptibility rates of 46 C. albicans isolates to MFG, CAS, AMB, 5-FC, FCZ, ICZ, VCZ and MCZ were 100.0% (46), 95.65% (44), 95.65% (44), 91.30% (42), 91.30% (42), 82.61% (38), 86.95% (40) and 100.0%, respectively. Some drug-resistant isolates were found. This yeast showed 4.35, 6.52 and 4.35% resistance to the FCZ, ICZ, and VCZ, respectively. The resistance rates of C. albicans to amphotericine B and 5-Flucytosine were 4.35% (Table 2).
Discussion
Vulvovaginal candidiasis (VVC) is the second most common vaginal infection in reproductive age women [31]. The results from different studies indicate that the prevalence of vulvovaginal candidiasis varies between countries, depending on the country, region, and population [14, 31,32,33]. In cases of complicated VVC, vaginal cultures are necessary to confirm clinical diagnosis and identify unusual species, because patients are more likely to have an infection with non-albicans Candida strains, which may require different treatment [13, 34] but in Vietnam this issue has received very little attention. Therefore, the current study has been performed to determine the prevalence, species distribution of yeast causing vaginal discharge and antifungal susceptibility pattern of C. albicans among symptomatic non-pregnant women of reproductive age in Ha Noi city, Vietnam.
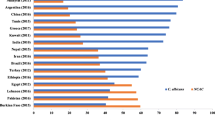
Multiple previous studies showed the prevalence rate of VVC among reproductive age women varies between countries and different regions, ranging from 12 to 72% [32, 35]. In this study, the incidence of VVC in Ha Noi city, Vietnam was found to be 51.30%. Although the prevalence rate of VVC in our study was within the reported range, it was higher than the prevalence rates reported by Anh et al. (1996) [36] that also performed in Ha Noi and Lien et al. (2002) in Hue, central Vietnam [37]. The reasons for such varying prevalence of VVC in Vietnam might be explained the investigation of different geographical locations, profile of the population being studied and period of time in these studies [31]. Therefore, more studies are required to determine the specific rate of VVC in Vietnam.
The availability of epidemiological data from around the world showed that the prevalence rate of VVC among reproductive age women had many differences [5, 32, 35]. The results of our study were similar to that of previous researches in Turkey (49.2%) [38], Egypt (50.4%) [21], Iran (50.5 and 51.6%) [39, 40]. Lower yeast prevalence was reported from Greece (12,1%) [41], UAE (13.88%) [42], India (20.0%) [43], Gabon (28.52%) [44], Ghana (36.5%) [45], Lebanon (39.0 and 44.8%) [1, 33], and Tanzania (45.7%) [46]. Studies from Yemen, Ethiopia reported also a lower prevalence of VVC among non-pregnant reproductive-aged women than the current study [20, 47]. Higher prevalence rates have been reported in Saudi (53.5%) [48], Nigeria (57.3%) [5, 32], and Brazil (72.7%) [35]. Varying prevalence could be due to multiple factors, including socio-demographic characteristics, immune status of patients, treating patients with broad spectrum antibiotics and immune suppressive drugs, and hormonal influences, etc. [20]. VVC affects women globally, and therefore, more studies are required to a better knowledge of the incidence of VVC [5].
Identification of Candida species is becoming increasingly important because there has been a notable shift in the etiology of candidiasis with non-albicans Candida (NAC) species gaining prominence [9]. In the current investigation, nice yeast species were isolated, including C. albicans, C. parapsilosis, C. glabrata, C. tropicalis, C. krusei, C. africana, S. cerevisiae, C. nivariensis, and C. lusitaniae. Among that, C. albicans was the most dominant isolated species (51.37%), whereas the overall prevalence of NAC species was 48.63%. Many previous studies have reported findings that agree with our results, including Guzel et al. (50.4 and 49.6%) [38], Hazirolan et al. (53.9 and 46.1%) [49], and Bitew et al. (58.6 and 41.4%) [20]. The data in different parts of the world have recorded higher rates of C. albicans in VVC (75–90%) [7, 39, 42, 44, 50], while lower rates of 25.9, 41.7 and 44.21% were reported from Ghana [51], Pakistan [52] and Iran [17], respectively. According to previous reports, C. albicans was responsible for 85–95% of VVC patients; however, most studies, published during the last years, reported incidence of C. albicans below 85% and in some regions even below 50% [5]. In fact, the distribution of Candida species isolated from women with VVC varies greatly depending on the location as well as the study population [1, 2]. In general, comparing the results of our study with those other studies which demonstrated, our incidence of candidiasis caused by of NAC were quite high. Notably, the most common NAC species in this study are C. parapsilosis accounting for 25.88% of all Candida species. Our result differs from other studies that implicate C. grabrata as the predominant NAC species causing VVC. According to most previous studies, the frequency rates of VVC attributed to C. glabrata were around 5–25% of cases [5, 9, 14, 50]. However, other NAC species have been reported as the most prevalent, such as C. tropicalis and C. krusei in Pakistan [52], C. krusei in Ethiopia [20, 53], and C. famata in Gabon [44].
Multiple studies have demonstrated the prevalence of Candida coinfection among VVC patients varies from 1 to 10% [5]. In the current study, Candida coinfection was observed in 17 (7.17%) patients. Lower coninfection prevalence was reported from China (2.2%) [54], Tunisia (3%) [55] and United States (4.8%) [30]. Higher prevalence rates reported in Iran (10.3 and 28%) [17, 56], Australia (13.4%) [57] and Turkey (14.1%) [38]. The prevalence of Candida coinfection in Vietnam are not very different from those in other parts of the world. Notably, the majority of the coinfection, 7 out of 17 (41.18%), were C. albicans and C. parapsilosis. These results of our study were slightly different from reports from around the world. According to previous studies, C. albicans and C. glabrata co-infection was the most common mixed infection [5, 30, 38]. These differences may be due to the high prevalence of C. albicans and C. parapsilosis.
The results of this study indicated that C. albicans was susceptible to most of the tested antifungals. The resistance rates of C. albicans isolates to azoles were lower than 6.25%. Multiple studies around the world also showed good in vitro activity of azoles against C. albicans isolates from VVC [20, 22, 58,59,60]. In contrast, the results previous researches in China [16, 61,62,63], Ethiopia [53] and Pakistan [52] indicated that susceptibility of C. albicans isolates from VVC to azoles were lower than those in other regions. In our study, all the C. albicans strains were susceptible to MFG and MCZ. The susceptibility rate of C. albicans to CAS was 96.65%. These results have shown that MFG, CAS and MCZ may provide an opportunity for treating azole-resistant VVC. Our results also showed good in vitro activity of AMB against C. albicans isolates from VVC. These results were in agreement with previously published reports from China [22, 63], Egypt [21], Lebanon [1] and Iran [39]. According to Philips (2005), amphotericin B vaginal suppositories have been successfully used in cases of azole-resistant Candida species [64]. Our findings also showed the prevalence of flucytosine resistance in C. albicans isolates were low. However, the speed at which yeast can develop resistance to flucytosine has driven clinicians to use the compound in combination with mainly amphotericin B [65].
Conclusion
There is a high prevalence of VVC among symptomatic non-pregnant women of reproductive age in Vietnam. Our findings also showed a high incidence of non- albicans Candida strains causing vulvovaginitis in the study population, which should be looked at as both novel and alarming. Resistance rates of vaginal C. albicans isolates to antifungal agents was low. Extensive surveillance studies of changes in species distribution and antifungal susceptibility should be routinely screened and treated.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AMB:
-
Amphotericin B
- ATCC:
-
American Type Culture Collection
- CAS:
-
Caspofungin
- CLSI:
-
Clinical and Laboratory Standards Institute
- FCZ:
-
Fluconazole
- GM:
-
Geometric mean
- ICZ:
-
Itraconazole
- ITS:
-
Internally Transcribed Spacer
- MFG:
-
Micafungin
- MIC:
-
Minimal Inhibitory Concentration
- MCZ:
-
Miconazole
- PCR:
-
Polimerase Chain Reaction
- RFLP:
-
Restriction Fragment Length Polymorphism
- SDA:
-
Sabouraud Dextrose Agar
- SDD:
-
Susceptible dose dependent
- VVC:
-
Vulvovaginal candidiasis
- VCZ:
-
Voriconazole
- 5-FC:
-
Flucytosine
References
Ghaddar N, Anastasiadis E, Halimeh R, Ghaddar A, Dhar R, AlFouzan W, et al. Prevalence and antifungal susceptibility of Candida albicans causing vaginal discharge among pregnant women in Lebanon. BMC Infect Dis. 2020;20(1):32. https://doi.org/10.1186/s12879-019-4736-2.
Achkar JM, Fries BC. Candida infections of the genitourinary tract. Clin Microbiol Rev. 2010;23(2):253–73. https://doi.org/10.1128/CMR.00076-09.
Sobel JD, Faro S, Force RW, Foxman B, Ledger WJ, Nyirjesy PR, et al. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998;178(2):203–11. https://doi.org/10.1016/S0002-9378(98)80001-X.
Geiger AM, Foxman B. Risk factors for vulvovaginal candidiasis: a case-control study among university students. Epidemiology. 1996;7(2):182–7. https://doi.org/10.1097/00001648-199603000-00013.
Gonçalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. 2016;42(6):905–27. https://doi.org/10.3109/1040841X.2015.1091805.
Røttingen J-A, William Cameron D, Garnett GP. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex Transm Dis. 2001;28(10):579–7.
Rezaei-Matehkolaei A, Shafiei S, Zarei-Mahmoudabadi A. Isolation, molecular identification, and antifungal susceptibility profiles of vaginal isolates of Candida species. Iran J Microbiol. 2016;8(6):410–7.
Sobel JD. Vulvovaginal candidosis. Lancet (London, England). 2007;369(9577):1961–71.
Makanjuola O, Bongomin F, Fayemiwo SA. An update on the roles of non-albicans Candida species in Vulvovaginitis. J Fungi (Basel). 2018;4(4):121. https://doi.org/10.3390/jof4040121.
Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12(1):80–96. https://doi.org/10.1128/CMR.12.1.80.
Mendling W, Brasch J, Cornely OA, Effendy I, Friese K, Ginter-Hanselmayer G, et al. Guideline: vulvovaginal candidosis (AWMF 015/072), S2k (excluding chronic mucocutaneous candidosis). Mycoses. 2015;58(Suppl 1):1–15. https://doi.org/10.1111/myc.12292.
Dovnik A, Golle A, Novak D, Arko D, Takac I. Treatment of vulvovaginal candidiasis: a review of the literature. Acta dermatovenerologica Alpina, Pannonica, et Adriatica. 2015;24(1):5–7.
Workowski KA, Bolan GA, Centers for Disease C. Prevention: sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137.
Ilkit M, Guzel AB. The epidemiology, pathogenesis, and diagnosis of vulvovaginal candidosis: a mycological perspective. Crit Rev Microbiol. 2011;37(3):250–61. https://doi.org/10.3109/1040841X.2011.576332.
Mendling W, Brasch J. Guideline vulvovaginal candidosis (2010) of the german society for gynecology and obstetrics, the working group for infections and infectimmunology in gynecology and obstetrics, the german society of dermatology, the board of german dermatologists and the german speaking mycological society. Mycoses. 2012;55(s3):1–13.
Shi XY, Yang YP, Zhang Y, Li W, Wang JD, Huang WM, et al. Molecular identification and antifungal susceptibility of 186 Candida isolates from vulvovaginal candidiasis in southern China. J Med Microbiol. 2015;64(Pt 4):390–3. https://doi.org/10.1099/jmm.0.000024.
Hashemi SE, Shokohi T, Abastabar M, Aslani N, Ghadamzadeh M, Haghani I. Species distribution and susceptibility profiles of Candida species isolated from vulvovaginal candidiasis, emergence of C. lusitaniae. Curr Med Mycol. 2019;5(4):26–34. https://doi.org/10.18502/cmm.5.4.2062.
Kiasat N, Rezaei-Matehkolaei A, Zarei Mahmoudabadi A, Hamidavi Mohamadpour K, Molavi S, Khoshayand N. Prevalence of vulvovaginal candidiasis in Ahvaz, Southwest Iran: a semi-large scale study. Jundishapur J Microbiol. 2019;12(3):e89815.
Nagashima M, Yamagishi Y, Mikamo H. Antifungal susceptibilities of Candida species isolated from the patients with vaginal candidiasis. J Infect Chemother. 2016;22(2):124–6. https://doi.org/10.1016/j.jiac.2015.08.008.
Bitew A, Abebaw Y. Vulvovaginal candidiasis: species distribution of Candida and their antifungal susceptibility pattern. BMC Womens Health. 2018;18(1):94. https://doi.org/10.1186/s12905-018-0607-z.
ElFeky DS, Gohar NM, El-Seidi EA, Ezzat MM, AboElew SH. Species identification and antifungal susceptibility pattern of Candida isolates in cases of vulvovaginal candidiasis. Alexandria J Med. 2016;52(3):269–77. https://doi.org/10.1016/j.ajme.2015.10.001.
Shi Y, Zhu Y, Fan S, Liu X, Liang Y, Shan Y. Molecular identification and antifungal susceptibility profile of yeast from vulvovaginal candidiasis. BMC Infect Dis. 2020;20(1):287. https://doi.org/10.1186/s12879-020-04985-w.
Romeo O, Criseo G. First molecular method for discriminating between Candida africana, Candida albicans, and Candida dubliniensis by using hwp1 gene. Diagn Microbiol Infect Dis. 2008;62(2):230–3. https://doi.org/10.1016/j.diagmicrobio.2008.05.014.
Mirhendi H, Makimura K, Khoramizadeh M, Yamaguchi H. A one-enzyme PCR-RFLP assay for identification of six medically important Candida species. Nihon Ishinkin Gakkai zasshi =. Japanese J Med Mycol. 2006;47(3):225–9. https://doi.org/10.3314/jjmm.47.225.
Fontecha G, Montes K, Ortiz B, Galindo C, Braham S. Identification of cryptic species of four Candida complexes in a culture collection. J Fungi (Basel). 2019;5(4):117. https://doi.org/10.3390/jof5040117.
Diba K, Namaki A, Ayatolahi H, Hanifian H. Rapid identification of drug resistant Candida species causing recurrent vulvovaginal candidiasis. Med Mycol J. 2012;53(3):193–8. https://doi.org/10.3314/mmj.53.193.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition, vol. 28; 2008.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement, vol. 32; 2012.
Mady OY, Donia AM, Al-Madboly LA. Miconazole-urea in a buccal film as a new trend for treatment of resistant mouth fungal white patches. Front Microbiol. 2018;9:837. https://doi.org/10.3389/fmicb.2018.00837.
Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol. 2005;43(5):2155–62. https://doi.org/10.1128/JCM.43.5.2155-2162.2005.
Zeng X, Zhang Y, Zhang T, Xue Y, Xu H, An R. Risk factors of vulvovaginal candidiasis among women of reproductive age in Xi'an: a cross-sectional study. Biomed Res Int. 2018;2018:9703754.
Sustr V, Foessleitner P, Kiss H, Farr A. Vulvovaginal candidosis: current concepts, challenges and perspectives. J Fungi. 2020;6(4):267. https://doi.org/10.3390/jof6040267.
Ghaddar N, El Roz A, Ghssein G, Ibrahim J-N. Emergence of vulvovaginal candidiasis among Lebanese pregnant women: prevalence, risk factors, and species distribution. Infect Dis Obstet Gynecol. 2019;2019:5016810.
Paladine HL, Desai UA. Vaginitis: diagnosis and treatment. Am Fam Physician. 2018;97(5):321–9.
Linhares LM, Witkin SS, Miranda SD, Fonseca AM, Pinotti JA, Ledger WJ. Differentiation between women with vulvovaginal symptoms who are positive or negative for Candida species by culture. Infect Dis Obstet Gynecol. 2001;9(4):221–5. https://doi.org/10.1155/S1064744901000369.
Anh PK, Khanh NTN, Ha DT, Chien DT, Thuc PTB, Luong PH, et al. Prevalence of lower genital tract infection among women attending maternal and child health and family planning clinics in Hanoi, Vietnam. Southeast Asian J Trop Med Publ Health. 2003;34(2):367–73.
Thi Lien P, Elias C, Thi Loi N, Thi Chi B, Hua Phuc N, Gardner M. The prevalence of reproductive tract infections in hue, Vietnam. Stud Fam Plan. 2002;33(3):217–26. https://doi.org/10.1111/j.1728-4465.2002.00217.x.
Guzel AB, Ilkit M, Akar T, Burgut R, Demir SC. Evaluation of risk factors in patients with vulvovaginal candidiasis and the value of chromID Candida agar versus CHROMagar Candida for recovery and presumptive identification of vaginal yeast species. Med Mycol. 2011;49(1):16–25. https://doi.org/10.3109/13693786.2010.497972.
Shokoohi G, Rasekh-Jahromi A, Solhjoo K, Hasannezhad A, Nouripour-Sisakht S, Ahmadi B, et al. Molecular characterization and antifungal susceptibility of Candida species isolated from vulvovaginitis in Jahrom City, south of Iran. Jundishapur J Microbiol. 2020;13(10):e106825.
Gharaghani M, Ahmadi B, Taheripour Sisakht M, Ilami O, Aramesh S, Mouhamadi F, et al. Identification of Candida species isolated from vulvovaginal candidiasis patients by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) in Yasuj southwestern Iran. Jundishapur J Microbiol. 2018;11(8):e65359.
Grigoriou O, Baka S, Makrakis E, Hassiakos D, Kapparos G, Kouskouni E. Prevalence of clinical vaginal candidiasis in a university hospital and possible risk factors. Eur J Obstet Gynecol Reprod Biol. 2006;126(1):121–5. https://doi.org/10.1016/j.ejogrb.2005.09.015.
Hamad M, Kazandji N, Awadallah S, Allam H. Prevalence and epidemiological characteristics of vaginal candidiasis in the UAE. Mycoses. 2014;57(3):184–90. https://doi.org/10.1111/myc.12141.
Rathod SD, Klausner JD, Krupp K, Reingold AL, Madhivanan P. Epidemiologic features of vulvovaginal candidiasis among reproductive-age women in India. Infect Dis Obstet Gynecol. 2012;2012:859071.
Bignoumba M, Onanga R, Bivigou Mboumba B, Gafou A, Mouanga Ndzime Y, Lendamba RW, et al. Vulvovaginal candidiasis among symptomatic women of childbearing age attended at a medical analysis Laboratory in Franceville, Gabon. J de Mycologie Médicale. 2019;29(4):317–9. https://doi.org/10.1016/j.mycmed.2019.100895.
Konadu DG, Owusu-Ofori A, Yidana Z, Boadu F, Iddrisu LF, Adu-Gyasi D, et al. Prevalence of vulvovaginal candidiasis, bacterial vaginosis and trichomoniasis in pregnant women attending antenatal clinic in the middle belt of Ghana. BMC Pregnancy Childbirth. 2019;19(1):341. https://doi.org/10.1186/s12884-019-2488-z.
Namkinga LA, Matee MI, Kivaisi AK, Moshiro C. Prevalence and risk factors for vaginal candidiasis among women seeking primary care for genital infections in Dar Es Salaam, Tanzania. East Afr Med J. 2005;82(3):138–43. https://doi.org/10.4314/eamj.v82i3.9270.
Abdul-Aziz M, Mahdy MAK, Abdul-Ghani R, Alhilali NA, Al-Mujahed LKA, Alabsi SA, et al. Bacterial vaginosis, vulvovaginal candidiasis and trichomonal vaginitis among reproductive-aged women seeking primary healthcare in Sana’a city, Yemen. BMC Infect Dis. 2019;19(1):879. https://doi.org/10.1186/s12879-019-4549-3.
Yassin MT, Mostafa AA, Al-Askar AA, Bdeer R. In vitro antifungal resistance profile of Candida strains isolated from Saudi women suffering from vulvovaginitis. Eur J Med Res. 2020;25(1):1. https://doi.org/10.1186/s40001-019-0399-0.
Hazirolan G, Altun HU, Gumral R, Gursoy NC, Otlu B, Sancak B. Prevalence of Candida africana and Candida dubliniensis, in vulvovaginal candidiasis: first Turkish Candida africana isolates from vulvovaginal candidiasis. J de Mycologie Médicale. 2017;27(3):376–81. https://doi.org/10.1016/j.mycmed.2017.04.106.
Rosati D, Bruno M, Jaeger M, Ten Oever J, Netea MG. Recurrent vulvovaginal candidiasis: An immunological perspective. Microorganisms. 2020;8(2):144. https://doi.org/10.3390/microorganisms8020144.
Waikhom SD, Afeke I, Kwawu GS, Mbroh HK, Osei GY, Louis B, et al. Prevalence of vulvovaginal candidiasis among pregnant women in the ho municipality, Ghana: species identification and antifungal susceptibility of Candida isolates. BMC Pregnancy Childbirth. 2020;20(1):266. https://doi.org/10.1186/s12884-020-02963-3.
Khan M, Ahmed J, Gul A, Ikram A, Lalani FK. Antifungal susceptibility testing of vulvovaginal Candida species among women attending antenatal clinic in tertiary care hospitals of Peshawar. Infect Drug Resist. 2018;11:447–56. https://doi.org/10.2147/IDR.S153116.
Tsega A, Mekonnen F. Prevalence, risk factors and antifungal susceptibility pattern of Candida species among pregnant women at Debre Markos referral hospital, Northwest Ethiopia. BMC Pregnancy Childbirth. 2019;19(1):527. https://doi.org/10.1186/s12884-019-2494-1.
Fan SR, Liu XP, Li JW. Clinical characteristics of vulvovaginal candidiasis and antifungal susceptibilities of Candida species isolates among patients in southern China from 2003 to 2006. J Obstet Gynaecol Res. 2008;34(4):561–6. https://doi.org/10.1111/j.1447-0756.2008.00817.x.
Amouri I, Sellami H, Borji N, Abbes S, Sellami A, Cheikhrouhou F, et al. Epidemiological survey of vulvovaginal candidosis in Sfax, Tunisia. Mycoses. 2011;54(5):e499–505. https://doi.org/10.1111/j.1439-0507.2010.01965.x.
Mahmoudi Rad M, Zafarghandi S, Abbasabadi B, Tavallaee M. The epidemiology of Candida species associated with vulvovaginal candidiasis in an Iranian patient population. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):199–203. https://doi.org/10.1016/j.ejogrb.2010.11.022.
Willinger B, Manafi M. Evaluation of CHROMagar Candida for rapid screening of clinical specimens for Candida species. Mycoses. 1999;42(1–2):61–5. https://doi.org/10.1046/j.1439-0507.1999.00406.x.
Gamarra S, Morano S, Dudiuk C, Mancilla E, Nardin ME, de los Angeles Méndez E, et al. Epidemiology and antifungal susceptibilities of yeasts causing Vulvovaginitis in a teaching hospital. Mycopathologia. 2014;178(3):251–8. https://doi.org/10.1007/s11046-014-9780-2.
Kalkanci A, Güzel AB, Khalil IIJ, Aydin M, Ilkit M, Kuştimur S. Yeast vaginitis during pregnancy: susceptibility testing of 13 antifungal drugs and boric acid and the detection of four virulence factors. Med Mycol. 2012;50(6):585–93. https://doi.org/10.3109/13693786.2012.662597.
Dota KFD, Consolaro MEL, Svidzinski TIE, Bruschi ML. Antifungal activity of Brazilian propolis microparticles against yeasts isolated from vulvovaginal candidiasis. Evid Based Complement Alternat Med. 2011;2011:201953.
Ying C, Zhang H, Tang Z, Chen H, Gao J, Yue C. Antifungal susceptibility and molecular typing of 115 Candida albicans isolates obtained from vulvovaginal candidiasis patients in 3 Shanghai maternity hospitals. Med Mycol. 2015;54(4):394–9. https://doi.org/10.1093/mmy/myv082.
Liu XP, Fan SR, Peng YT, Zhang HP. Species distribution and susceptibility of Candida isolates from patient with vulvovaginal candidiasis in southern China from 2003 to 2012. J de Mycologie Médicale. 2014;24(2):106–11. https://doi.org/10.1016/j.mycmed.2014.01.060.
Yan L, Wang X-D, Seyedmousavi S, Yuan J-N, Abulize P, Pan W-H, et al. Antifungal susceptibility profile of Candida albicans isolated from vulvovaginal candidiasis in Xinjiang province of China. Mycopathologia. 2019;184(3):413–22. https://doi.org/10.1007/s11046-018-0305-2.
Phillips AJ. Treatment of non-albicans Candida vaginitis with amphotericin B vaginal suppositories. Am J Obstet Gynecol. 2005;192(6):2009–12. https://doi.org/10.1016/j.ajog.2005.03.034.
Kanafani ZA, Perfect JR. Resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis. 2008;46(1):120–8. https://doi.org/10.1086/524071.
Acknowledgments
We gratefully thank all the staff of the Department of Obstetrics and Gynecology (103 Military Hospital) who kindly contributed to patient handling and sample preparation. We are also indebted to the Department of Parasitology and Entomology (Vietnam Military Medical University) for providing the equipment used for the molecular analysis of the samples.
Funding
This work was partially supported by the Department of Science and Technology of Hanoi city, Vietnam, (grant no. 01C-08/01–2019-3, to DNA).
Author information
Authors and Affiliations
Contributions
DNA, NTV and NDB designed the study, carried out the experiments and analyzed the data statistically. DNH, VTS, NTNQ, NVT, LQT, NKL, and LTA collected the samples. NTNQ, NVT, and DNA identified species of the responsible agent. DNA and DMT drafted the manuscript. DNA and VTS prepared Figs. 1, 2, 3 and 4. TVT, VND, and NVL revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All subjects included in the study were informed of the purpose, benefits of the research and the procedures to be used in collecting the data. The inclusion criteria were non-pregnant women of reproductive age: (i) agreed to participate in the study, (ii) signed a written informed consent, didn’t take antifungal drugs within 2 weeks and (iii) gave informed consent were eventually included in the study. This study was approved by the institutional review board of Military Medical University under 4021/QĐ-HVQY and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. A written informed consent was obtained from all eligible women before entering the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Anh, D.N., Hung, D.N., Tien, T.V. et al. Prevalence, species distribution and antifungal susceptibility of Candida albicans causing vaginal discharge among symptomatic non-pregnant women of reproductive age at a tertiary care hospital, Vietnam. BMC Infect Dis 21, 523 (2021). https://doi.org/10.1186/s12879-021-06192-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-021-06192-7